Unreliability of Serum- or Plasma-based Assays of D-dimer or Fibrin (Fibrinogen) Degradation Product for Diagnosing Periprosthetic Joint Infection: A Prospective Parallel Study
Grant sources: This work is supported by the Regional Innovation & Cooperation Program of the Science &Technology Department of Sichuan Province (2021YFQ0028), and Technological Innovation R&D project of Chengdu Science and Technology Bureau (2022-YF05-01836-SN). These funding sources did not play a role in investigation.
Disclosure: No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
Abstract
Objective
The ability of D-dimer to diagnose periprosthetic joint infection (PJI) before revision hip or knee arthroplasty is still controversial, and the differences in diagnostic ability between serum- or plasma-based assays of D-dimer and fibrin (fibrinogen) degradation product (FDP) are uncertain. The prospective parallel study was performed to determine the ability of D-dimer to diagnose PJI before revision hip or knee arthroplasty, and the differences in diagnostic ability between serum- or plasma-based assays of D-dimer and FDP.
Methods
Patients undergoing knee or hip arthroplasty at our institution were prospectively enrolled into the following groups: those without inflammatory diseases who were undergoing primary arthroplasty (“Prim” group), those with inflammatory arthritis who were undergoing primary arthroplasty (“Prim/Inflam”), those undergoing revision arthroplasty because of aseptic failure (“Rev/Asept”), or those undergoing revision arthroplasty because of PJI (“Rev/PJI”). The ability of preoperative levels of D-dimer or FDP in serum or plasma to diagnose PJI in each group was assessed using areas under receiver operating characteristic curves (AUCs) and other diagnostic performance indicators. The diagnostic performance of these assays was compared with that of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
Results
In the final analysis, Prim included 42 patients; Prim/Inflam, 40; Rev./Asept, 62; and Rev./PJI, 47. D-dimer assays led to AUCs of 0.635 in serum and 0.573 in plasma, compared to 0.593 and 0.607 for FDP. Even in combination with CRP or ESR, these assays failed to perform as well as the combination of CRP and ESR for diagnosing PJI.
Conclusion
Levels of D-dimer or FDP in serum or plasma, whether used alone or together with CRP or ESR, are unreliable for diagnosing PJI before revision arthroplasty.
Introduction
Periprosthetic joint infection (PJI) is a catastrophic complication after total hip arthroplasty (THA) and total knee arthroplasty (TKA). Its incidence has been increasing rapidly with the number of primary arthroplasties around the world, placing a tremendous burden on patients, surgeons, and healthcare systems.1, 2 Early and reliable diagnosis for PJI is extremely important for optimizing treatment, preserving the implant, and managing patients' expectations of their prognosis.3 However, no single test can currently diagnose PJI, so the search continues for reliable biomarkers of PJI that can be assessed before revision arthroplasty.4
Blood markers, which are relatively easy and inexpensive to assay, play an important role in diagnosing PJI. Inflammatory markers, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are usually used to diagnose PJI.5 All these markers, however, can lack selectivity because they may appear normal even in infections with weakly virulent pathogens such as Propionibacterium acnes and Mycobacterium tuberculosis.6, 7 Some have argued for diagnosing PJI based on D-dimer levels: Shahi et al. reported that serum D-dimer is promising for diagnosing PJI, with diagnostic performance even higher than that of CRP and ESR.8 Furthermore, serum D-dimer has been recommended by the International Consensus Meeting (ICM) as a biomarker to use alongside CRP for diagnosing PJI in 2018.5 However, some studies question the reliability of D-dimer assays.9, 10 Some of the controversy around the diagnostic performance of D-dimer assays arises because some studies have focused on serum,8-12 others on plasma,7, 13-15 and still others may have failed to differentiate between the two blood fractions, as Pannu et al. suspect.16 This challenges the reliability of meta-analyses comparing the diagnostic ability of D-dimer levels in serum or plasma.17-19
Since an increase in fibrin (fibrinogen) degradation product (FDP) after TKA has been linked to the observed increase in CRP,20 we wondered whether FDP levels might be useful for diagnosing PJI. Our previous work suggests that FDP levels in plasma are not fit for this purpose.7 We are unaware of reports assessing the diagnostic ability of FDP levels in serum.
Therefore, we prospectively examined patients at our hospital in order to assess: (i) whether preoperative levels of D-dimer or FDP, in serum or plasma, may be sufficiently accurate to diagnose PJI before revision knee or hip arthroplasty; (ii) differences in D-dimer or FDP levels between serum and plasma; and (iii) the effect of inflammatory diseases on the levels of serum and plasma levels of D-dimer and FDP.
Materials and Methods
Study Design
This prospective, single-center, parallel-arm study was performed to evaluate the ability of serum- or plasma-based D-dimer or FDP to diagnose PJI. The study was approved by our hospital's review board (Approval number: 2020859), and written informed consent was obtained from each patient before enrollment. The study protocol was published in advance,21 and the study was registered in the Chinese Clinical Trial Registry (Registration number: ChiCTR2000038547). In addition, the work has been reported in line with the STARD (Standards for the Reporting of Diagnostic accuracy studies) criteria.
Inclusion and Exclusion Criteria
Patients were prospectively enrolled from October 2020 to September 2021 into four groups: (i) “Prim,” patients undergoing primary THA for primary osteoarthritis, osteonecrosis of femoral head or developmental dysplasia of the hip, or patients undergoing primary TKA for primary osteoarthritis; (ii) “Prim/Inflam,” patients undergoing primary THA or TKA for inflammatory arthritis such as rheumatoid arthritis; (iii) “Rev/Asept,” patients undergoing revision arthroplasty because of aseptic failure; and (iv) “Rev/PJI,” patients undergoing revision arthroplasty because of PJI. Comparisons within Prim allowed us to evaluate differences in levels of D-dimer or FDP between serum and plasma. Comparisons between Prim and Prim/Inflam allowed us to evaluate effects of inflammatory diseases on levels of D-dimer or FDP. Comparisons between Rev./Asept and Rev./PJI allowed us to evaluate the ability of D-dimer or FDP in serum or plasma to detect PJI.
Patients were excluded if they did not consent to participate in the study: (i) if they had been diagnosed with deep vein thrombosis or hemophilia; (ii) if they were taking oral anticoagulants or antiplatelet drugs; or (iii) if they had received antibiotics within two weeks before the operation.
Diagnosis of PJI and Laboratory Evaluations
We defined PJI according to the 2013 International Consensus Meeting (ICM) criteria on PJI.5 After patients provided written informed consent, fasting venous blood samples were obtained on the day of admission and assayed for D-dimer and FDP in plasma or serum in the Department of Laboratory Medicine at our hospital, using immunoturbidimetry or a commercial test kit (Siemens, Berlin, Germany), respectively. In parallel, levels of CRP in serum and ESR were determined.
Patients undergoing revision arthroplasty in our study underwent preoperative joint aspiration. Technicians aspirated the hip joint under ultrasound guidance, while surgeons directly aspirated the knee joint in the ward. All procedures were performed under sterile conditions. The synovial fluid obtained was promptly processed for routine tests (white blood cell count, differential neutrophil count, polymorphonuclear neutrophil percentage) and culturing for bacteria and fungi under aerobic and anaerobic conditions (usually for 21 days). If the clinical manifestations and imagological examination suggest tuberculosis infection, such as the history of tuberculosis, low fever, night sweats and emaciation, more importantly, insect-like bone destruction and dead bone in ***x-ray or CT tests, synovial fluid was sent for culturing tuberculosis. And cultures for tuberculosis were maintained for 42 days routinely.
In the case of patients undergoing revision procedures, at least four soft tissues around the prosthesis were biopsied and analyzed by histology and culturing. Histology was considered to indicate PJI if >5 neutrophils were observed per high-power field in five high-power fields at 400× magnification.5
Data Extraction and Outcomes
The primary outcome in our study was the area under the receiver operating characteristic curve (AUC) for diagnosing PJI. Secondary outcomes were the levels of D-dimer or FDP in serum or plasma in patients with or without inflammatory diseases, as well as the AUCs for combinations of D-dimer or FDP with CRP or ESR.
We also recorded the following patient details: (i) clinical diagnosis, age, sex, comorbidities (hypertension, diabetes, chronic obstructive pulmonary disease, coronary heart disease, and inflammatory diseases such as rheumatoid arthritis and ankylosing spondylitis), and history of cancer surgery; (ii) levels of D-dimer, FDP and CRP in serum; (iii) levels of D-dimer and FDP in plasma; (iv) ESR; (v) results of tests and cultures of synovial fluid; and (vi) results of histology analysis and cultures of biopsied soft tissues.
Sample Size Calculation
The minimal sample needed to provide the primary outcome was calculated using MedCalc 12.7 (MedCalc Software, Ostend, Belgium) and the AUC reported for plasma D-dimer (0.657),13 since this was much lower than the AUC reported for serum D-dimer.8 When the type I (significant) error was set at 0.05 and type II (1-power) error was set at 0.2, we calculated the minimal sample sizes to be 47 in Rev./Asept or Rev./PJI.
We also enrolled two groups of patients who underwent primary arthroplasties and either had inflammatory arthritis or did not (at least 40 patients in each group). These additional groups were meant to provide further data for our comparison of levels of D-dimer or FDP between serum and plasma, and our comparison of levels in the presence or absence of inflammatory disease.
Statistical Analysis
Normally distributed, continuous data were presented as mean and standard deviation (SD), while skewed continuous data were presented as median and interquartile range (IQR), and categorical data were presented as frequencies and percentages. Differences among the four groups were assessed for significance using one-way ANOVA in the case of normally distributed continuous data, the Wilcoxon Mann–Whitney U test in the case of continuous data that showed skew or unequal variance, or the Pearson χ2 and Fisher exact tests in the case of categorical data. Levels of D-dimer or FDP in Prim were compared between serum and plasma using the paired-samples t test, while AUCs were compared using the z-test. Differences associated with p < 0.05 were considered significant.
The ability of biomarkers to diagnose PJI was assessed using receiver operating characteristic curves and the resulting AUCs, for which 95% confidence intervals (CIs) were also calculated. The Youden index was used to determine the optimal predictive cut-offs for D-dimer and FDP, whereas the cut-offs for CRP and ESR were taken from the recommendations of the 2013 ICM Criteria on PJI.5 Positive predictive value (PPV) and negative predictive value (NPV) were also determined. All statistical analyses were performed using SPSS 24 (IBM, Armonk, NY, USA), except the z-test, for which MedCalc was used. Scatterplots were drawn using GraphPad Prism 8.3 for Mac OS X (GraphPad Software, San Diego, CA, USA).
Results
Patient Recruitment and Demographics
From October 2020 to September 2021, 203 patients were recruited, of whom two declined to participate in the trial, two were diagnosed with deep vein thrombosis, three were taking oral anticoagulants or antiplatelet drugs, two had hemophilia, and three used antibiotics within two weeks before surgery. In the end, 191 patients were included in the final analysis: 42 in Prim, 40 in Prim/Inflam, 62 in Rev./Asept and 47 in Rev./PJI (Figure 1, Table 1).
| Characteristic | Prim (n = 42) | Prim/Inflam (n = 40) | Rev/Asept (n = 62) | Rev/PJI (n = 47) | p value |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age, years | 62.07 ± 11.64 | 51.10 ± 13.72 | 62.15 ± 13.30 | 58.13 ± 14.30 | <0.001* |
| Female | 25 (59.52) | 24 (69.0) | 45 (72.58) | 18 (38.30) | 0.005* |
| Comorbidities | |||||
| Hypertension | 15 (35.71) | 9 (22.5) | 22 (25.48) | 5 (10.64) | 0.013* |
| Diabetes | 5 (11.90) | 2 (5.00) | 9 (14.52) | 2 (4.26) | 0.206 |
| COPD | 5 (11.90) | 6 (15.00) | 5 (8.06) | 5 (10.64) | 0.743 |
| CHD | 2 (4.76) | 0 | 3 (4.84) | 0 | 0.231 |
| Inflammatory disease | 0 | 40 (100) | 8 (12.90) | 4 (8.51) | <0.001* |
| History of cancer surgery | 0 | 4 (10.00) | 2 (3.23) | 1 (2.13) | 0.090 |
| Hip joint involvement | 22 (52.38) | 21 (52.50) | 47 (75.81) | 40 (85.11) | 0.001* |
- Notes: Values are n (%) or mean ± SD, unless otherwise noted.
- Abbreviations: CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease.
- * p < 0.05.
The Levels of D-Dimer and FDP in Different Groups
First, we compared levels of D-dimer or FDP between serum and plasma of four groups, respectively. The level of D-dimer in serum was significantly higher than that in plasma in Prim (p = 0.001), but no significant difference was found in the other groups (p = 0.190–0.336). The level of FDP in serum was significantly higher than that in plasma in Prim, Rev./Asept and Rev./PJI (p ≤ 0.016), while there was no significant difference in Prim/Inflam (p = 0.052).
We performed pairwise comparisons between Prim and Prim/Inflam in order to determine effects of inflammatory diseases on the level of serum D-dimer and FDP, as well as plasma D-dimer and FDP (Table 2, Figure 2). Levels of CRP and FDP in serum, as well as levels of D-dimer and FDP in plasma, were significantly higher in Prim/Inflam than in Prim.
| Marker | Prim (n = 42) | Prim/Inflam (n = 40) | Rev/Asept (n = 62) | Rev/PJI (n = 47) | p0 value | p1 value | p2 value |
|---|---|---|---|---|---|---|---|
| CRP (mg/L) | 2.52 (1.97–3.91) | 9.41 (3.70–13.70) | 3.16 (1.91–6.29) | 16.90 (5.28–32.3) | <0.001* | <0.001* | <0.001* |
| ESR (mm/1 h) | 25.00 (15.00–39.00) | 27.00 (15.00–44.50) | 14.00 (7.00–25.00) | 47.00 (29.00–68.00) | <0.001* | 0.578 | <0.001* |
| Serum D-dimer (mg/L) | 0.82 (0.53–1.41) | 1.12 (0.61–1.83) | 1.08 (0.66–1.94) | 2.03 (0.88–3.17) | 0.001* | 0.084 | 0.019* |
| Plasma D-dimer (mg/L) | 0.47 (0.34–0.69) | 0.86 (0.44–1.59) | 1.07 (0.61–1.72) | 1.46 (0.76–2.49) | <0.001* | 0.015* | 0.048* |
| Serum FDP (mg/L) | 2.10 (1.40–3.70) | 3.45 (1.75–6.45) | 3.20 (1.80–6.10) | 4.50 (2.51–7.50) | 0.001* | 0.033* | 0.141 |
| Plasma FDP (mg/L) | 1.25 (1.25–1.25) | 1.25 (1.25–3.95) | 1.25 (1.25–3.50) | 3.10 (1.25–5.85) | <0.001* | 0.022* | 0.013* |
- Notes: Data are presented as median (interquartile range).
- p0: p value among all four groups; p1: p value of group Prim versus Prim/Inflam; p2: p value of group Rev./Asept versus Rev./PJI.
- Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FDP, fibrin degradation product.
- * p < 0.05.
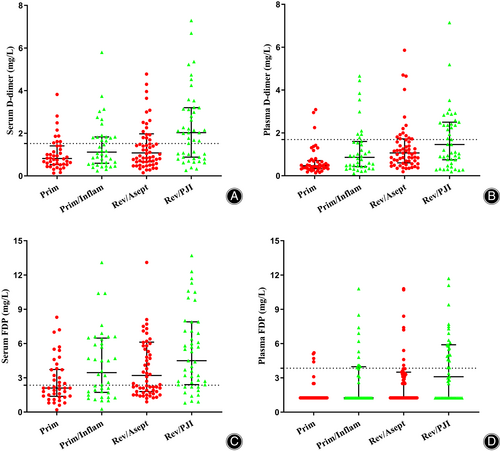
We also performed pairwise comparisons between Rev./Asept and Rev./PJI in order to determine effects of infection on the level of tested biomarkers. Levels of D-dimer in serum or plasma as well as levels of FDP in plasma were significantly higher in Rev./PJI than in Rev./Asept.
The Ability of the Various Biomarkers to Identify PJI Individually
Next, we evaluated the ability of the various biomarkers to identify PJI, benchmarking them against the widely used markers CRP and ESR (Table 3, Figure 3A). With its recommended cut-off of 10 mg/L, serum CRP showed an AUC of 0.811 (95% CI 0.728–0.893), sensitivity of 61.7% and specificity of 91.9%. With its recommended cut-off of 30 mm/h, ESR gave an AUC of 0.820 (95% CI 0.740–0.899), sensitivity of 72.3% and specificity of 75.8%. These two widely used markers showed similar AUCs (z = 0.222, p = 0.824). D-dimer did not perform as well, either in serum or plasma. With an optimal cut-off of 1.52 mg/L based on the Youden index, serum D-dimer gave an AUC of 0.635 (95% CI 0.530–0.741), sensitivity of 61.7% and specificity of 67.7%. This AUC was significantly lower than those of CRP (z = 3.381; p < 0.001) and ESR (z = 3.252; p = 0.001). With an optimal cut-off of 1.69 mg/L based on the Youden index, plasma D-dimer gave an AUC of 0.573 (95% CI 0.459–0.688), sensitivity of 46.8% and specificity of 74.2%. This AUC was again significantly lower than those of CRP (z = 4.022; p < 0.001) and ESR (z = 3.952; p < 0.001). AUCs were similarly low for serum- or plasma-based assays of D-dimer (z = 1.204; p = 0.229).
| Marker | AUC (95% CI) | Youden index | Predictive cutoff | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| Serum CRP (mg/L) | 0.811 (0.728–0.893) | 0.536 | 10.00* | 61.7 | 91.9 | 85.2 | 76.0 |
| ESR (mm/h) | 0.820 (0.740–0.899) | 0.481 | 30.00* | 72.3 | 75.8 | 69.4 | 78.3 |
| Serum D-dimer (mg/L) | 0.635 (0.530–0.741) | 0.294 | 1.52# | 61.7 | 67.7 | 59.2 | 70.0 |
| Plasma D-dimer (mg/L) | 0.573 (0.459–0.688) | 0.210 | 1.69# | 46.8 | 74.2 | 57.9 | 64.8 |
| Serum FDP (mg/L) | 0.593 (0.485–0.701) | 0.222 | 2.35# | 78.7 | 43.5 | 51.4 | 72.9 |
| Plasma FDP (mg/L) | 0.607 (0.497–0.716) | 0.307 | 3.85# | 46.8 | 83.9 | 68.8 | 67.5 |
- Abbreviations: AUC, area under the receiver operating characteristic curve; 95% CI, 95% confidence interval; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FDP, fibrin degradation product; NPV, negative predictive value; PPV, positive predictive value.
- * Taken from the 2013 International Consensus Meeting criteria on periprosthetic joint infection.5
- # Determined based on the Youden index.
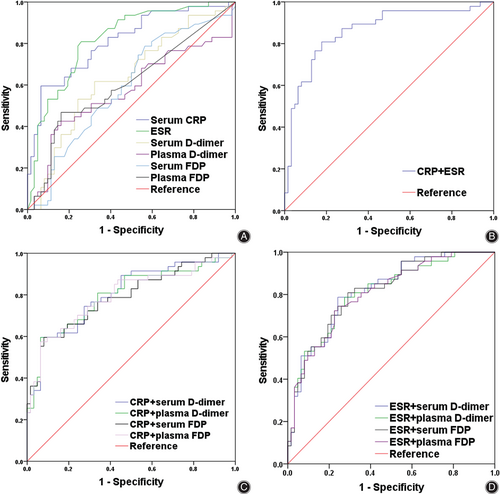
Similar results were observed for FDP. With an optimal cut-off of 2.35 mg/L based on the Youden index, serum FDP gave an AUC of 0.593 (95% CI 0.485–0.701), sensitivity of 78.7% and specificity of 43.5%. This AUC was significantly lower than those of CRP (z = 3.924; p < 0.001) and ESR (z = 3.890; p < 0.001). With an optimal cut-off of 3.85 mg/L based on the Youden index, plasma FDP gave an AUC of 0.607 (95% CI 0.497–0.716), sensitivity of 46.8% and specificity of 83.9%. This AUC was again significantly lower than those of CRP (z = 3.640; p < 0.001) and ESR (z = 3.583; p < 0.001). AUCs were similarly low for serum- or plasma-based assays of FDP (z = 0.245; p = 0.806).
The Ability of Combinations of the Various Biomarkers to Identify PJI
Finally, we evaluated the diagnostic performance of different combinations of D-dimer and FDP with CRP or ESR (Table 4, Figure 3B–D). All the combinations involving D-dimer or FDP, in serum or plasma, failed to perform better than the combination of CRP with ESR, which gave an AUC of 0.862 (95% CI 0.788–0.935), sensitivity of 80.9% and specificity of 82.3%.
| Combination | AUC (95% CI) | Youden index | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| CRP + ESR | 0.862 (0.788–0.935) | 0.632 | 80.9 | 82.3 | 77.6 | 85.0 |
| Combinations with serum CRP | ||||||
| CRP + serum D-dimer | 0.805 (0.721–0.889) | 0.531 | 59.6 | 93.5 | 87.4 | 75.3 |
| CRP + plasma D-dimer | 0.797 (0.709–0.885) | 0.531 | 59.6 | 93.5 | 87.4 | 75.3 |
| CRP + serum FDP | 0.790 (0.702–0.885) | 0.515 | 59.6 | 91.9 | 84.8 | 75.0 |
| CRP + plasma FDP | 0.794 (0.705–0.883) | 0.509 | 57.4 | 93.5 | 87.0 | 74.3 |
| Combinations with ESR | ||||||
| ESR + serum D-dimer | 0.817 (0.739–0.896) | 0.545 | 78.7 | 75.8 | 71.1 | 82.4 |
| ESR + plasma D-dimer | 0.808 (0.727–0.890) | 0.513 | 78.7 | 72.6 | 68.5 | 81.8 |
| ESR + serum FDP | 0.815 (0.735–0.894) | 0.519 | 80.9 | 71.0 | 67.9 | 83.1 |
| ESR + plasma FDP | 0.806 (0.725–0.887) | 0.503 | 74.5 | 75.8 | 70.0 | 79.7 |
- Abbreviations: AUC, area under the curve; 95% CI, 95% confidence interval; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FDP, fibrin degradation product; NPV, negative predictive value; PPV, positive predictive value.
Discussion
To our knowledge, this is the first prospective parallel study to compare the ability of D-dimer or FDP in serum or plasma to diagnose PJI. Our results suggest that preoperative D-dimer and FDP levels are higher in serum than in plasma in patients without inflammatory diseases who undergo primary arthroplasty. Furthermore, we found that inflammatory diseases can increase levels of D-dimer and FDP in serum and plasma. More importantly, we found that neither D-dimer nor FDP, whether assayed in serum or plasma, is reliable for diagnosing PJI.
The Generation and Ability for Diagnosing PJI of D-dimer and FDP
D-dimer is generated by the cleavage of cross-linked fibrin under the work of plasmin22 and is therefore a specific marker of fibrinolysis. It is quite useful for ruling out venous thrombo-embolism,23, 24 and it may be useful in the diagnosis of systemic or local infection22, 25 because fibrinolysis is strongly associated with infection and inflammation.26, 27 FDP, for its part, is generated when plasmin cleaves fibrinogen, fibrin monomer and cross-linked fibrin,28 implying that it can serve as a marker analogous to D-dimer. However, our results suggest that neither D-dimer nor FDP is reliable enough for diagnosing PJI.
We previously reported that FDP levels in plasma are ineffective at identifying PJI.7 In addition, several studies have reported that D-dimer, whether in serum or plasma, performs unreliably when predicting PJI,9, 10, 12, 13, 15 including work from our own laboratory.7 While some studies have reported serum D-dimer to show good performance,8, 11 other studies have come to different conclusions.17, 29 At least part of this divergence in the literature may be due to failure to take into account whether protein levels were assayed in serum or plasma.16 In addition, Pannu et al. reported that plasma D-dimer paradoxically increases before reimplantation while ESR/CRP decrease, and he suggested that surgeons shall adopt caution using D-dimer to make clinical decisions.30
The Levels of D-dimer and FDP in Different Specimens and Populations
We found that preoperative levels of D-dimer and FDP were higher in serum than in plasma in patients without inflammatory diseases who undergo primary arthroplasty. This may reflect a secondary fibrinolytic reaction: when blood clots, the fibrinolytic system is activated, which increases levels of D-dimer and FDP in serum. In addition, our finding that patients with inflammatory arthritis had higher levels of D-dimer and FDP in serum and plasma than those without inflammatory arthritis may reflect a chronically higher state of inflammation.31 We observed the same for CRP, which is line with previous work.32
Our study supports the recommendations of the 2013 ICM Criteria on PJI to diagnose PJI using serum CRP and ESR.5 These biomarkers are fast and convenient to assay, in contrast to the gold-standard procedures of joint aspiration and culturing of synovial fluid,33 making them more desirable for preliminary PJI screening, particularly of outpatients.34, 35
Strengths and Limitations
This was the first prospective study that directly compared plasma and serum D-dimer and FDP for the diagnosis of PJI, and the results are reliable. However, the fact that our patients came from a single medical center and that all assays were performed in one clinical laboratory may mean that they are not generalizable to other patient populations or healthcare contexts. We are also uncertain whether our results apply to the patient subgroups that we excluded from our study. Second, the optimal time for assaying serum D-dimer and FDP is unclear: levels may increase as secondary fibrinolytic reactions progress, but then later decrease as the proteins degrade further. Although future studies should verify and extend our findings while taking these questions into account, we feel confident in concluding that levels of D-dimer or FDP, in serum or plasma, are unreliable for diagnosing PJI.
Conclusions
Preoperative D-dimer and FDP levels are higher in serum than in plasma, and inflammatory diseases can increase their levels in serum and plasma. However, neither D-dimer nor FDP, whether assayed in serum or plasma, is reliable for diagnosing PJI.
Acknowledgments
We thank A. Chapin Rodríguez, PhD, from Creaducate Enterprises Ltd. for editing the English text of a draft of this manuscript.
Ethics Statement
The Ethics Committee of West China Hospital of Sichuan University approved the study. Written informed consent was deemed unnecessary by the hospital's institutional review board.
Author Contributions
Hong Xu and Jing Zhou: conceptualization, data curation, methodology, software and writing—original draft. Qiang Huang and Zeyu Huang: formal analysis, methodology, software and writing—review &and editing. Jinwei Xie: investigation, conceptualization, project administration, validation and visualization and writing—review. Zongke Zhou: conceptualization, funding acquisition, resources, supervision and writing—review. All authors read and approved the final manuscript.
Open Research
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to some of them remaining unpublished and confidential but are available from the first or corresponding author on reasonable request.




