Emerging Roles of Macrophage Polarization in Osteoarthritis: Mechanisms and Therapeutic Strategies
Abstract
Osteoarthritis (OA) is the most common chronic degenerative joint disease in middle-aged and elderly people, characterized by joint pain and dysfunction. Macrophages are key players in OA pathology, and their activation state has been studied extensively. Various studies have suggested that macrophages might respond to stimuli in their microenvironment by changing their phenotypes to pro-inflammatory or anti-inflammatory phenotypes, which is called macrophage polarization. Macrophages accumulate and become polarized (M1 or M2) in many tissues, such as synovium, adipose tissue, bone marrow, and bone mesenchymal tissues in joints, while resident macrophages as well as other stromal cells, including fibroblasts, chondrocytes, and osteoblasts, form the joint and function as an integrated unit. In this study, we focus exclusively on synovial macrophages, adipose tissue macrophages, and osteoclasts, to investigate their roles in the development of OA. We review recent key findings related to macrophage polarization and OA, including pathogenesis, molecular pathways, and therapeutics. We summarize several signaling pathways in macrophage reprogramming related to OA, including NF-κB, MAPK, TGF-β, JAK/STAT, PI3K/Akt/mTOR, and NLRP3. Of note, despite the increasing availability of treatments for osteoarthritis, like intra-articular injections, surgery, and cellular therapy, the demand for more effective clinical therapies has remained steady. Therefore, we also describe the current prospective therapeutic methods that deem macrophage polarization to be a therapeutic target, including physical stimulus, chemical compounds, and biological molecules, to enhance cartilage repair and alleviate the progression of OA.
Introduction
Osteoarthritis (OA) is the most common chronic degenerative joint disease in middle-aged and elderly people, characterized by joint pain and dysfunction.1, 2 It is estimated that 240 million individuals worldwide have symptomatic OA currently, with women aged 60 and older having a 1.80-fold higher prevalence than men.3 Several risk factors have been reported to be associated with OA development, including gender, age, hereditary factors, diabetes, and obesity.4-8 In addition, the prevalence of OA is related to one's lifestyle and habits.9 Unfortunately, the prevalence of OA has been increasing in younger people in recent years, with a large increase in the proportion of patients under 65 years old.10 Thereafter, more and more people suffer from OA, which has a negative effect on the quality of their later life, resulting in a huge burden for individuals, families, and society.
It is widely acknowledged that inflammation plays a vital role in the development of OA. Macrophages, a kind of innate immune cells, regulate the immune and inflammatory process in the physiological state of a variety of diseases. Macrophages can be classified into two subtypes: activated macrophages (M1) and the other alternatively activated macrophages (M2). Generally, M1 is a kind of proinflammatory cell, whereas M2 plays opposite roles in the inflammation process. The transformation of macrophages from M1 to M2 in response to inflammatory stimuli is known as macrophage polarization, involved in the pathophysiological processes of many disorders, such as autoimmune diseases,11 infections,12 liver diseases,13 tumors,14 and OA.
Herein, we review recent key findings related to macrophage polarization and OA, including pathogenesis, molecular pathways, and therapeutics. After scanning, we summarize the macrophage polarization and underlying potential mechanism in different joint tissues such as synovium, infrapatellar fat pad (IPFP), and bone during the progression of OA. Despite the increasing availability of treatments for OA, like intra-articular (IA) injections, surgery, and cellular therapy, the demand for more effective clinical therapies remains high. Therefore, we also describe the current prospective therapeutic methods that deem macrophage polarization as a therapeutic target to enhance cartilage repair and alleviate the progression of OA.
Macrophage Classification in Osteoarthritis
Osteoarthritis is a disease that affects most joint tissues. Inflammation and the most abundant immune cell type within the joint, macrophages, have been recognized as possible players in disease development and progression. In the context of macrophage origin, macrophages emerge from two distinct lineages. Tissue-resident macrophages exhibit local self-renewal, independent of adult hematopoiesis,15-17 while short-lived monocyte-derived macrophages originate from adult hematopoietic stem cells, predominantly accumulating in inflamed lesions.18 The precise contribution of these macrophage lineages to the progression of OA remains unclear.
Numerous investigations have proposed that macrophages undergo phenotypic alterations toward proinflammatory or anti-inflammatory states in response to diverse forms of functional activation dictated by signals within their microenvironment.19-21 Specific phenotypes of macrophages might differentially modulate the anabolic or catabolic responses of different cell types with the onset or progression of OA.
Polarized Macrophage Classification
Broadly, macrophages are commonly categorized into two functionally activated forms, often denoted as exhibiting an M1 or M2 phenotype.22 These distinct phenotypes play divergent roles in either the initiation or advancement of OA.
M1 Macrophages
M1 macrophages, or classically activated macrophages, serve as antimicrobial and pro-inflammatory players, which are activated in response to stimuli from T helper type 1 (Th1) cells.23 M1 macrophages are usually activated by pattern recognition receptors (PRR), such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs), when they recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). Thereafter, this phenotype initiates the downstream inflammatory signaling pathways, such as nuclear factor (NF)-κB signaling, thereby inducing a massive release of pro-inflammatory cytokines and chemokines.24, 25
Studies have shown that enhanced synovial M1 macrophage polarization is responsible for the increased severity of OA.26 The destruction of cartilage mediated by M1 macrophages in the progression of OA has been highlighted in previous studies. Fahy et al.27 stated that the M1-associated cytokines IL-6, IL-1b, TNF-α, and oncostatin M (OSM) induced destructive processes in chondrocytes, including downregulation of collagen type II and aggrecan synthesis. In addition, synovial M1 macrophages were also shown to upregulate the production of proteolytic enzymes, such as matrix metalloproteinase (MMP)-1, MMP3, MMP13, MMP9 aggrecanases, and cyclooxygenase-2, which contribute to articular degeneration.28, 29 Inflammation of synovial membranes might be another etiology in which M1 macrophages participate. Sun et al. showed that obesity, as a generally acknowledged pathogenic factor for OA, is associated with spontaneous and local inflammation of the synovial membranes, which was followed by a predominant elevation of pro-inflammatory M1 macrophages and increased synovitis.30, 31 All of these abovementioned studies seem to prove that the promotion of M1 macrophage polarization can aggravate OA progression.26 However, conditional macrophage deletion has been demonstrated to have no effect on the development of OA.32 Interventions for macrophage M1 polarization to attenuate the severity of OA are awaiting investigation.
It is worth mentioning that an M1 macrophage-provoked excessive inflammatory response usually results in tissue destruction, which manifests as joint pain in OA patients. Li et al. found that IL-1β, IL-6, and TNF-α played critical roles in pain in the early stages of knee OA and were correlated with pain.33 Because pain is one of the most prominent symptoms of OA and a primary motivator of clinical decision-making, etiological research into pain is crucial not just for understanding OA but also for developing new medications to relieve it.
M2 Macrophages
M2 macrophages, also known as selectively activated macrophages, are primarily activated by “M2-related” polarizing factors such as IL-4, IL-10, TGF-β, and dexamethasone,34 which have pro-healing or anti-inflammatory effects. M2 macrophages are further subdivided into M2a, M2b, and M2c, which exhibit anti-inflammatory characteristics and contribute to tissue repair and remodeling.35 Activation of mechanistic target of rapamycin complex 1 (mTORC1) increased synovial M1 macrophage polarization, which exacerbated cartilage degeneration and osteophyte formation in experimental OA models. Inhibition of mTORC1, in contrast, increased M2 polarization and attenuated OA development.26
In recent years, much progress has been made regarding the mechanism of macrophage polarization, which is a promising strategy in the treatment of OA. Zheng et al. reported that IA administration of D0 exosomes could lead to mitochondrial dysfunction and polarize macrophage response toward an M2 phenotype, thereby successfully preventing the development of OA.36 Bailey et al. reported that in macrophage-depleted macrophage Fas-induced apoptosis (MaFIA) mice, macrophage polarity shifted to the dominance of M1 macrophages and reduction of M2 macrophages in the synovial stroma, indicating a shift in the M1/M2 macrophage ratio in the joint following injury.37 However, macrophage depletion of both M1 and M2 subtypes has a confusing effect on OA progression. Wu et al. demonstrated that macrophage depletion by a small molecule (AP20187) in a MaFIA-transgenic mouse model exhibited decreased osteophyte formation immediately following depletion but did not attenuate the severity of OA.32 Those studies indicated that the failure to transform from the M1 to M2 subtype might play a larger role in the progression of OA than the quantity of activated macrophages.
Macrophages at Different Physiological Sites
Macrophages accumulate and become polarized (M1 or M2) in many tissues such as synovium, adipose tissue, bone marrow, mucosa, and bone mesenchymal tissues in joints, which comprise the appendicular skeleton, synovium, cartilage, tendons, ligaments, joint capsules, and their associated lymphatics and vasculature, while resident macrophages and other stromal cells, including fibroblasts, endothelial cells, chondrocytes, and osteoblasts, form the joint and function as an integrated unit.38 In the following sections, we focus exclusively on synovial macrophages, adipose tissue macrophages, and osteoclasts (Figure 1).
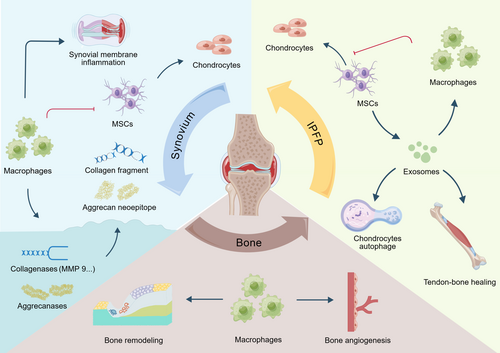
Synovium
The synovial membrane has a two-layer structure, which is divided into the inner surface layer and the bottom layer. The inner surface layer is composed of two to three layers of macrophages and fibro-like synovial cells, wherein the synovial cells can secrete synovial fluid and the macrophages can phagocytic worn debris. The bottom layer consists of fibrous connective tissue, blood vessels, a few lymphocytes, and macrophages.
The role of polarized macrophages in synovium tissue during the process of OA has been highlighted. It has been demonstrated that M1 macrophage polarization in the synovium exacerbates the progression of OA.39 In investigating the mechanism involved in this macrophage-driven degradative progression, Bondeson and his colleagues discovered that depletion of macrophages in OA synovial explants significantly reduced the production of several cytokines, including TNF, IL-1, IL-6, and IL-8. Importantly, macrophage depletion and neutralization of macrophage-derived TNF and IL-1 could downregulate MMP production, thereby linking synovial macrophages to cartilage degradation.40 Moreover, Blom et al. confirmed that synovial macrophages were involved in MMP-mediated cartilage degeneration in the murine collagenase-induced model of OA.41 These findings could indicate that OA synovial macrophages play an important role in perpetuating the production of proinflammatory cytokines and destructive enzymes. In recent years, further studies have been conducted to explore the roles and regulatory mechanisms of synovial macrophages and their polarization in the development of OA. Sun et al. (2022) reported that synovial M1 macrophage polarization was stimulated by mTORC1 activation and, in turn, exacerbated cartilage degeneration and osteophyte formation in experimental OA, whereas M2 polarization was enhanced by mTORC1 inhibition and attenuated OA development.35 Synovial macrophages, which serve as modulators and producers of nerve growth factor (NGF) in joint synovial tissue, have been shown to be associated with pain sensitivity in OA joints.42 Further, it is reported that the role of synovial macrophages is regulated by TNF-α, which can alleviate joint pain in OA by upregulating the NGF signal transduction produced by synovial macrophages in OA joints.43
In addition to synovium tissue, macrophages were also detected in the synovial fluid of OA patients.44 Kraus et al. detected M1 (iNOS) and/or M2 (TGF-b) macrophage markers in synovial fluid cells of two OA patients by immunostaining.21 Kulkarni et al. (2022) induced U937 cells with synovial fluid of progressive KL grades and indicated that synovial fluid in OA joints acted as a niche and facilitated M1 phenotype polarization by providing a proinflammatory microenvironment.45 Using transgenic mouse models with enhanced M1- or M2-polarized macrophages, Zhang et al. showed that synovial macrophage M1 polarization exacerbated experimental collagenase-induced OA while M2 polarization attenuated OA development.26 The findings of the above mentioned studies reveal the critical role of synovial M1 and M2 macrophages in the development of OA.
Infrapatellar Fat Pad
The infrapatellar fat pad (IPFP), also known as Hoffa's fat pad, is located below the patella and fills the potential space between the posterior patellar tendon, the condyle of the femur, and the anterior tibial plateau. It is a structure of extra-synovial fat tissue within the bursae of the knee joint, composed of adipose tissue similar to subcutaneous fat. IPFP acts as an important organizational structure involved in the occurrence and development of OA,46 and the resident macrophages of IPFP contribute to the onset and progression of inflammatory joint diseases.47
The macrophages in IPFP participate in the pathogenesis of OA and other joint diseases. Not only the increasing number of macrophages but also M1-like (CD11c+) and M2-like (CD206+) macrophage markers have been reported in this tissue.48, 49 People with IPFP signal intensity alteration have a high risk of accelerated OA, which is characterized by local inflammation.50 Wei et al. suggested that macrophages harvested from the IPFP of diseased joints inhibit chondrogenesis of mesenchymal stem cells (MSCs),51 supporting the notion that IPFP macrophages play a potentially detrimental role in cartilage regeneration. Recent studies showed that the exosomes derived from several types of MSCs could maintain chondrocyte homeostasis and ameliorate the pathological severity of OA.52, 53 In an acute synovial/IPFP inflammation rat model, IPFP-MSC exosome therapeutic treatment resulted in robust macrophage polarization toward an anti-inflammatory therapeutic M2 phenotype within the synovium/IFP tissues.47 Wu et al. showed that miR-100-5p-abundant exosomes derived from IPFP-MSCs promote autophagy of chondrocytes through mTOR inhibition.54 IPFP MSC-derived exosomes accelerate tendon–bone healing and IA graft remodeling after anterior cruciate ligament reconstruction, which might result from the immunomodulation of macrophage polarization.55
Bone
According to earlier OA research, cartilage breakdown is no longer the only pathogenic alteration in OA. In contrast, it is a whole joint condition encompassing cartilage as well as non-cartilage tissues such as subchondral bone. Microstructural analysis suggested that cartilage and bone alterations occur concurrently,56 while analysis of the biomechanical properties of subchondral bone revealed that bone changes might occur before cartilage damage.57 Using novel microstructural analysis techniques, a comparative study by Chen et al. identified a drastic loss of rod-like trabeculae and thickening of plate-like trabeculae in all regions of the tibial plateau even if the cartilage was still intact.58 Thus, it is conceivable that changes in bone density and/or bone microstructure could be early pathologic characteristics of OA.
In the subchondral bone, rapid bone loss after traumatic injuries and bone sclerosis at the end stage are well-recognized hallmarks of OA. There is a large body of evidence supporting that enhanced subchondral bone turnover plays an active and pivotal role in the onset and progression of OA in which the subchondral bone compartment undergoes active remodeling, a process that is partially influenced by macrophages.59, 60 A study in 2020 showed that abnormally increased platelet-derived growth factor (PDGF)-BB secretion by mononuclear preosteoclasts induced subchondral bone angiogenesis, which contributed to OA development.61 Another study demonstrated that platelet-related scaffold for tissue engineering could promote osteochondral repair through immune regulation by M2 polarization and is a potential candidate for osteochondral tissue engineering.62 These findings indicated that some newly developed molecules or materials targeted at macrophage polarization might be effective biological reagents to prevent and treat OA in the clinic.
Signaling Pathways in Macrophage Polarization
Signaling pathways are essential for OA development. A growing body of literature has clarified the effect of signaling pathways in OA and provides evidence that targeting these axes might be a viable therapeutic approach. However, it seems that simply activating or inhibiting these signaling pathways could be a double-edged sword, with unavoidable side effects.
As mentioned before, macrophage polarization is dynamically affected by the various stimuli in the microenvironment. However, the underlying mechanism of this process remains obscure. Understanding the regulators and effectors of these signaling pathways on macrophage polarization would be useful to precisely target the polarized macrophage subsets, resulting in improved therapeutic efficacy with less toxicity in OA treatment.
Herein, we summarized several signaling pathways in macrophage reprogramming, including NF-κB, MAPK, TGF-β, JAK/STAT, PI3K/Akt/mTOR, and NLRP3 (Figure 2). More in-depth studies of more specific molecular mechanisms in the macrophage polarization will likely help translate it from bench to clinic since signaling pathways have multiple effectors and regulators in our body, which leads to complex and dynamic processes in the body.
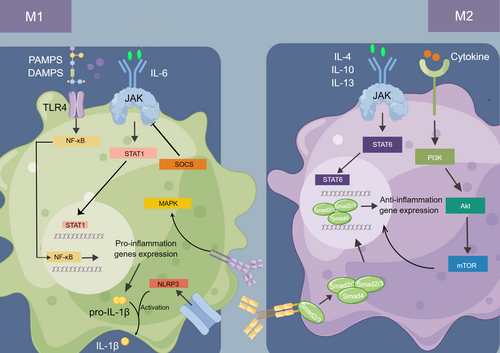
NF-κB Signaling Pathway
The NF-κB is generally acknowledged as a family of ubiquitous transcription factors in a great range of cells. It is involved in the adaptive and innate immune response and the pathogenesis of inflammation-related disease, cancer, and other diseases.63 It is well established that the NF-κB family consists of five distinct proteins, including p50, p52, Rel A (p65), Rel B, and c-Rel, which yield homodimers and heterodimers, thus affecting cells’ biological process.64
Generally, the NF-κB activation has the classical and alternative pathways. The classical one responds to external stimulus and mediates the activation of p50, p65, and c-Rel, whereas p52 and Rel-B are activated in the alternative pathway.65, 66 Then, the phosphorylation of the inhibitor of NF-κB (IκB) proteins by the IκB kinase (IKK) complex (IKKα, ΙΚΚβ, ΙΚΚγ) is followed by degradation of IκB and release of the NF-κB dimers from inhibition, which activate the transcription of target genes.67, 68 In contrast, the alternate pathway depends on NF-κB inducing kinase (NIK), resulting in the phosphorylation of p52, and finally RelB/p52 dimer translocates into nuclear toward specific gene activation.66, 69
Emerging evidence has shown the critical role of the NF-κB pathway in M1 polarization and subsequently inflammatory cytokine release, such as IL-1β, IL-6 and TNF-α.70, 71 In OA, when PAMPs from microorganisms or DAMPs from damaged tissue are released, the activation of Toll-like receptors (TLRs) leads to the M1 polarization, which is regulated via the TLR/NF-κB signaling pathway.72 This implies that the block of TLRs might be a potential target for not only M1 polarization but also a promising OA therapy. Berberine was found to inhibit M1 polarization through a block of the TLR4/MyD88/NF-κB signaling pathway and attenuate inflammation in the early phase.73 Likewise, Mo et al. (2023) found that knockdown of pellino1 (Peli1) could not only inhibit M1 polarization through the TLR signaling pathway but also produce anti-apoptotic effects.74 Moreover, Barreto et al. demonstrated that lumican co-stimulation with lipopolysaccharide provided a pro-inflammatory stimulus, upregulating macrophage polarization toward the M1-like phenotype.75 Consistently, chondrocyte death was also significantly upregulated in a TLR4-dependent manner, which is a feature of OA, often through apoptosis and autophagy mechanisms.76 Beyond TLRs, p65 could also serve as a target for M1 polarization through inhibition of the NF-κB pathway. Li et al. (2023) described the role of PCAF in NF-κB activation during M1 polarization with regulation p65 activation through its pivotal role in p65 acetylation.77 Additionally, peroxisome proliferator receptors (PPAR) comprise a family of nuclear hormone receptors, which can evoke anti-inflammatory outcomes and promote M2 polarization.78 Previous studies have demonstrated that PPAR directly interacts with p65 and p50, which prevents them from binding to specific DNA sequences.79 However, the link between inhibition of NF-κB and enhancement of M2 polarization remains obscure and needs further investigation.
Transforming Growth Factor β Signaling Pathway
Transforming growth factor β (TGF-β) and related growth factors are secreted pleiotropic factors that play critical roles in embryogenesis and adult tissue homeostasis by regulating cell proliferation, differentiation, migration, and death.80 The TGF-β superfamily comprises 33 members, including TGF-β, Nodal, Activin, and bone morphogenetic proteins (BMPs).81 Binding TGF-β family proteins to heteromeric complexes composed of two type I and two type II receptors on the plasma membrane, the type I transmembrane kinase is phosphorylated and activated following the activation of specific type II receptors. Activated type I receptor initiates intracellular signaling through phosphorylating the C termini of specific SMAD proteins, R-Smads, the intracellular signaling effectors.82, 83 This event activates the R-Smads and enables the formation of heteromeric complexes between two R-Smads (SMAD2 and/or SMAD3) and one common-Smad (co-Smad), Smad4, and then translocates into the nucleus to direct transcriptional response and exert the transcription of target genes.84, 85 In addition, the inhibitory Smads (I-Smads), Smad6 and Smad7, antagonize the signaling mediated by R-Smads and co-Smad.86
An increasing number of studies have suggested that macrophage polarization is associated with the TGF-β signaling pathway. Numerous studies describe the role of M2-phenotype tumor-associated macrophage (TAM) in distinct cancers’ progression.87 CD155+ TAMs show an M2 phenotype and promote colorectal cancer cell migration, invasion, and tumor growth, regulated by TGF-β-induced STAT3 activation-mediated release of matrix metalloproteinases (MMP) 2 and MMP9 in CRC cells.88 Similarly, the pro-tumor roles of M2 TAMs by TGF-β signaling have been described in breast cancer.89 Strikingly, constitutive miR-182 deficiency and conditional knockout in macrophages impair M2-like TAMs and breast tumor development, while reconstitution of miR-182-expressing macrophages promotes tumor growth.
A similar phenomenon of M2 polarization with an elevated level of the TGF-β pathway has been observed in other diseases, such as OA.90-92 In Dai et al., squid type II collagen immunomodulates M2 macrophage polarization to skew the local OA microenvironment toward a pro-chondrogenic atmosphere, with elevation of TGF-β1 and TGF-β3, and promotes cartilage repair under inflammatory conditions.90 Moreover, previous studies showed that eucommia ulmoides polysaccharides promoted the expression of TGF-β accompanied with decreased M1 polarization and accumulating M2 polarization.91 Further, Tissue Gene-C (TG-C), a novel cell and gene therapy, has been used in the treatment of OA, which caused increased levels of TGF-β1 and IL-10, induced the expression of arginase 1, a marker of M2 macrophages, and decreased the expression of CD86, a marker of M1 macrophages.92 This evidence suggests a strong link between TGF-β and macrophage polarization. However, how TG-C induces an M2 macrophage-dominant microenvironment through TGF-β signaling was not mentioned in this study.92
Mitogen-Activated Protein Kinase Signaling Pathway
Mitogen-activated protein kinase (MAPK) signaling pathways play a crucial role in human diseases, including inflammation and cancer,93 which are cascade reactions of three kinases. Initially, the most upstream kinase (MAPKKK) receives various extracellular and intracellular signals and activates the middle kinase (MAPKK) through direct phosphorylation. Then, MAPKK exclusively phosphorylate and activate MAPK to make its substrates execute specific cell fate decisions.94-96 MAPK, including extracellular signal-regulated kinases (ERKs), c-JUN NH2-terminus kinases (JNKs), and p38 MAPK, typically affect cell proliferation, differentiation, apoptosis, and metabolism, regulating the biological behavior of cells through various signaling pathways.97
Emerging studies highlight the vital role of MAPK signaling in macrophage polarization in a variety of diseases. Over the past few years, a series of studies examined the macrophage polarization in the cancer, mechanistically regulated by MAPK signaling. In 2023, Zheng et al. identified that FBXO38 promotes macrophage immunosuppressive function by upregulating the expression of M2-like genes through MAPK signaling, which affects the development of cancer or colitis.98 Qiu et al. discuss the pro-tumor role of exosome miR-519a-3p, which activates the MAPK/ERK pathway, thereby causing M2-like polarization of macrophages, accelerating the gastric cancer liver metastasis by inducing angiogenesis and promoting intrahepatic premetastatic niche formation.99
The regulatory roles of MAPK signaling in macrophage polarization and cartilage degradation have also been demonstrated in the pathogenesis and progression of OA.100, 101 Wu et al. found that the increase of IL-6 and TNF-α production, which are released by M1 macrophages and further deteriorate synovial inflammation, is mediated via the ERK, p38, and JNK signaling pathways.40, 102 Further, a recent study has shown that inhibition of the phosphorylation of MAPK in macrophages attenuates the infiltration of pro-inflammatory M1-type macrophages and articular cartilage degeneration,103 which suggests that the MAPK pathway might serve as a promising target for OA treatment. More interestingly, the MAPK pathway might become involved in the complication of wear particles in total joint replacements. This is evident in the case of M1 polarization induced by orthopaedic implant materials,104 where pre-treatment of macrophages with MAPK inhibitors not only prevented M1 polarization but also pro-inflammatory gene expression. This finding provides the basis for further improving our understanding of the signaling cascades activated by wear particles and paves the way for new treatments in the future.
JAK/STAT Signaling Pathway
The JAK/STAT pathway is one of the crucial nodes that demonstrates how extracellular communication works, thus leading to multiple biological responses. A range of cytokines are recognized as ligands that activate the JAK/STAT pathway, which consequently regulates the target gene expression, including interleukins, colony-stimulating factors (CSFs), hormone-like cytokines, and growth factors.105, 106 JAK is a family of non-transmembrane tyrosine kinases, including JAK1, JAK2, JAK3, and Tyk2. As signals transmit via receptors, the receptor-associated JAKs activate, mediate tyrosine phosphorylation of receptors, and subsequently recruit STATs for further activation, tyrosine phosphorylation, and dimerization. The STAT family consists of STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6, which are activated by different cytokines and show distinct biological effects. Thereafter, the dimers translocate to the nucleus and finally regulate transcription.107
A wealth of evidence has described that macrophage polarization regulates the proinflammation and anti-inflammation via the JAK/STAT pathway in vitro and in many diseases, such as rheumatoid arthritis,108, 109 inflammatory bowel disease,110 and cancers.111 Previous studies have analyzed different cytokines that induce M1 or M2 polarization through distinct JAK/STAT pathways. To sum up, proinflammatory cytokines like IL-6 activate the M1 phenotype through the JAK2/STAT3 pathway,112 whereas pro-healing or anti-inflammatory cytokines like IL-4, IL-10, and IL-13 promote alternative macrophage polarization via the JAK/STAT pathway.113 IL-10 was reported to act as a signal through JAK1/STAT3, which was responsible for higher negative feedback control of inflammation.114 In addition, both IL-4 and IL-13 were found to activate the JAK3-STAT6 signaling inducing M2 polarization.115 Meanwhile, in Bhattacharjee et al., IL-13 was found to utilize JAK2-STAT3 and Tyk2-STAT1/STAT6, whereas IL-4 could only use JAK1-STAT3/STAT6.116
Suppressors of cytokine signaling (SOCS) proteins are a family of negative regulators of the JAK/STAT pathway, including CIS, SOCS1, SOCS2, SOCS3, SOCS4, SOCS5, SOCS6, and SOCS7. They function as an impediment or inhibitor by binding to the phosphorylated JAKs and their associated receptors. Therefore, SOCS proteins could be a prospective target for controlling JAK/STAT signaling in diseases.117 Likewise, SOCS might also have an effect on macrophage polarization by regulating the JAK/STAT pathway. For instance, downregulated SOCS1 expression was found to activate the JAK1/STAT1 pathway, thereby promoting the polarization of macrophages into M1 type.118 Fascinatingly, an increasing body of literature supports the strong link between altered cellular metabolism and macrophage polarization as an adaptation for alternative cellular functions. Several studies have described how the JAK/STAT pathway was involved in this process.119-121 Creatine was found to reprogram macrophage polarization toward M2 phenotype by suppressing the JAK/STAT1 pathway, while supporting IL-4/STAT6/activated arginase 1 expression by enhancing chromatin remodeling.120 These findings reveal the underlying mechanism of the connection between changes in metabolism and macrophage polarization.
During the pathogenesis of OA, the JAK/STAT pathway has participated in the inflammatory and immune responses, cartilage remodeling, and metabolism.122, 123 Macrophage polarization is acknowledged as a crucial mechanism for inflammation in OA, as described above. Hu et al.124 and Dai et al.90 reported STAT6-regulated M2 polarization in vitro and in vivo, which could be a potential target for OA treatment. More studies on OA mechanisms and using JAK/STAT as a therapeutic target are urgently needed.
PI3K/Akt/mTOR Signaling Pathway
The PI3K includes three classes of enzymes: class I, II, and III. Among them, class I PI3Ks attract wide concentration from researchers. Initiated by growth factors, cytokines, and insulin, PI3Ks are downstream molecules of tyrosine kinases, G protein-coupled receptors and GTPases, which activate PI3Ks.125 Later, activated PI3Ks catalyze the phosphorylation of phosphatidylinositol-4,5-bisphosphate into phosphatidylinositol-3,4,5-trisphosphate, which is a second messenger and further activates downstream effectors like protein kinases (e.g., Akts). Akts, consisting of Akt1, Akt2 and Akt3, are recruited to the inner surface of cytoplasmic membrane, where it is phosphorylated by phosphoinositide-dependent kinase-1. Thereafter, it activates various downstream molecules, such as mammalian target of rapamycin (mTOR), thus engaging in the biological process, including cell proliferation, survival, metabolism, apoptosis, and motility.126
Several studies emphasize the significance of the PI3K/Akt/mTOR pathway in cancer, with its dysregulation in almost all human cancers, including breast cancer, colorectal cancer, and hematologic malignancies.127 In recent years, a growing number of studies have focused on the PI3K/Akt pathway in macrophage polarization during cancer progression. In Geraldo et al., SLIT2 promoted microglia tumor-supportive polarization through ROBO1- and ROBO2-mediated PI3K-γ activation, regulating the glioblastoma microenvironment and immunotherapeutic target for brain tumors.128 A similar phenomenon has been observed in esophageal squamous cell carcinoma.129 Interestingly, FOXO1(+) tumor-induced M2 macrophages promoted tumor proliferation through the FAK–PI3K–AKT pathway and could be impeded by the PI3K inhibitor. Beyond the cancer, the PI3K/Akt pathway mediated macrophage polarization also becomes involved in the pathogenesis of other diseases, such as rheumatoid disease,130 pancreatitis,131 and OA.132
A wealth of studies reveal the correlation between the PI3K/Akt/mTOR signaling pathway and OA pathogenesis, including versatile functions in cartilage, synovial tissue, and subchondral bone.133 Of note, previous studies have demonstrated that PI3K/Akt/mTOR engaged in the process of macrophage reprogramming in OA.132 Several studies have demonstrated that the PI3K/Akt/mTOR pathway is a critical step for M2 polarization. Liu et al.134 used 3D-M-EF scaffolds to induce M2 polarization through PI3K/Akt signaling in vitro, which provided a beneficial microenvironment for tissue regeneration and engineering. Similarly, Li et al.135 modulated the PI3K/Akt to alter macrophage to M2 phenotype through mRNA mediation in an OA model. In addition, PI3K/Akt signaling potentially regulated M1 macrophages to exert anti-inflammatory roles. In 2023, metformin was found to hamper the M1 polarization of synovial sublining macrophages through the PI3K/Akt pathway, thus lessening cartilage loss and finally attenuating OA development.136 However, related findings have rarely been reported so far, which means further exploration of the underlying mechanism is needed.
NLRP3 Pathway
NLRP3 is a critical Nod-like receptor (NLR) family member that forms the inflammasomes, protein complexes following the recognition of PAMPs and DAMPs during infection or stress.137 Inflammasomes consist of a sensor receptor, the adaptor apoptosis-associated speck-like protein containing a CARD (ASC), and the effector protease caspase 1.138 Most inflammasome receptors are NLR sensors, an essential family of PRRs, including molecules like NLRP1, NLRP3, and NLRP6. Among them, NLRP3 is the most characterized inflammasome, which comprises a pyrin domain (PYD), a NACHT domain hydrolyzing nucleotides with ATPase activity, and a leucine-rich repeat (LRR) domain.139 Generally, the classical activation of NLRP3 pathway occurs in two signal steps. The first one is a prime signal, which requires activation of TLRs and the NF-κB pathway, thus increasing the pro-IL-1β, pro-IL-18, and NLRP3 protein in the cytosol.140 Then, triggered by a great range of PAMPs or DAMPs, the second signal induces NLRP3 oligomerization and recruits downstream molecules, ASC and procaspase-1, to come together to form the inflammasome.137 Ultimately, the NLRP3 activates caspase-1, which cleaves pro-inflammatory cytokines into an active form, thereby leading to an immune response.141 Furthermore, active caspase-1 also triggers the cleavage of inflammatory cytokines IL-1β and IL-18 and gasdermin D, which induces a proinflammatory programmed cell death known as pyroptosis.142 Additionally, dysregulation of NLRP3 inflammasome could drive a variety of inflammation responses associated with diseases such as OA.143
Emerging evidence has shown that NLRP3 might be one of the regulatory mechanisms of macrophage polarization in diseases. Several previous studies have supported this suggestion in vivo and in vitro. Wisitpongpun et al. (2022) found that oleamide promoted M1 macrophage polarization and increased IL-1β production by activating the NLRP3 inflammasome in primary monocyte-derived macrophages.144 Similarly, M1 macrophage polarization requiring NLRP3 inflammasome activation mediated Th1 and Th17 differentiation, thus leading to choline-metabolized trimethylamine N-oxide-induced graft-versus-host disease. In addition, Liu et al. showed that ubiquitin-specific protease 19 (USP19) acts as an anti-inflammatory switch that inhibits inflammatory responses and promotes M2-like macrophage polarization by stabilizing NLRP3 inflammasome in vitro and in a peritonitis model in vivo.145 Appealingly, similar results concerned critical role of NLRP3 inflammasome in macrophage polarization have been reported in OA, which provides a promising therapeutic strategy for OA. Sun et al. (2022) revealed that inhibition of transient receptor potential channel subfamily V member 4 (TRVP4), an ion channel, inhibited M1 macrophage polarization through the ROS/NLRP3 pathway, consequently attenuating OA progression.146 In the same manner, Luo et al. demonstrated that IL-37 induces M2 macrophage polarization through a process that requires IL-1R8/NLRP3, which appears to be a potential therapy target for temporomandibular joint OA.147
Application of Macrophage Polarization in the Treatment of Osteoarthritis
As is well known, treatments for OA are increasingly diverse, but there is still a need for more effective ways to alleviate the inflammatory progression. There are many ways to treat OA, including physical stimulus, chemical compounds, and biological molecules. Studies have demonstrated that most of these methods are related to macrophage polarization. Thus, we summarize the treatment of OA by targeting macrophage polarization as a potential target (Table 1).
| Type | Name | Study sample | Mechanism; function | Signaling pathway; target | Efficacy | Reference | |
|---|---|---|---|---|---|---|---|
| Physical Stimulus | Moderate physical activity | MIA-induced OA rat model | MPA generates synovial fluid and lipids in the infrapatellar fat pad, producing LXA4 in the knee joint. LXA4 act on synovial macrophages to promote M2 polarization | – | M2↑ | 148 | |
| Low intensity pulsed ultrasound | DMM-induced OA mouse model; THP-1 cells, RAW264.7 cells | LIPUS downregulates the high expression of LPS-induced p-JNK and p-p65 proteins; inhibites NF-κB nuclear translocation in osteoblasts induced by LPS | JNK; NF-κB | M1↓, M2↑ | 149 | ||
| Chemical Compounds | Hyaluronic acid | Human primary synoviocytes obtained from knee joint OA patients; THP-1 cells | HMW-HA can inhibit the expression of GRP78 and the activiation of NF-κB | GRP78/NF-κB; target: CD44 receptor | M1↓, M2↑ | 70 | |
| Rapamycin | CIOA and DMM-induced OA mouse model | Rapamycin inactivates the complexes of the mTOR. Activated mTORC1 increases M1 polarization in both CIOA and DMM models, while inhibited mTORC1 enhanced M2 polarization and alleviated CIOA in mice | Target: mTORC1 | M1↓, M2↑ | 26 | ||
| Itaconate | DMM-induced OA mouse model; RAW264.7 macrophages | Exogenous supplementation of itaconate can activate Nrf2, and accordingly inhibit the STING-dependent NF-κB pathway | Nrf2/STING/ NF-κB | M1↓, M2↑ | 150 | ||
| Platelet-Rich Plasma | CIOA-induced OA mouse model | PRP contains high levels of IL-1Ra that can inhibit acute inflammation caused by IL-1 | Target: IL-IR | M2↑ | 151 | ||
| Single compounds derived from TCM | Fargesin | CIOA-induced OA mouse model; RAW264.7 macrophages | M1 macrophages treated with fargesin reduced the expression of catabolic markers (MMP13, RUNX2, and ColX) and increases chondrogenic markers (Col2a1 and SOX9) in OA chondrocytes | p38/ERK MAPK p65/NF-κB |
M1↓, M2↑ | 152 | |
| Frugoside | CIOA-induced OA mouse model; RAW264.7 cells | Frugoside inhibits macrophage M1 polarization by partially downregulating miR-155 expression | Target: miR-155 | M1↓ | 153 | ||
| Quercetin | OA mouse model induced by removing the medial meniscus and transecting the anterior meniscotibial ligament; RAW264.7 cells | Quercetin inhibites the expression of inflammatory mediators and matrix degrading proteases and upregulates expression of cartilage anabolic factors in IL-1β-induced rat chondrocytes | Akt/NF-κB | M2↑ | 124 | ||
| Biological Molecules | Mesenchy-me stem cells and their exosomes | BMSC-derived exosomes | OA mouse model induced by the modified Hulth technique; RAW264.7 cells | BMSC-derived exosomes promote the transformation of synovial macrophages from M1 to M2, reducing the infiltration of synovial inflammatory cells | – | M1↓, M2↑ | 154 |
| hUCMSCs | ACLT-induced OA rat model | hUCMSCs-EVs could alleviate OA progression likely via transferring key proteins and miRNAs to regulate the PI3K-Akt signaling pathway | PI3K-Akt | M1↓, M2↑ | 135 | ||
| AMSCs | Synovial tissues obtained from OA patients; subcutaneous abdominal fat obtained from healthy patients; human monocytes | PGE2, produced by ASC, directly inhibits the inflammatory cytokines TNFα and IL-6 and can induce production of the anti-inflammatory cytokine IL-10 and expression of CD163 and CD206 | PGE2/COX2 | M1↓, M2↑ | 155 | ||
| IPFP MSC-derived exosomes | Infrapatellar fat pad and articular cartilage obtained from OA patients | Exosomes derived from mesenchymal stem cells in IPFP can inhibit the mTOR signaling pathway through miR-100-5p, enhance autophagy, inhibit apoptosis, promote extracellular matrix synthesis | mTOR target: miR-100-5p | M1↓ | 54 | ||
| Artificial M2 macrophage | Papain solution-induced OA mouse model; RAW264.7 cells | AM2M could decrease joint surface erosion, chondrocyte apoptosis and loss of glycosaminoglycan content, and downregulate the secretion level of the inflammatory factors such as IL-Iβ, IL-6, and IL-17 | – | M2↑ | 156 | ||
| Meta-Defensome | Patients with OA; RAW264.7 cells | Meta-defensomes reprogram the mitochondrial metabolism of M1 macrophages by scavenging mitochondrial reactive oxygen species and inhibiting mitochondrial NO synthase, thereby increasing mitochondrial transcription factor A expression and restoring aerobic respiration. | – | M1↓, M2↑ | 157 | ||
| Modified ZIF-8 Nanoparticles | ACLT-induced OA rat model | Infiltration of the synovial M1 macrophages (CD16/32-positive cells is decreased after treatment with modified NPs, and NPs upregulates synovial M2 phenotype macrophages (CD206-positive cells) | – | M1↓, M2↑ | 158 | ||
- Abbreviations: ACLT, anterior cruciate ligament transection; Akt, protein kinase B; AM2M, artificial M2 macrophage; AMSCs, adipose-derived mesenchymal stem cells; BMSC, bone marrow mesenchymal stem cell; CIOA, collagenase-induced OA; ColX, collagen X; Col2a, collagen Type II Alpha 1; COX2, cyclooxygenase-2; DMM, destabilization of the medial meniscus; ERK, extracellular regulated protein kinase; EVs, extracellular vesicles; GRP78:78-kD glucose-regulated protein; HMW-HA, high molecular weight hyaluronic acid; hUCMSCs, human umbilical cord mesenchymal stem cells; IL-IR, interleukin-1 receptor; IPFP-MSC, infrapatellar fat pad mesenchymal stem cell; JNK, Jun N-terminal kinase; LIPUS, low intensity pulsed ultrasound; LPS, lipopolysaccharide; LXA4, lipoxin A4; MAPK, mitogen-activated protein kinase; MIA, monoiodoacetic acid; miR-100-5p, microRNA-100-5p; miR-155, microRNA-155; MMP13, matrix metalloproteinase protein 13; MPA, moderate physical activity; mTORC1, mammalian target of rapamycin C1; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NPs, nanoparticles; Nrf2, nuclear factor-erythroid 2-related factor 2; OA, osteoarthritis; PGE2, prostaglandin E2; PI3K, phosphatidylinositol 3-kinase; RUNX2, Runt-related transcription factor 2; STING, stimulator of interferon genes; PRP, platelet rich plasma; TNFα, tumor necrosis factor alpha.
Physical Stimulus Targeted at Macrophage Polarization
Moderate Physical Activity
Moderate physical activity (MPA) refers to activity that requires 3.0–5.9 times higher energy than that of the resting state and plays a crucial role in joint health.159, 160 In recent years, MPA has been widely used as a treatment in patients after arthroplasty due to increasing muscle strength, reducing postoperative pain, recovery times, and costs.161, 162 In a rat model with peroxisome proliferator-activated receptors γ (PPARγ) deletion in myeloid cells, Silveira et al. found that PPARγ was essential to exercise-induced M2 peritoneal macrophage recruitment and polarization, which strongly supported the association between exercise and macrophage polarization.163 There is also evidence showing that MPA produces a hypoxic environment in blood vessels, which promotes platelet aggregation, lipoxygenase activation, and LXA4 generation. In the above study, LXA4 has also been proven to promote M2 polarization and reduce inflammatory cytokines in the synovium, thereby relieving OA.148 In summary, MPA could facilitate M2 polarization of synovial macrophages, which is a potential therapeutic tool for OA. However, there are many forms of MPA. Additionally, treadmill exercise in animals cannot mimic the activities or common exercises that OA patients might perform. Further, participation in any physical activity must be balanced with the injury risk, and extreme fitness trends of the past result in more knee OA cases. As such, mitigating injury risk is paramount for participating in physical activity.164, 165
Low-Intensity Pulsed Ultrasound
Low-intensity pulsed ultrasound (LIPUS), a kind of non-invasive and safe physical therapy, has been widely used in the treatment of fractures and other musculoskeletal diseases.166 A large number of clinical studies suggest that LIPUS can alleviate the degeneration of knee cartilage. Therefore, it is regarded as a potential treatment for OA.167 LIPUS was found to increase the specific protein CD163, one of the major changes in macrophages converted to the M2 subtype, when it was used at the later stage of tendon–bone interface (TBI) healing. This evidence demonstrated that macrophage polarization might be a potential mechanism of LIPUS treatment.168 Sun et al. (2020) also showed that LIPUS treatment decreases the proportion of M1 macrophages and increases the M2 macrophages in the joint synovium through suppressing the JNK and NF-κB signaling pathways.149 Above all, LIPUS has been considered an effective strategy for patients with OA.
The main limitation of this therapy is that no clinical studies have been reported on its role in the treatment of OA patients. Scientific research is generally carried out on animals, whose knees have different anatomical and physiological characteristics to humans. On the one hand, this might affect the reproducibility of results obtained in human clinical studies in the future, and on the other hand, there is a lack of parameter and dosage information for giving this physical therapy to humans, which has implications for safety.169
Chemical Compounds Targeted at Macrophage Polarization
Hyaluronic Acid
Intra-articular injection of hyaluronic acid (HA) is a common treatment of knee OA for patients. Nonsteroidal anti-inflammatory drugs are not effective or contraindicated to replace the lost HA in synovial fluid.170 Macrophage-derived inflammatory and degenerative molecules, including TNF-α, IL-6, IL-1β, MMPs, and ADAMTS, which lead to synovitis, have significantly reduced expression after using HA alone or in combination.171-173 Jin et al. (2022) showed that the ratio of M1 to M2 was steadily increased after HA intra-articular injection, which means intra-articular hyaluronic acid (IAHA) might play an anti-inflammatory functional role through macrophage polarization.173 Furthermore, Lee et al. found that high molecular weight HA (HMW-HA) could affect the polarization of synovial macrophages through the inhibition of the GRP78/NF-κB pathway, which increased the M2/M1 ratio and decreased pro-inflammatory cytokines.70 However, Rayahin et al. demonstrated that hyaluronic acid has molecular weight-dependent effects in modulating the macrophage phenotype.174 HMW-HA increases the M2 phenotype, whereas HA of a low molecular weight increases the M1 phenotype.70
However, until now, the effects of age, symptoms, and severity of OA patients on the clinical efficacy of HA have been unclear. Some studies have shown that older patients and those with advanced arthritis are less likely to benefit from intra-articular HA injections.175 Other studies have shown that younger patients with moderate symptoms or only early radiological signs of OA do not appear to gain any benefit from HA injections.176 Therefore, long-term follow up results after such injections remain to be investigated in future studies.
Rapamycin
Rapamycin, a potential inhibitor of T cell proliferation, is widely used as a medication to prevent organ transplant rejection through inactivating the mTOR. The mTOR, a ubiquitous serine/threonine kinase, plays a crucial role in regulating cell growth, proliferation, and survival.177 There is increasing evidence that the mTOR is closely related to macrophage polarization.178-182 There are two interacting complexes of the mTOR, mTORC1 and mTORC2, which regulate T cell lineage specification and macrophage differentiation.177 The present study demonstrates that treatment with rapamycin in vitro reduces mTORC1 and enhances mTORC2 activity in T cells of healthy individuals. However, whether long-term treatment with rapamycin can also activate mTORC2 in vivo is unclear. Therefore, it is uncertain whether the therapeutic effect of rapamycin in OA patients stems solely from the blocking of mTORC1 or also involves the activation of mTORC2.177 As mentioned above, Zhang et al. found that enhancement of M1 macrophages was associated with the mTORC1 activation in the synovium of OA patients.26 They also concluded that mTORC1-induced M1 polarization stimulates inflammatory cytokine production and Rspo2 secretion, which promotes a series of characteristic changes in OA, such as degradation of matrix proteoglycan and cartilage.26
Itaconate
Itaconate, regulated by immune-responsive gene 1 (Irg1), is an unsaturated dicarboxylic acid derived from the tricarboxylic acid cycle.183 A previous study has shown that itaconate can be highly expressed in macrophages to inhibit their inflammatory activities, thus playing a role in the regulation of macrophage function.184 There is mounting evidence demonstrating that itaconate can reduce the degree of inflammation as an Nrf2 agonist.183-187
Itaconate was found to have the ability to alleviate the progression of OA by reducing the destruction of cartilage and regulating macrophage polarization. Pan et al. found that four-octyl itaconate (4-OI) could improve autophagy in chondrocytes by regulating the PI3K/AKT/mTOR signaling pathway.188 Interestingly, Guo et al. (2022) also demonstrated that itaconate could promote M2 polarization in macrophages, reducing the release of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, thereby relieving synovial inflammation and chondrocyte apoptosis.150
Nevertheless, most of the current studies have only investigated the effects of itaconate on the inflammation and injury of articular cartilage in the Nrf2 pathway.189, 190 Therefore, more experiments should be performed in future to reveal the signaling pathway of itaconate in chondrocyte protection.
Platelet-Rich Plasma
Platelet-rich plasma (PRP) is a platelet concentrate obtained from autologous whole blood after centrifugation that contains a large number of growth factors such as PDGF, transforming growth factor β (TGF-β), insulin-like growth factor 1 (IGF-1), and vascular endothelial growth factor (VEGF).191 PRP releases various growth factors (mentioned above) that are involved in the regulation of bone regeneration in the local microenvironment of bone defects to accelerate wound healing and the tissue repair process.192 The result of a previous study that PRP from OA patients could contribute to the inhibition of chondrocyte matrix synthesis and enhanced macrophage inflammation in vitro demonstrated the strong link between PRP and OA treatment.193
Of note, data from a mouse OA model suggested that the articular cavity of knees might have fewer proinflammatory and more anti-inflammatory macrophages after injection of PRP. Surprisingly, Khatab et al. demonstrated that PRP suppresses IL-1-induced acute inflammation and promotes M2 macrophage polarization due to the high level of IL-1 receptor antagonist it contains.151 Furthermore, Uchiyama et al. proved that injection of PRP in knees could transform M1 macrophages into M2 macrophages in different ways, such as directly polarizing M1 macrophages to M2 macrophages, aggregating macrophages and monocytes around the joint, and unifing polarizing into M2 macrophages.194 These results demonstrate that PRP is a therapeutic target of OA through its function on macrophage polarization in the joint and synovium.
Overall, most of the low-quality research evidence suggests that intra-articular injection of PRP is a safe treatment with the potential to provide symptomatic benefits for OA in the short term, and there are studies suggesting that younger patients and those with less structural changes in the knee joint might be more sensitive to PRP.195 However, these results need to be confirmed in high-quality clinical trials, and further research is needed to identify patient characteristics that make them suitable for PRP. Due to the lack of research in this area, there is currently no recommendation on the optimal PRP protocol for patients with OA.
Single Compounds Derived from Traditional Chinese Medicine
The formulations used in traditional Chinese medicine (TCM) are very intricate and include a wide range of effective substances.196 It is crucial to separate the components and purify them to create powerful single compounds that can be used to increase medicine efficacy, lessen adverse effects, and study the pharmacological process. As a result, numerous researchers have investigated and analyzed tiny compounds derived from TCM to prevent inflammation and degeneration against OA.197-200
Moreover, in terms of macrophage polarization, several studies have focused on the active ingredients of TCM herbs targeted at macrophage reprogramming for the treatment of OA. Fargesin, which is one of the main components of Magnolia fargesii, has traditionally been used to treat sinusitis and inflammation.201 According to a recent study, fargesin could promote the phenotypic transformation of macrophages from M1 to M2 and partially prevent cartilage degeneration by downregulating p38/ERK MAPK and p65/NF-κB signaling.152 In addition, a number of extractions, including frugoside from Calotropis gigante and quercetin, a naturally occurring flavonoid present in a large variety of fruits and vegetables, have been shown to retard the degradation of cartilage and extracellular matrix as well as chondrocyte hypertrophy by inhibiting M1 polarization or inducing M2 polarization.124, 153 Nevertheless, the translation of herbal substances into clinics has a number of challenges, including potential liver and kidney toxicity, the difficulty of standardization, and the low stability or solubility of tiny molecules in serum or synovial fluid.196, 202 The efficacy of TCM herbs for the treatment of OA patients cannot be adequately evaluated.203 Therefore, sufficient representative animal models and preclinical experiments are required for further exploration.
Biomaterial-Based Applications Targeted at Macrophage Polarization
Biomaterials of both synthetic and natural origin have been investigated in the context of tissue regeneration.204 When exposed to synthetic biomaterials, macrophages are polarized toward anti-inflammatory functions as opposed to their normal pro-inflammatory reaction to natural biomaterials, such as the dermis of mammalian origin.205, 206 Further, a number of variables, such as the surface morphology, design, and chemical composition, will have an effect on the in vivo and in vitro behavior of macrophages, which will further influence the development of tissue regeneration.204
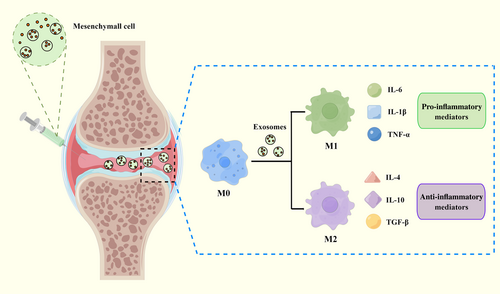
Mesenchyme Stem Cells and their Exosomes
Due to their chondrogenic potential and immunomodulatory capacities, mesenchyme stem cells (MSCs) are being studied extensively as a potential therapy for OA.207 A growing number of studies have come to the conclusion that the paracrine mechanism is the principal role of MSCs, and exosomes generated from MSCs serve as an important medium to exert MSCs’ therapeutic effects.208 MSC-derived exosomes can also influence macrophages, leading them to become more polarized into M2 macrophages and produce more anti-inflammatory cytokines.209 Zhang et al. found that exosomes derived from bone mesenchyme stem cells might delay the progression of OA by promoting a switch of macrophages from M1 to M2 phenotype.154 A similar phenomenon was observed by Cosenza et al. through a mouse OA model induced by intra-articular injection collagenase.210 In addition to bone marrow, extracellular vesicles from other origins, including infrapatellar fat pads,54 human umbilical cord,135 and adipose tissue,155 have been demonstrated to influence macrophage polarization and relieve OA. Notably, although diverse exosomes generated by different tissues have been demonstrated to effectively prevent OA progression through altering the macrophage phenotype, the precise nature of their effects is not fully understood, particularly the specific role of miRNA and proteins in these microparticles.135 Therefore, further thorough studies are needed to identify the crucial internal contents of exosomes and specific molecular mechanisms on OA (Figure 3).
Artificial M2 Macrophages
Current data suggest that M1 phenotype macrophages are mainly involved in harmful and crippling chondrocyte conditions, whereas the M2 phenotype can aid in cartilage restoration. As a result, macrophage M2 polarization has significant promise for treating OA, albeit with low expression of some pro-inflammatory cytokines that can cause cartilage damage.211-213 To regulate the beneficial secretion of M2 macrophages, an artificial M2 macrophage, mainly consisting of macrophage membrane as the “shell” and inflammation-responsive nanogel as the “yolk,” was proposed and fabricated by Ma et al. to enhance the therapeutic efficacy of M2 macrophages in the treatment of OA.156 They demonstrated that this artificial M2 macrophage could break the severe circle of OA owing to its prominent characteristics, including its ability to target inflammation, the customizable rate of medication release in response to OA activity, and the prolonged retention duration in the joint cavity. Transplantation of exogenous M2 macrophages could be considered for treatment of OA, with the major challenge being high adaptability and plasticity in the microenvironment, which might lead to the loss of macrophage phenotype. Therefore, Liang et al. (2022) created a stable exogenous macrophage model by knocking out tumor necrosis factor receptor 1 (TNFR1) and using Cas9-ribonuclear protein (Cas9-RNP) complexes and electroporation to overexpress IL-4, thus locking the interstitial synovial macrophage in the M2a phenotype (L-M2a).214 Similarly, the modified L-M2a macrophages retained a superior anti-inflammatory and pro-regenerative capacity in the inflammatory OA microenvironment, thus representing an ideal new strategy for the disease-modifying therapy of OA.
Metabolic Reprogramming Compounds
Currently, several studies on the treatment of OA have focused on mitochondrial metabolism and how it relates to M1/M2 imbalance.157, 158 It has been reported that oxidative stress and mitochondrial dysfunction both play an important role in the dysregulation of the inflammatory response.215, 216 The process of oxidative phosphorylation is decreased by the activation of inflammatory M1 macrophages, which prevents their repolarization.217 Moreover, in disorders associated with inflammation, mitochondrial reactive oxygen species (mtROS) is essential for driving macrophage polarization to the inflammatory M1 phenotype.218, 219 Consequently, improving the reprogramming of inflammatory M1 macrophages into anti-inflammatory M2 macrophages to control the disease might be possible by therapeutically restoring mitochondrial activity. In line with this, Zhang et al. (2022) have developed a novel meta-defensome by metabolically engineering PLGA nanoparticles (MMP) enclosed in macrophage membrane to target M1 macrophages for selective cargo release in mitochondria.157 In vivo, in a collagenase-induced OA mice model, the OA synovium was successfully defended by the meta-defensome against inflammatory stress, and cartilage was shielded from deterioration by scavenging mtROS, restoring aerobic respiration and successfully repolarizing the M2 phenotype. In addition, the modified zeolitic imidazolate framework-8 (ZIF-8) nanoparticles synthesized by Zhou et al. was demonstrated to effectively catalyze H2O2 to produce O2 and eliminate NO, hence inhibiting hypoxia-inducible factor 1α and further rescuing mitochondrial function. More importantly, through this metabolic reprogramming pathway, these modified nanoparticles upregulated M2 macrophage infiltration in the synovium, further inhibiting cartilage degeneration. Therefore, a metabolic reprogramming strategy targeted at mitochondria might pave the way for new OA therapy.158
Future Directions
Macrophage polarization, as first stated by Mills et al.,220 is a kind of classification for the description of macrophage phenotypical and functional diversity in physiological and disease states, which is mainly based on the arginine metabolism. For decades, a growing literature has demonstrated the limitations of this concept, as the binary classifications could be an oversimplification, and there is a continuum of macrophage phenotypes between M1 and M2 macrophages in vivo.221, 222 However, studies continue to utilize M1 or M2 markers in the classification of macrophages because there is extensive experience in reprogramming macrophages based on their polarization, which is certainly an attractive strategy in many diseases.
Macrophages have functional plasticity, and when stimulated in the microenvironment of a specific tissue, they switch from one functional phenotype to another, which can be used as a target for disease treatment. For OA, applying this property to therapy requires understanding the microenvironment of OA, as well as the factors within it that might influence plasticity. Studies have shown that in addition to the M1 and M2 types of macrophages, M2 can also be divided into four subtypes with different functions, all with different cell expression markers.223 Therefore, studying the expression of macrophage surface markers and the production of specific biological factors at different stages of OA can be helful in determining disease progression.
In recent years, the rapid progress in single-cell RNA sequencing, spatial transcriptomics, fate-mapping, and multiplexed immunohistochemical technologies has revealed the unexpected diversity in macrophages. Indeed, several studies have demonstrated the synovial macrophage diversity during the pathogenesis in the OA,224-226 and in-depth research focusing on the distinct functions, origins, and disease kinetics of macrophage subtypes would be indispensable for targeting these versatile cells.
Conclusion
Osteoarthritis, as a prevalent chronic degenerative joint disease in middle-aged and elderly individuals, is significantly influenced by the behavior of macrophages within joint tissues. Macrophage polarization, where these cells adapt to pro-inflammatory or anti-inflammatory states in response to environmental stimuli, plays a crucial role in the pathogenesis and progression of OA. This review's focus on synovial macrophages, adipose tissue macrophages, and osteoclasts reveals their substantial roles in OA development. Key findings in the field have highlighted various signaling pathways, such as NF-κB, MAPK, and TGF-β, involved in macrophage polarization related to OA. The potential for reprogramming macrophages based on these targets has emerged as a promising avenue for enhancing cartilage repair and mitigating OA progression.
Author Contributions
YZ and XY conceived the concept of the manuscript. ZMY was responsible for the initial manuscript writing. DCJ, MZY and JT revised the manuscript. All authors participated the manuscript writing and approved the final manuscript.
Conflict of Interest Statement
All authors have declared that there is no potential conflict of interest.
Funding Informations
This study was support by the Department of Science and Technology of Sichuan Province (23ZDYF2641), Health Commission of Sichuan Province (2023-118), Department of Science and Technology of Chengdu (2023-GH02-00075-HZ), and National key research and development program of the Ministry of Science and Technology (2023YFB4606700).
Ethics Statement
The data utilized for this study were derived from databases that are publicly accessible and do not contain any personally identifiable information. Consequently, ethical parameters are not applicable.




