Are avoidance diets still warranted in children with atopic dermatitis?
Corresponding Author
Philippe A. Eigenmann
Department of Woman, Child and Adolescent, Pediatric Allergy Unit, University Hospitals of Geneva, Geneva, Switzerland
Correspondence
Philippe A. Eigenmann, Pediatric Allergy Unit, University Hospitals of Geneva, Rue Willy-Donze 6, CH-1211 Geneva 14, Switzerland.
Email: [email protected]
Search for more papers by this authorKirsten Beyer
Department of Pediatric Pneumology, Immunology and Intensive Care, Charité Universitätsmedizin Berlin, Berlin, Germany
Search for more papers by this authorGideon Lack
Department of Pediatric Allergy, Division of Asthma, Allergy and Lung Biology, King’s College London and Guy’s and St Thomas’ National Health Service Foundation Trust, London, UK
Search for more papers by this authorAntonella Muraro
Department of Woman and Child Health, Food Allergy Centre, Padua University Hospital, Padua, Italy
Search for more papers by this authorPeck Y. Ong
Keck School of Medicine, Children's Hospital Los Angeles, University of Southern California, Los Angeles, CA, USA
Search for more papers by this authorScott H. Sicherer
Department of Pediatrics, Jaffe Food Allergy Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Search for more papers by this authorHugh A. Sampson
Department of Pediatrics, Jaffe Food Allergy Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Search for more papers by this authorCorresponding Author
Philippe A. Eigenmann
Department of Woman, Child and Adolescent, Pediatric Allergy Unit, University Hospitals of Geneva, Geneva, Switzerland
Correspondence
Philippe A. Eigenmann, Pediatric Allergy Unit, University Hospitals of Geneva, Rue Willy-Donze 6, CH-1211 Geneva 14, Switzerland.
Email: [email protected]
Search for more papers by this authorKirsten Beyer
Department of Pediatric Pneumology, Immunology and Intensive Care, Charité Universitätsmedizin Berlin, Berlin, Germany
Search for more papers by this authorGideon Lack
Department of Pediatric Allergy, Division of Asthma, Allergy and Lung Biology, King’s College London and Guy’s and St Thomas’ National Health Service Foundation Trust, London, UK
Search for more papers by this authorAntonella Muraro
Department of Woman and Child Health, Food Allergy Centre, Padua University Hospital, Padua, Italy
Search for more papers by this authorPeck Y. Ong
Keck School of Medicine, Children's Hospital Los Angeles, University of Southern California, Los Angeles, CA, USA
Search for more papers by this authorScott H. Sicherer
Department of Pediatrics, Jaffe Food Allergy Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Search for more papers by this authorHugh A. Sampson
Department of Pediatrics, Jaffe Food Allergy Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Search for more papers by this authorAbstract
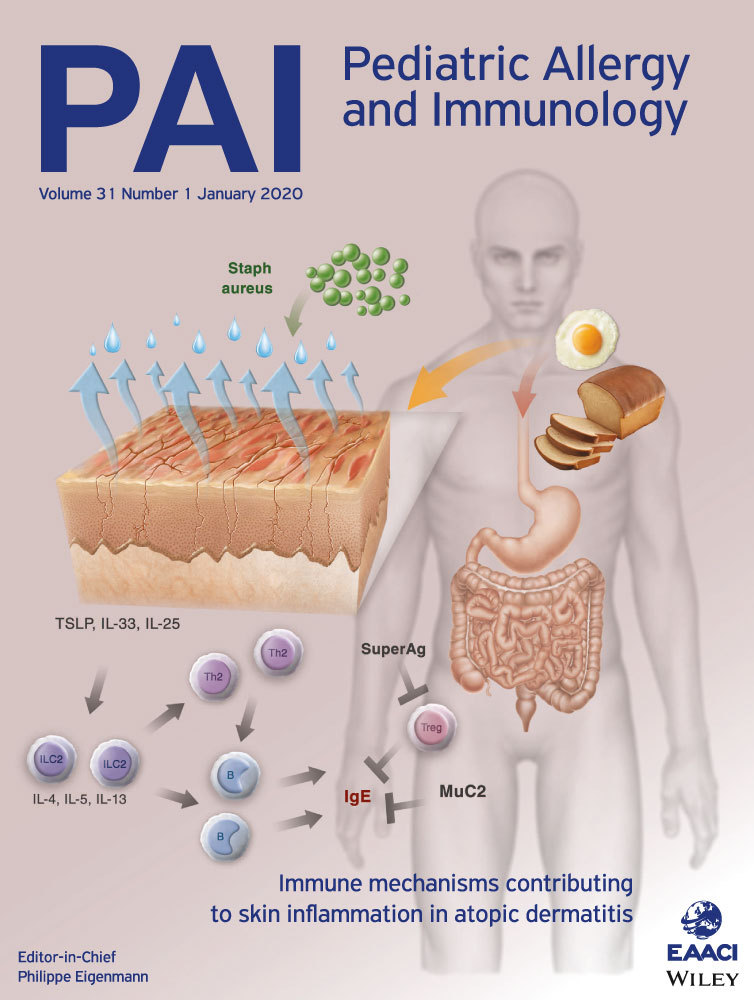
Nearly 40% of children with moderate-to-severe atopic dermatitis (AD) have IgE-mediated food allergy (FA). This clinical observation has been extensively documented by experimental data linking skin inflammation in AD to FA, as well as by food challenges reproducing symptoms and avoidance diets improving AD. Although food avoidance may improve AD, avoidance diets do not cure AD, may even have detrimental effects such as progression to immediate-type allergy including anaphylactic reactions, and may significantly reduce the quality of life of the patient and the family. AD care should focus upon optimal medical management, rather than dietary elimination. Food allergy testing is primarily indicated when immediate-type allergic reactions are a concern. In recalcitrant AD, if food is being considered a possible chronic trigger, a limited panel of foods may be tested. An avoidance diet is only indicated in patients clearly identified as food allergic by an appropriate diagnostic food challenge, and after adequately informing the family of the limited benefits, and possible harms of an elimination diet.
CONFLICT OF INTERESTS
PAE received speaker's honoraria and research support from Thermo Fisher Scientific, consultant fees from Nestlé, Abbott, DBV Technologies, reports royalty payments from UpToDate and Elsevier, and holds stock options in DBV Technologies. KB reports grants and personal fees from Aimmune, ALK, Danone, Hycor, and InfectoPharm, grants from DBV, Good Mills, Hipp, Thermo Fisher, and VDI, and personal fees from Allergopharma, Bausch & Lomb, Bencard Allergie, Di-Text, Hammer und Rall Media, Mabylon AG, Meda Pharma, Med Update, Mabylon, Meda Pharma, Nestlé, and Thermo Fisher. GL received research support from the National Institute of Allergy and Infectious Diseases, Food Allergy and Research Education, MRC and Asthma UK Centre, UK Department of Health through National Institute for Health Research Biomedical Research Centre based in Guy's and St Thomas' National Health Service Foundation Trust and King's College London, National Peanut Board, Food Standards Agency, and Action Medical Research, has stock/stock options with DBV Technologies, and is a shareholder in Mission Mighty Me. AM received speaker's fee from Mylan, DBV Technologies, and Aimmune, PYO reports research funding from Atopic Dermatitis Research Network, Regeneron, Pfizer, and consultant fees from Pfizer. SHS reports royalty payments from UpToDate and from Johns Hopkins University Press; grants to his institution from the National Institute of Allergy and Infectious Diseases, from Food Allergy Research and Education, and from HAL Allergy; and personal fees from the American Academy of Allergy, Asthma and Immunology, outside of the submitted work. HAS reports being a part-time employee of DBV Technologies, receiving consultant fees from N-Fold Therapeutics, holding stock options in DBV Technologies, and N-Fold Therapeutics, receiving grants from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, and co-owning a patent on FAHF-2.
REFERENCES
- 1Mastrorilli C, Caffarelli C, Hoffman-Sommergruber K. Food allergy and atopic dermatitis: prediction, progression, and prevention. Pediatr Allergy Immunol. 2017; 28: 831-840.
- 2Meyer R. Nutritional disorders resulting from food allergy in children. Pediatr Allergy Immunol. 2018; 29: 689-704.
- 3Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. J Allergy Clin Immunol. 1998; 101: S241-S241.
- 4Martin PE, Eckert JK, Koplin JJ, et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clin Exp Allergy. 2015; 45: 255-264.
- 5Tordesillas L, Berin MC, Sampson HA. Immunology of food allergy. Immunity. 2017; 47: 32-50.
- 6White MI, Jenkinson DM, Lloyd DH. The effect of washing on the thickness of the stratum corneum in normal and atopic individuals. Br J Dermatol. 1987; 116: 525-530.
- 7Werner Y, Lindberg M. Transepidermal water loss in dry and clinically normal skin in patients with atopic dermatitis. Acta Derm Venereol. 1985; 65: 102-105.
- 8Elias PM, Wakefield JS. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol. 2014; 134: 781-791.e1.
- 9Dainichi T, Kitoh A, Otsuka A, et al. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat Immunol. 2018; 19: 1286-1298.
- 10Leung D, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014; 134: 769-779.
- 11Leiferman KM, Ackerman SJ, Sampson HA, Haugen HS, Venencie PY, Gleich GJ. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis. Comparison with onchocerciasis. N Engl J Med. 1985; 313: 282-285.
- 12Nygaard U, Hvid M, Johansen C, et al. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J Eur Acad Dermatol Venereol. 2016; 30: 1930-1938.
- 13Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol. 2008; 121: 1331-1336.
- 14Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015; 372: 803-813.
- 15Matsumoto K, Mori R, Miyazaki C, Ohya Y, Saito H. Are both early egg introduction and eczema treatment necessary for primary prevention of egg allergy? J Allergy Clin Immunol. 2018; 141: 1997-2001.e3
- 16Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol. 2009; 123: 231-238.e4.
- 17Tordesillas L, Goswami R, Benedé S, et al. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J Clin Invest. 2014; 124: 4965-4975.
- 18Leung DY, Harbeck R, Bina P, et al. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest. 1993; 92: 1374-1380.
- 19Ong PY. Association between egg and staphylococcal superantigen IgE sensitizations in atopic dermatitis. Allergy Asthma Proc. 2014; 35: 346-348.
- 20Burgess JA, Dharmage SC, Allen K, et al. Age at introduction to complementary solid food and food allergy and sensitization: a systematic review and meta-analysis. Clin Exp Allergy. 49(6): 754–769.
- 21Sampson HA. The role of food allergy and mediator release in atopic dermatitis. J Allergy Clin Immunol. 1988; 81: 635-645.
- 22Sampson HA. Food sensitivity and the pathogenesis of atopic dermatitis. J R Soc Med. 1997; 90(Suppl 30): 2-8.
- 23Ahrens B, Niggemann B, Wahn U, Beyer K. Organ-specific symptoms during oral food challenge in children with food allergy. J Allergy Clin Immunol. 2012; 130: 549-551.
- 24Suomalainen H, Soppi E, Isolauri E. Evidence for eosinophil activation in cow’s milk allergy. Pediatr Allergy Immunol. 1994; 5: 27-31.
- 25Niggemann B, Beyer K, Wahn U. The role of eosinophils and eosinophil cationic protein in monitoring oral challenge tests in children with food-sensitive atopic dermatitis. J Allergy Clin Immunol. 1994; 94: 963-971.
- 26Abernathy-Carver KJ, Sampson HA, Picker LJ, Leung DY. Milk-induced eczema is associated with the expansion of T cells expressing cutaneous lymphocyte antigen. J Clin Invest. 1995; 95: 913-918.
- 27Beyer K, Castro R, Feidel C, Sampson HA. Milk-induced urticaria is associated with the expansion of T cells expressing cutaneous lymphocyte antigen. J Allergy Clin Immunol. 2002; 109: 688-693.
- 28Lever R, MacDonald C, Waugh P, Aitchison T. Randomised controlled trial of advice on an egg exclusion diet in young children with atopic eczema and sensitivity to eggs. Pediatr Allergy Immunol. 1998; 9: 13-19.
- 29Bath-Hextall F, Delamere FM, Williams HC. Dietary exclusions for established atopic eczema. Cochrane Database Syst Rev. 2008;(1): CD005203.
- 30Atherton DJ, Soothill JF, Sewell M, Wells RS, Chilvers CE. A double-blind controlled crossover trial of an antigen-avoidance diet in atopic eczema. Lancet. 1978; 311(8061): 401-403.
10.1016/S0140-6736(78)91199-6 Google Scholar
- 31Juto P, Engberg S, Winberg J. Treatment of infantile atopic dermatitis with a strict elimination diet. Clin Exp Allergy. 1978; 8: 493-500.
- 32Businco L, Businco E, Cantani A, Galli E, Infussi R, Benincori N. Results of a milk and/or egg free diet in children with atopic dermatitis. Allergol Immunopathol (Madr). 1982; 10: 283-288.
- 33Broberg A, Engström I, Kalimo K, Reimers L. Elimination diet in young children with atopic dermatitis. Acta Derm Venereol. 1992; 72: 365-369.
- 34Niggemann B, Binder C, Dupont C, Hadji S, Arvola T, Isolauri E. Prospective, controlled, multi-center study on the effect of an amino-acid-based formula in infants with cow’s milk allergy/intolerance and atopic dermatitis. Pediatr Allergy Immunol. 2001; 12: 78-82.
- 35David TJ. Anaphylactic shock during elimination diets for severe atopic eczema. Arch Dis Child. 1984; 59: 983-986.
- 36Flinterman AE, Knulst AC, Meijer Y, Bruijnzeel-Koomen CA, Pasmans SG. Acute allergic reactions in children with AEDS after prolonged cow’s milk elimination diets. Allergy. 2006; 61: 370-374.
- 37Larramendi CH, Esteban MM, Marcos CP, Fiandor A, Díaz Pena JM. Possible consequences of elimination diets in asymptomatic immediate hypersensitivity to fish. Allergy. 1992; 47: 490-494.
- 38Barbi E, Gerarduzzi T, Longo G, Ventura A. Fatal allergy as a possible consequence of long-term elimination diet. Allergy. 2004; 59: 668-669.
- 39Nachshon L, Goldberg MR, Elizur A, Appel MY, Levy MB, Katz Y. Food allergy to previously tolerated foods: course and patient characteristics. Ann Allergy Asthma Immunol. 2018; 121: 77-81.e1.
- 40Du Toit G, Sampson HA, Plaut M, Burks AW, Akdis CA, Lack G. Food allergy: update on prevention and tolerance. J Allergy Clin Immunol. 2018; 141: 30-40.
- 41Ho H-E, Chehade M. Development of IgE-mediated immediate hypersensitivity to a previously tolerated food following its avoidance for eosinophilic gastrointestinal diseases. J Allergy Clin Immunol Pract. 2018; 6: 649-650.
- 42Dharma C, Lefebvre DL, Tran MM, et al. Patterns of allergic sensitization and atopic dermatitis from 1 to 3 years: effects on allergic diseases. Clin Exp Allergy. 2018; 48: 48-59.
- 43Chang A, Robison R, Cai M, Singh AM. Natural history of food-triggered atopic dermatitis and development of immediate reactions in children. J Allergy Clin Immunol Pract. 2016; 4(2): 229-236.e1.
- 44Berry MJ, Adams J, Voutilainen H, Feustel PJ, Celestin J, Järvinen KM. Impact of elimination diets on growth and nutritional status in children with multiple food allergies. Pediatr Allergy Immunol. 2015; 26: 133-138.
- 45Sinai T, Goldberg MR, Nachshon L, et al. Reduced final height and inadequate nutritional intake in cow’s milk-allergic young adults. J Allergy Clin Immunol Pract. 2019; 7: 509-515.
- 46Tuokkola J, Luukkainen P, Nevalainen J, et al. Eliminating cows’ milk, but not wheat, barley or rye, increases the risk of growth deceleration and nutritional inadequacies. Acta Paediatr. 2017; 106(7): 1142-1149.
- 47Groetch M, Henry M, Feuling MB, Kim J. Guidance for the nutrition management of gastrointestinal allergy in pediatrics. J Allergy Clin Immunol Pract. 2013; 1: 323-331.
- 48Hobbs CB, Skinner AC, Burks AW, Vickery BP. Food allergies affect growth in children. J Allergy Clin Immunol Pract. 2015; 3(1): 133-134.e1.
- 49Mehta H, Ramesh M, Feuille E, Groetch M, Wang J. Growth comparison in children with and without food allergies in 2 different demographic populations. J Pediatr. 2014; 165: 842-848.
- 50Boyce JA, Assa'ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010; 126: S1-58.
- 51Muraro A, Werfel T, Hoffmann-Sommergruber K, et al. EAACI Food Allergy and Anaphylaxis Guidelines: diagnosis and management of food allergy. Allergy. 2014; 69: 1008-1025.
- 52Sova C, Feuling MB, Baumler M, et al. Systematic review of nutrient intake and growth in children with multiple IgE-mediated food allergies. Nutr Clin Pract. 2013; 28: 669-675.
- 53Jhamnani RD, Levin S, Rasooly M, et al. Impact of food allergy on the growth of children with moderate-severe atopic dermatitis. J Allergy Clin Immunol. 2018; 141(4): 1526-1529.e4.
- 54Renz H, Allen KJ, Sicherer SH, et al. Food allergy. Nat Rev Dis Primer. 2018; 4: 17098.
- 55Lieberman JA, Sicherer SH. Quality of life in food allergy. Curr Opin Allergy Clin Immunol. 2011; 11: 236-242.
- 56DunnGalvin A, Polloni L, Le Bovidge J, et al. Preliminary development of the food allergy coping and emotions questionnaires for children, adolescents, and young people: qualitative analysis of data on IgE-mediated food allergy from five countries. J Allergy Clin Immunol Pract. 2018; 6(2): 506-513.e11.
- 57Muraro A, Dubois A, DunnGalvin A, et al. Food Allergy and Anaphylaxis Guidelines. Food allergy health-related quality of life measures. Allergy. 2014; 69: 845-853.
- 58King RM, Knibb RC, Hourihane JO. Impact of peanut allergy on quality of life, stress and anxiety in the family. Allergy. 2009; 64: 461-468.
- 59LeBovidge JS, Strauch H, Kalish LA, Schneider LC. Assessment of psychological distress among children and adolescents with food allergy. J Allergy Clin Immunol. 2009; 124: 1282-1288.
- 60du Toit G, Sayre PH, Roberts G, et al. Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About Peanut Allergy study cohort. J Allergy Clin Immunol. 2018; 141: 1343-1353.
- 61Du Toit G, Roberts G, Sayre PH, et al. Identifying infants at high risk of peanut allergy: The Learning Early About Peanut Allergy (LEAP) screening study. J Allergy Clin Immunol. 2013; 131: 135-143.e12.
- 62Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017; 72: 1133-1147.
- 63Blumchen K, Trendelenburg V, Ahrens F, et al. Efficacy, safety, and quality of life in a multicenter, randomized, placebo-controlled trial of low-dose peanut oral immunotherapy in children with peanut allergy. J Allergy Clin Immunol Pract. 2019; 7(2): 479-491.e10.
- 64 PALISADE Group of Clinical Investigators, Vickery BP, Vereda A, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018; 379: 1991-2001.
- 65Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007; 62: 1261-1269.
- 66Burks AW, Jones SM, Wood RA, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012; 367: 233-243.
- 67Nowak-Węgrzyn A, Wood RA, Nadeau KC, et al. Multicenter, randomized, double-blind, placebo-controlled clinical trial of vital wheat gluten oral immunotherapy. J Allergy Clin Immunol. 2019; 143(2): 651-661.e9.
- 68Chu DK, Wood RA, French S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet. 2019; 393(10187): 2222-2232.