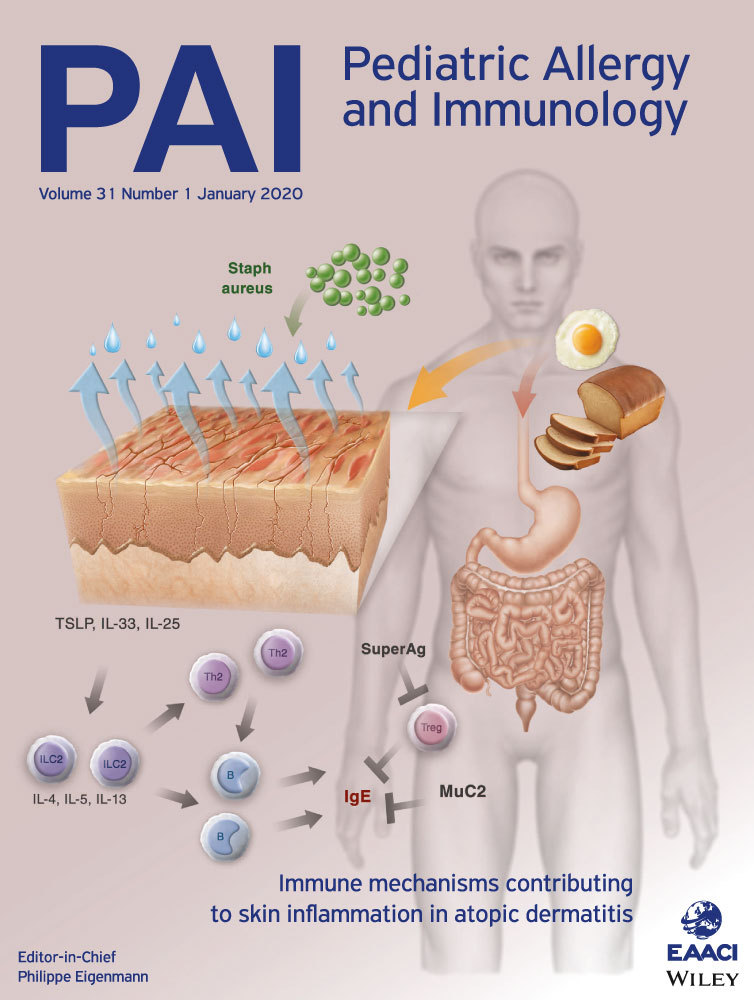
Epicutaneous sensitization to food allergens in atopic dermatitis: What do we know?
Corresponding Author
Elizabeth Huiwen Tham MMed, MCI
Department of Paediatrics, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Khoo Teck Puat-National University Children’s Medical Institute, National University Hospital, National University Health System, Singapore, Singapore
Correspondence
Elizabeth Huiwen Tham, Division of Allergy & Immunology, Department of Paediatrics, Yong Loo Lin School of Medicine, National University of Singapore, 1E Kent Ridge Road, Level 12 NUHS Tower Block, Singapore 119228, Singapore.
Email: [email protected]
Search for more papers by this authorMohana Rajakulendran MMed, MRCPCH
Khoo Teck Puat-National University Children’s Medical Institute, National University Hospital, National University Health System, Singapore, Singapore
Search for more papers by this authorBee Wah Lee MD
Department of Paediatrics, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Search for more papers by this authorHugo P. S. Van Bever PhD
Department of Paediatrics, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Khoo Teck Puat-National University Children’s Medical Institute, National University Hospital, National University Health System, Singapore, Singapore
Search for more papers by this authorCorresponding Author
Elizabeth Huiwen Tham MMed, MCI
Department of Paediatrics, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Khoo Teck Puat-National University Children’s Medical Institute, National University Hospital, National University Health System, Singapore, Singapore
Correspondence
Elizabeth Huiwen Tham, Division of Allergy & Immunology, Department of Paediatrics, Yong Loo Lin School of Medicine, National University of Singapore, 1E Kent Ridge Road, Level 12 NUHS Tower Block, Singapore 119228, Singapore.
Email: [email protected]
Search for more papers by this authorMohana Rajakulendran MMed, MRCPCH
Khoo Teck Puat-National University Children’s Medical Institute, National University Hospital, National University Health System, Singapore, Singapore
Search for more papers by this authorBee Wah Lee MD
Department of Paediatrics, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Search for more papers by this authorHugo P. S. Van Bever PhD
Department of Paediatrics, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Khoo Teck Puat-National University Children’s Medical Institute, National University Hospital, National University Health System, Singapore, Singapore
Search for more papers by this authorFunding information
Tham EH is funded by the National Medical Research Council (NMRC) Research Training Fellowship Grant [MH 095:003\008-225] from the National Medical Research Council (NMRC), Singapore.
Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disease mainly affecting children, which has no definitive curative therapy apart from natural outgrowing. AD is persistent in 30%-40% of children. Epithelial barrier dysfunction in AD is a significant risk factor for the development of epicutaneous food sensitization, food allergy, and other allergic disorders. There is evidence that prophylactic emollient applications from birth may be useful for primary prevention of AD, but biomarkers are needed to guide cost-effective targeted therapy for high-risk individuals. In established early-onset AD, secondary preventive strategies are needed to attenuate progression to other allergic disorders such as food allergy, asthma, and allergic rhinitis (the atopic march). This review aims to describe the mechanisms underpinning the development of epicutaneous sensitization to food allergens and progression to clinical food allergy; summarize current evidence for interventions to halt the progression from AD to food sensitization and clinical food allergy; and highlight unmet needs and directions for future research.
CONFLICT OF INTEREST
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors do not have any conflicts of interests to declare.
REFERENCES
- 1Kelleher M, Dunn-Galvin A, Hourihane JO, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015; 135(4): 930-5.e1.
- 2Rennick GJ, Moore E, Orchard DC. Skin prick testing to food allergens in breast-fed young infants with moderate to severe atopic dermatitis. Australas J Dermatol. 2006; 47(1): 41-45.
- 3Tsakok T, Marrs T, Mohsin M, et al. Does atopic dermatitis cause food allergy? A systematic review. J Allergy Clin Immunol. 2016; 137(4): 1071-1078.
- 4Johansson EK, Bergstrom A, Kull I, et al. IgE sensitization in relation to preschool eczema and filaggrin mutation. J Allergy Clin Immunol. 2017; 140(6): 1572-9.e5.
- 5Johansson E, Hershey G. Contribution of an impaired epithelial barrier to the atopic march. Ann Allergy Asthma Immunol. 2018; 120(2): 118-119.
- 6Hill DA, Spergel JM. The atopic march: critical evidence and clinical relevance. Ann Allergy Asthma Immunol. 2018; 120(2): 131-137.
- 7Host A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow's milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol. 2002; 13(s15): 23-28.
- 8Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. 1999; 103(6): 1173-1179.
- 9Berdyshev E, Goleva E, Bronova I, et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight. 2018; 3(4):e98006.
- 10Barker JN, Palmer CN, Zhao Y, et al. Null mutations in the filaggrin gene (FLG) determine major susceptibility to early-onset atopic dermatitis that persists into adulthood. J Investig Dermatol. 2007; 127(3): 564-567.
- 11Ashley SE, Tan HT, Vuillermin P, et al. The skin barrier function gene SPINK5 is associated with challenge-proven IgE-mediated food allergy in infants. Allergy. 2017; 72(9): 1356-1364.
- 12Marenholz I, Esparza-Gordillo J, Lee YA. The genetics of the skin barrier in eczema and other allergic disorders. Curr Opin Allergy Clin Immunol. 2015; 15(5): 426-434.
- 13Kim BE, Leung D. Significance of skin barrier dysfunction in atopic dermatitis. Allergy Asthma Immunol Res. 2018; 10(3): 207-215.
- 14Gavrilova T. Immune dysregulation in the pathogenesis of atopic dermatitis. Dermatitis. 2018; 29(2): 57-62.
- 15Czarnowicki T, Krueger JG, Guttman-Yassky E. Skin barrier and immune dysregulation in atopic dermatitis: an evolving story with important clinical implications. J Allergy Clin Immunol Pract. 2014; 2(4): 371-379; quiz 80–1.
- 16Noda S, Suarez-Farinas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015; 136(5): 1254-1264.
- 17Sanyal RD, Pavel AB, Glickman J, et al. Atopic dermatitis in African American patients is TH2/TH22-skewed with TH1/TH17 attenuation. Ann Allergy Asthma Immunol. 2019; 122(1): 99-110.e6.
- 18Totte JE, van der Feltz WT, Hennekam M, van Belkum A, van Zuuren EJ, Pasmans SG. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol. 2016; 175: 687-695.
- 19Spaulding AR, Salgado-Pabon W, Kohler PL, Horswill AR, Leung DY, Schlievert PM. Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev. 2013; 26(3): 422-447.
- 20Tauber M, Balica S, Hsu CY, et al. Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J Allergy Clin Immunol. 2016; 137(4): 1272-4.e3.
- 21Leung D, Calatroni A, Zaramela LS, et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci Transl Med. 2019; 11(480):eaav2685.
- 22Bunikowski R, Mielke M, Skarabis H, et al. Prevalence and role of serum IgE antibodies to the Staphylococcus aureus-derived superantigens SEA and SEB in children with atopic dermatitis. J Allergy Clin Immunol. 1999; 103(1 Pt 1): 119-124.
- 23Leung DY, Harbeck R, Bina P, et al. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest. 1993; 92(3): 1374-1380.
- 24Hauk PJ, Hamid QA, Chrousos GP, Leung DY. Induction of corticosteroid insensitivity in human PBMCs by microbial superantigens. J Allergy Clin Immunol. 2000; 105(4): 782-787.
- 25Leung DY, Hanifin JM, Pariser DM, et al. Effects of pimecrolimus cream 1% in the treatment of patients with atopic dermatitis who demonstrate a clinical insensitivity to topical corticosteroids: a randomized, multicentre vehicle-controlled trial. Br J Dermatol. 2009; 161(2): 435-443.
- 26Brauweiler AM, Goleva E, Leung D. Th2 cytokines increase Staphylococcus aureus alpha toxin-induced keratinocyte death through the signal transducer and activator of transcription 6 (STAT6). J Invest Dermatol. 2014; 134(8): 2114-2121.
- 27Classen A, Kalali BN, Schnopp C, et al. TNF receptor I on human keratinocytes is a binding partner for staphylococcal protein A resulting in the activation of NF kappa B, AP-1, and downstream gene transcription. Exp Dermatol. 2011; 20(1): 48-52.
- 28Brauweiler AM, Goleva E, Leung D Staphylococcus aureus lipoteichoic acid damages the skin barrier through an IL-1 mediated pathway. J Invest Dermatol. 2019; 139(8): 1753-1761.e4.
- 29Nakatsuji T, Chen TH, Two AM, et al. Staphylococcus aureus exploits epidermal barrier defects in atopic dermatitis to trigger cytokine expression. J Invest Dermatol. 2016; 136(11): 2192-2200.
- 30Gonzalez T, Biagini Myers JM, Herr AB, Khurana Hershey GK. Staphylococcal biofilms in atopic dermatitis. Curr Allergy Asthma Rep. 2017; 17(12): 81.
- 31Byrd AL, Deming C, Cassidy S, et al. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med. 2017; 9(397):eaal4651.
- 32Forbes-Blom E, Camberis M, Prout M, Tang SC, Le Gros G. Staphylococcal-derived superantigen enhances peanut induced Th2 responses in the skin. Clin Exp Allergy. 2012; 42(2): 305-314.
- 33Jones AL, Curran-Everett D, Leung D. Food allergy is associated with Staphylococcus aureus colonization in children with atopic dermatitis. J Allergy Clin Immunol. 2016; 137(4): 1247-8.e3.
- 34Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol. 2008; 121(6): 1331-1336.
- 35Chan SM, Turcanu V, Stephens AC, Fox AT, Grieve AP, Lack G. Cutaneous lymphocyte antigen and alpha4beta7 T-lymphocyte responses are associated with peanut allergy and tolerance in children. Allergy. 2012; 67(3): 336-342.
- 36Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011; 241(1): 241-259.
- 37Venkataraman D, Soto-Ramirez N, Kurukulaaratchy RJ, et al. Filaggrin loss-of-function mutations are associated with food allergy in childhood and adolescence. J Allergy Clin Immunol. 2014; 134(4): 876-82.e4.
- 38Kelleher MM, Dunn-Galvin A, Gray C, et al. Skin barrier impairment at birth predicts food allergy at 2 years of age. J Allergy Clin Immunol. 2016; 137(4): 1111-6.e8.
- 39Lowe AJ, Abramson MJ, Hosking CS, et al. The temporal sequence of allergic sensitization and onset of infantile eczema. Clin Exp Allergy. 2007; 37(4): 536-542.
- 40Martin PE, Eckert JK, Koplin JJ, et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clin Exp Allergy. 2015; 45(1): 255-264.
- 41Gray CL, Levin ME, du Toit G. Egg sensitization, allergy and component patterns in African children with atopic dermatitis. Pediatr Allergy Immunol. 2016; 27(7): 709-715.
- 42Minami T, Fukutomi Y, Sekiya K, Akasawa A, Taniguchi M. Hand eczema as a risk factor for food allergy among occupational kitchen workers. Allergol Int. 2018; 67(2): 217-224.
- 43Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003; 348(11): 977-985.
- 44Brough HA, Liu AH, Sicherer S, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol. 2015; 135(1): 164-170.
- 45Brough HA, Simpson A, Makinson K, et al. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol. 2014; 134(4): 867-75.e1.
- 46Fukutomi Y, Taniguchi M, Nakamura H, Akiyama K. Epidemiological link between wheat allergy and exposure to hydrolyzed wheat protein in facial soap. Allergy. 2014; 69(10): 1405-1411.
- 47Yokooji T, Kurihara S, Murakami T, et al. Characterization of causative allergens for wheat-dependent exercise-induced anaphylaxis sensitized with hydrolyzed wheat proteins in facial soap. Allergol Int. 2013; 62(4): 435-445.
- 48Jimenez-Saiz R, Ellenbogen Y, Koenig J, et al. IgG1(+) B-cell immunity predates IgE responses in epicutaneous sensitization to foods. Allergy. 2019; 74(1): 165-175.
- 49Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin Exp Allergy. 2005; 35(6): 757-766.
- 50Bartnikas LM, Gurish MF, Burton OT, et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J Allergy Clin Immunol. 2013; 131(2): 451-460.e6.
- 51Noti M, Kim BS, Siracusa MC, et al. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J Allergy Clin Immunol. 2014; 133(5): 1390-1399, 9, e1–6.
- 52Hussain M, Borcard L, Walsh KP, et al. Basophil-derived IL-4 promotes epicutaneous antigen sensitization concomitant with the development of food allergy. J Allergy Clin Immunol. 2018; 141(1): 223-34.e5.
- 53Galand C, Leyva-Castillo JM, Yoon J, et al. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J Allergy Clin Immunol. 2016; 138(5): 1356-1366.
- 54Hua TC, Hwang CY, Chen YJ, et al. The natural course of early-onset atopic dermatitis in Taiwan: a population-based cohort study. Br J Dermatol. 2014; 170(1): 130-135.
- 55Carlsten C, Dimich-Ward H, Ferguson A, et al. Atopic dermatitis in a high-risk cohort: natural history, associated allergic outcomes, and risk factors. Ann Allergy Asthma Immunol. 2013; 110(1): 24-28.
- 56Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014; 134(4): 818-823.
- 57Horimukai K, Morita K, Narita M, et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol. 2014; 134(4): 824-830.e6.
- 58Glatz M, Jo JH, Kennedy EA, et al. Emollient use alters skin barrier and microbes in infants at risk for developing atopic dermatitis. PLoS ONE. 2018; 13(2):e0192443.
- 59Lowe AJ, Su JC, Allen KJ, et al. A randomized trial of a barrier lipid replacement strategy for the prevention of atopic dermatitis and allergic sensitization: the PEBBLES pilot study. Br J Dermatol. 2018; 178(1): e19-e21.
- 60Lowe A, Su J, Tang M, et al. PEBBLES study protocol: a randomised controlled trial to prevent atopic dermatitis, food allergy and sensitisation in infants with a family history of allergic disease using a skin barrier improvement strategy. BMJ Open. 2019; 9(3):e024594.
- 61Chalmers JR, Haines RH, Mitchell EJ, et al. Effectiveness and cost-effectiveness of daily all-over-body application of emollient during the first year of life for preventing atopic eczema in high-risk children (The BEEP trial): protocol for a randomised controlled trial. Trials. 2017; 18(1): 343.
- 62Lowe AJ, Leung D, Tang M, Su JC, Allen KJ. The skin as a target for prevention of the atopic march. Ann Allergy Asthma Immunol. 2018; 120(2): 145-151.
- 63Doege K, Grajecki D, Zyriax BC, Detinkina E, Zu Eulenburg C, Buhling KJ. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood–a meta-analysis. Br J Nutr. 2012; 107(1): 1-6.
- 64Wickens K, Black PN, Stanley TV, et al. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2008; 122(4): 788-794.
- 65Cuello-Garcia CA, Brozek JL, Fiocchi A, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015; 136(4): 952-961.
- 66Fiocchi A, Pawankar R, Cuello-Garcia C, et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): probiotics. World Allergy Organ J. 2015; 8(1): 4.
- 67Greer FR, Sicherer SH, Burks AW. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics. 2019; 143(4):e20190281.
- 68Waidyatillake NT, Dharmage SC, Allen KJ, et al. Association between the age of solid food introduction and eczema: a systematic review and a meta-analysis. Clin Exp Allergy. 2018; 48(8): 1000-1015.
- 69Ohya Y. Early Aggressive Intervention for Infantile Atopic Dermatitis to Prevent Development of Food Allergy: a Multicenter, Investigator-Blinded, Randomized, Parallel Group Controlled Trial (PACI Study). UMIN-CTR Trial Registration ID: UMIN000028043. http://paci-study.jp/english.htmlhttps://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000031912. Accessed 1 May, 2019.
- 70Greenhawt M. Environmental exposure to peanut and the risk of an allergic reaction. Ann Allergy Asthma Immunol. 2018; 120(5): 476-81.e3.
- 71Simonte SJ, Ma S, Mofidi S, Sicherer SH. Relevance of casual contact with peanut butter in children with peanut allergy. J Allergy Clin Immunol. 2003; 112(1): 180-182.
- 72Perry TT, Conover-Walker MK, Pomes A, Chapman MD, Wood RA. Distribution of peanut allergen in the environment. J Allergy Clin Immunol. 2004; 113(5): 973-976.
- 73Brough HA, Makinson K, Penagos M, et al. Distribution of peanut protein in the home environment. J Allergy Clin Immunol. 2013; 132(3): 623-629.
- 74Leonardi S, Pecoraro R, Filippelli M, et al. Allergic reactions to foods by inhalation in children. Allergy Asthma Proc. 2014; 35(4): 288-294.
- 75Furlong TJ, DeSimone J, Sicherer SH. Peanut and tree nut allergic reactions in restaurants and other food establishments. J Allergy Clin Immunol. 2001; 108(5): 867-870.
- 76Brough HA, Santos AF, Makinson K, et al. Peanut protein in household dust is related to household peanut consumption and is biologically active. J Allergy Clin Immunol. 2013; 132(3): 630-638.
- 77Chmielewska A, Piescik-Lech M, Shamir R, Szajewska H. Systematic review: early infant feeding practices and the risk of wheat allergy. J Paediatr Child Health. 2017; 53(9): 889-896.
- 78Arshad SH. Allergen avoidance and prevention of atopy. Curr Opin Allergy Clin Immunol. 2004; 4(2): 119-123.
- 79 American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000; 106(2 Pt 1): 346-349.
- 80Prescott S, Allen KJ. Food allergy: riding the second wave of the allergy epidemic. Pediatr Allergy Immunol. 2011; 22(2): 155-160.
- 81Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012; 9: Cd000133.
- 82Snijders BE, Thijs C, van Ree R, van den Brandt PA. Age at first introduction of cow milk products and other food products in relation to infant atopic manifestations in the first 2 years of life: the KOALA Birth Cohort Study. Pediatrics. 2008; 122(1): e115-e122.
- 83Poole JA, Barriga K, Leung DY, et al. Timing of initial exposure to cereal grains and the risk of wheat allergy. Pediatrics. 2006; 117(6): 2175-2182.
- 84Katz Y, Rajuan N, Goldberg MR, et al. exposure to cow's milk protein is protective against IgE-mediated cow's milk protein allergy. J Allergy Clin Immunol. 2010; 126(1): 77-82.e1.
- 85Du Toit G, Katz Y, Sasieni P, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008; 122(5): 984-991.
- 86Koplin JJ, Osborne NJ, Wake M, et al. Can early introduction of egg prevent egg allergy in infants? A population-based study. J Allergy Clin Immunol. 2010; 126(4): 807-813.
- 87Zeiger RS, Heller S. The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol. 1995; 95(6): 1179-1190.
- 88Kajosaari M. Atopy prophylaxis in high-risk infants. Prospective 5-year follow-up study of children with six months exclusive breastfeeding and solid food elimination. Adv Exp Med Biol. 1991; 310: 453-458.
- 89Zutavern A, Brockow I, Schaaf B, et al. Timing of solid food introduction in relation to atopic dermatitis and atopic sensitization: results from a prospective birth cohort study. Pediatrics. 2006; 117(2): 401-411.
- 90Greer FR, Sicherer SH, Burks AW, American Academy of Pediatrics Committee on Nutrition, American Academy of Pediatrics Section on Allergy and Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008; 121(1): 183-191.
- 91Boyce JA, Assa'a A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-Sponsored Expert Panel Report. Nutrition. 2011; 27(2): 253-267.
- 92Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015; 372(9): 803-813.
- 93Du Toit G, Sayre PH, Roberts G, et al. Effect of avoidance on peanut allergy after early peanut consumption. N Engl J Med. 2016; 374(15): 1435-1443.
- 94Fleischer DM, Sicherer S, Greenhawt M, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants. J Allergy Clin Immunol. 2015; 136(2): 258-261.
- 95Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017; 139(1): 29-44.
- 96Joshi PA, Smith J, Vale S, Campbell DE. The Australasian Society of Clinical Immunology and Allergy infant feeding for allergy prevention guidelines. Med J Aust. 2019; 210(2): 89-93.
- 97Ierodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016; 316(11): 1181-1192.
- 98Wei-Liang Tan J, Valerio C, Barnes EH, et al. A randomized trial of egg introduction from 4 months of age in infants at risk for egg allergy. J Allergy Clin Immunol. 2017; 139(5): 1621-1628 e8.
- 99Perkin MR, Logan K, Tseng A, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016; 374(18): 1733-1743.
- 100Natsume O, Kabashima S, Nakazato J, et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind, placebo-controlled trial. Lancet. 2017; 389(10066): 276-286.
- 101du Toit G, Sayre PH, Roberts G, et al. Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About Peanut Allergy study cohort. J Allergy Clin Immunol. 2018; 141(4): 1343-1353.
- 102Tham EH, Lee BW, Chan YH, et al. Food allergy prevalence despite delayed introduction of allergenic foods-data from the GUSTO cohort. J Allergy Clin Immunol Prac. 2017; 6: 466-475.e1.
- 103Tham EH, Leung A, Pacharn P, et al. Anaphylaxis – lessons learnt when East meets West. Pediatr Allergy Immunol. 2019. https://doi.org/10.1111/pai.13098
- 104Fedorova OS, Ogorodova LM, Fedotova MM, Evdokimova TA. The prevalence of food allergy to peanut and hazelnut in children in Tomsk Region. Vopr Pitan. 2014; 83(1): 48-54.
- 105Orhan F, Karakas T, Cakir M, Aksoy A, Baki A, Gedik Y. Prevalence of immunoglobulin E-mediated food allergy in 6-9-year-old urban schoolchildren in the eastern Black Sea region of Turkey. Clin Exp Allergy. 2009; 39(7): 1027-1035.
- 106Tham EH, Shek LP, Van Bever HP, et al. Early introduction of allergenic foods for the prevention of food allergy from an Asian perspective-An Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI) consensus statement. Pediatr Allergy Immunol. 2018; 29(1): 18-27.
- 107Obbagy JE, English LK, Wong YP, et al. Complementary feeding and food allergy, atopic dermatitis/eczema, asthma, and allergic rhinitis: a systematic review. Am J Clin Nutr. 2019; 109(Suppl 7): 890s-934s.
- 108Lanser BJ, Leung D. The current state of epicutaneous immunotherapy for food allergy: a comprehensive review. Clin Rev Allergy Immunol. 2018; 55(2): 153-161.
- 109Jones SM, Sicherer SH, Burks AW, et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol. 2017; 139(4): 1242-52.e9.
- 110Tordesillas L, Mondoulet L, Blazquez AB, Benhamou PH, Sampson HA, Berin MC. Epicutaneous immunotherapy induces gastrointestinal LAP(+) regulatory T cells and prevents food-induced anaphylaxis. J Allergy Clin Immunol. 2017; 139(1): 189-201.e4.
- 111Mondoulet L, Dioszeghy V, Puteaux E, et al. Specific epicutaneous immunotherapy prevents sensitization to new allergens in a murine model. J Allergy Clin Immunol. 2015; 135(6): 1546-57.e4.
- 112Dioszeghy V, Mondoulet L, Laoubi L, et al. Antigen uptake by langerhans cells is required for the induction of regulatory T cells and the acquisition of tolerance during epicutaneous immunotherapy in OVA-sensitized mice. Front Immunol. 2018; 9: 1951.
- 113Myles IA, Williams KW, Reckhow JD, et al. Transplantation of human skin microbiota in models of atopic dermatitis. JCI Insight. 2016; 1(10).
- 114Myles IA, Earland NJ, Anderson ED, et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight. 2018; 3(9).
- 115Nakatsuji T, Chen TH, Narala S, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017; 9(378):eaah4680.
- 116Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012; 129(2): 434-440, 40.e1–2.
- 117Chen CC, Chen KJ, Kong MS, Chang HJ, Huang JL. Alterations in the gut microbiotas of children with food sensitization in early life. Pediatr Allergy Immunol. 2016; 27(3): 254-262.
- 118Ling Z, Li Z, Liu X, et al. Altered fecal microbiota composition associated with food allergy in infants. Appl Environ Microbiol. 2014; 80(8): 2546-2554.
- 119Dzidic M, Abrahamsson TR, Artacho A, et al. Aberrant IgA responses to the gut microbiota during infancy precede asthma and allergy development. J Allergy Clin Immunol. 2017; 139(3): 1017-25.e14.
- 120Azad MB, Konya T, Guttman DS, et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy. 2015; 45(3): 632-643.
- 121Cummings JH. Short chain fatty acids in the human colon. Gut. 1981; 22(9): 763-779.
- 122Kovarik JJ, Holzl MA, Hofer J, et al. Eicosanoid modulation by the short-chain fatty acid n-butyrate in human monocytes. Immunology. 2013; 139(3): 395-405.
- 123Segain JP, Raingeard de la Bletiere D, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut. 2000; 47(3): 397-403.
- 124Liu L, Li L, Min J, et al. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell Immunol. 2012; 277(1–2): 66-73.
- 125Diakos C, Prieschl EE, Saemann MD, et al. n-Butyrate inhibits Jun NH(2)-terminal kinase activation and cytokine transcription in mast cells. Biochem Biophys Res Commun. 2006; 349(2): 863-868.
- 126Millard AL, Mertes PM, Ittelet D, Villard F, Jeannesson P, Bernard J. Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clin Exp Immunol. 2002; 130(2): 245-255.
- 127Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013; 504(7480): 451-455.
- 128Roduit C, Frei R, Ferstl R, et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy. 2019; 74(4): 799-809.
- 129Wopereis H, Sim K, Shaw A, Warner JO, Knol J, Kroll JS. Intestinal microbiota in infants at high risk for allergy: effects of prebiotics and role in eczema development. J Allergy Clin Immunol. 2018; 141(4): 1334-42.e5.
- 130Zhang GQ, Hu HJ, Liu CY, Zhang Q, Shakya S, Li ZY. Probiotics for prevention of atopy and food hypersensitivity in early childhood: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine. 2016; 95(8):e2562.
- 131Muraro A, Halken S, Arshad SH, et al. EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy. 2014; 69(5): 590-601.
- 132Grimshaw KE, Maskell J, Oliver EM, et al. Diet and food allergy development during infancy: birth cohort study findings using prospective food diary data. J Allergy Clin Immunol. 2014; 133(2): 511-519.