Prognosis of oral epithelial dysplasia in individuals with and without oral lichen planus
Abstract
Objectives
To investigate the role of oral lichen planus (OLP) on the long-term prognosis of oral epithelial dysplasia (OED).
Methods
Retrospective single-centre cohort study using the 2007–2019 database of the Head and Neck Cancer and Oral Medicine units of University College London Hospital. The exposure of interest was the presence of OLP, and the prognostic outcomes included the development of new primary episodes of OED, progression to malignancy and mortality. Cox proportional hazard and Poisson regression models were performed.
Results
A total of 299 patients, of whom 144 had OED arising on the background of OLP (OLP/OED) and 155 had OED without underlying OLP (non-OLP/OED), were included. A pre-existing diagnosis of OLP was significantly associated with a twofold increased risk of subsequent primary OED events (HR = 2.02, p = 0.04), which also developed faster (1.46 vs. 2.96 years, p = 0.04) and with more involvement of non-cancer-prone sites (p = 0.001) than in the non-OLP/OED group. There was no difference between groups in the progression to malignancy or mortality.
Conclusions
Oral lichen planus/OED patients are at higher risk of multiple episodes of primary OED, which can develop faster and at non-cancer-prone sites as compared to non-OLP/OED individuals. Further research is needed to clarify the effects of OLP upon progression to OSCC and mortality.
1 INTRODUCTION
Oral lichen planus (OLP) is a T-cell-mediated chronic inflammatory disease affecting approximately 1% of the population (González-Moles, Warnakulasuriya, González-Ruiz, González-Ruiz, et al., 2021; Li et al., 2020; Scully & Carrozzo, 2008). It presents with keratotic striation and painful ulcerative lesions, which are long standing and tend to fluctuate in severity over time (Carrozzo et al., 2019). Oral lichen planus is associated with an increased risk of oral cancer development with respect to the general population (El-Naggar et al., 2017; Holmstrup et al., 1988; Warnakulasuriya et al., 2007), with six recent systematic reviews/meta-analyses indicating development of oral squamous cell carcinoma (OSCC) in 1%–2% of affected individuals (Aghbari et al., 2017; Fitzpatrick et al., 2014; Giuliani et al., 2019; González-Moles et al., 2019; Idrees et al., 2021; Iocca et al., 2020; Warnakulasuriya et al., 2021). A number of small preliminary studies have also suggested that OSCC associated with OLP may have a worse prognosis than OSCC with no underlying OLP, including a higher tendency to develop multiple metachronous new primary OSCCs and lymph node metastases (González-Moles et al., 2020; Hietanen et al., 1999; Lo Muzio et al., 1998; Mignogna et al., 2006; Mignogna et al., 2001; Mignogna et al., 2002; Muñoz et al., 2007).
Little is known, however, regarding the prognosis of oral epithelial dysplasia (OED) in patients with OLP. This is a notably relevant issue as 10% to 25% of individuals with OLP or lichenoid disorders are suggested to develop OED at some point during the course of their disease (De Jong et al., 1984; González-Moles, Warnakulasuriya, González-Ruiz, Ayén, et al., 2021; Patil et al., 2015; Shearston et al., 2019).
Epithelial dysplasia represents the most commonly used predictor of an oral potentially malignant disease progressing to OSCC (McCarthy, Fedele, et al., 2021; Mehanna et al., 2009) and a fundamental intermediate step in the transition from normal mucosa to OSCC reflecting the accumulation of genetic abnormalities (Califano et al., 1996; Zhou et al., 2016).
Current data suggest that overall, and regardless of the type of underlying clinical disease, approximately 10% of individuals with OED eventually progress to OSCC (Mehanna et al., 2009; Shariff & Zavras, 2015). Interestingly, Rock et al. (2018) reported no notable difference in the progression rate of OED between individuals with and without OLP. Furthermore, Zhang et al. (2000) reported no differences in loss of heterozygosity associated with OED in individuals with and without oral lichen planus/lichenoid disorders. These preliminary studies seem to suggest that the progression of OED to cancer and the underlying genetic abnormalities might not be notably different between individuals with or without underlying OLP. However, evidence remains limited by the small sample size and the lack of other important prognostic endpoints such as the development of multiple and multifocal episodes of OED/OSCC (field cancerization) and mortality. Here, we report an observational study of a cohort of patients with OED aimed at assessing the effect of underlying OLP upon the (i) development of new dysplastic lesions, (ii) progression to malignancy and (iii) mortality.
2 MATERIALS AND METHODS
2.1 Study design and setting
The present retrospective cohort study used anonymized data collected as part of a service evaluation assessing the outcomes of care provided to patients with OED. The individuals of interest were identified from the Head and Neck Cancer Multidisciplinary Team (MDT) and the Oral Medicine clinic databases of the University College London Hospital (UCLH) relevant to the period between November 2007 and February 2019. Data were collected retrospectively from outpatient hospital notes. We used the Health Research Authority (HRA) decision tool (Health Research Authority, 2014) and engaged with the R&D office of UCLH to confirm that this analysis met the criteria for service evaluation and did not require HRA or research ethics review. Data collection started in November 2017 and ended in February 2019. This study was reported according to the Strengthening the Reporting of Observation Studies in Epidemiology (STROBE) guidelines (von Elm et al., 2007).
2.2 Study population, data collection, and definitions
Individuals diagnosed with OED were ascertained through the above databases and reviewed against the study inclusion and exclusion criteria (Table S1). In brief, we identified individuals with at least one episode of OED between November 2007 and February 2019 and no previous history of OSCC. Oral epithelial dysplasia was defined and graded as per WHO criteria (El-Naggar et al., 2017). We defined the first episode of OED (dysplastic event) as the index OED lesion and patients were stratified into two groups on the basis of their index OED lesion: low risk (mild dysplasia) and high risk (moderate/severe dysplasia; Warnakulasuriya et al., 2008).
A new primary episode of OED was defined as a new dysplastic event occurring after the previous episode of OED and (i) at a different intra-oral anatomical site or (ii) at the same anatomical site as the previous OED but ≥6 months after complete surgical excision with histopathologically confirmed disease-free margins. Subsequently, we identified within the group of OED the individuals who also had a diagnosis of OLP. The diagnosis of OLP was clinically and histologically confirmed based on the modified WHO diagnostic criteria (Van Der Meij & Van Der Waal, 2003). Individuals who were diagnosed with OLP after the index OED lesion or individuals being diagnosed with both OED and OLP at the same time were excluded. Individuals with a diagnosis of OSCC within 6 months after the index OED lesion were also excluded due to the likelihood of their malignant disease being synchronous to the OED (Lumerman et al., 1995). Figure S1 presents a flow chart of the process of database patient identification and selection. Details on the observation period, end of data collection and the list of the data retrieved from the hospital records are provided in the Appendix S1 document.
2.3 Study objective and outcomes
The main objective was to assess the number of outcomes relevant to the prognosis of OED. The outcomes of interest included (i) the development, after the index OED, of subsequent new primary OEDs and related time to onset; (ii) the total number of new primary episodes of OED, their site and degree; (iii) the progression to OSCC and related time to onset; (iv) the total number of OSCCs and (v) mortality related to OSCC and other causes.
2.4 Exposure of interest
The exposure of interest was the presence of OLP. The study population was divided into two groups based on the exposure of interest (exposed and non-exposed to pre-existing OLP).
2.5 Study covariates
Study covariates obtained at the baseline (at diagnosis of the index OED) were considered in the analyses as potential confounding factors including age, gender, smoking (no/yes) and alcohol consumption (no/yes) and the site and grade of index OED lesion. Sites of dysplastic lesions as a variable were grouped into cancer-prone (floor of mouth and tongue) and non-cancer-prone sites (buccal mucosa, gingivae, alveolar mucosa, labial mucosa and palate).
2.6 Statistical analysis
Statistical analyses were performed using STATA 15.1 (Stata Corporation). Demographics and clinicopathological data were described using mean and standard deviation or median and interquartile range (IQR) when data were not normally distributed. Baseline characteristics of OED patients with and without OLP were compared using chi-squared tests (for categorical variables), Student's t-test (for continuous variables) and Wilcoxon rank-sum test (for non-normally distributed continuous variables) as appropriate.
To investigate the risk of developing new primary OEDs and the risk of progression to OSCC after the index OED between subgroups (OED patients with OLP compared to those without OLP), Kaplan–Meier curves by pre-existing OLP were estimated and statistical significance was tested using log-rank tests. Univariate and multivariate Cox proportional hazard regression models were constructed and hazard ratios (HRs) and 95%CI for the risk of having additional new primary OEDs and the risk of progression to OSCC were calculated. The proportional hazards assumption was verified using Schoenfeld residual tests (Schoenfeld, 1982). In case of the proportional hazard assumption being violated for exposure of interest (OLP) or any of the covariates of the models (hazard ratio associated with that variable not being constant over time), we planned to split data into periods in which the hazard ratio remains constant (where the proportional hazard assumption holds) and fit separate Cox models for each period separately (Bellera et al., 2010; Koletsi & Pandis, 2017).
Univariate and multivariate Poisson regression models were utilized to investigate the relationship between OLP and the total number of additional new primary OEDs as well as the number of new primary OSCCs. To account for differences in observation periods for each individual, the Poisson models were adjusted using the “exposure” option within STATA (Long & Freese, 2014). Both crude and adjusted incidence rate ratios (IRR) and 95%CI were calculated.
To evaluate the effect of OLP on cause-specific mortality, competing risk analyses were carried out to appropriately account for a competing risk (death due to other causes) as recommended (Läärä et al., 2017). Patients who were found to have died during the study period were grouped according to causes of death – OSCC and all other causes (competing risks). Since Kaplan–Meier curves are not valid when the competing risks are present, cumulative incidence function curves were, therefore, estimated for each cause of death. Cox proportional hazards regression on the cause-specific hazards of death from OSCC and other causes were fitted and cause-specific hazard ratios and 95%CI were calculated.
All multivariate models were adjusted using a stepwise approach with a view to control for the following potential confounding factors: age at diagnosis, gender, smoking and alcohol consumption, sites and degree of index OED. We planned to use multiple imputations by chain equation (MICE) in order to handle missing values and 40 imputed datasets were generated (m = 40) for variables with missing values assuming missing at random (MAR) mechanism, and imputed values were combined using Rubin's rule (Pedersen et al., 2017). All statistical tests were two-tailed and p-value ≤0.05 was considered statistically significant.
3 RESULTS
3.1 Patient characteristics
This study included a total of 299 patients, of whom 144 (48.16%) were patients with OED arising on the background of OLP (OLP/OED) and 155 (51.84%) had OED without underlying OLP (non-OLP/OED). There was a significant difference in gender distribution between OLP/OED and non-OLP/OED patients (p < 0.001), with the percentage of females in OLP/OED and non-OLP/OED groups being 65.97% and 45.16% respectively. Patients in the OLP/OED group were significantly older than those in the non-OLP/OED group (mean age 63.49 vs. 59.72, p = 0.02). Moreover, smoking and alcohol consumption were significantly less predominant among OLP/OED individuals compared to those with OED alone (p < 0.001 and p = 0.04, respectively). With respect to the oral mucosal sites of the index OED, non-cancer-prone sites were significantly more common among OLP/OED patients (63.19% vs. 50.32%, p = 0.03). Regarding the grade and treatment of the index OED and subsequent OED, no statistically significant difference was found between OLP/OED and non-OLP/OED groups (Table 1). Full baseline demographics and clinicopathological characteristics are reported in Table 1. There was no difference in the median follow-up between the OLP/OED and non-OLP/OED group (4.54 vs. 3.77 years, p = 0.08).
| Parameters | All | OLP/OED group | Non-OLP/OED group | p-value |
|---|---|---|---|---|
| Total | 299 (100%) | 144 (100%) | 155 (100%) | |
| Gender (%) | ||||
| Male | 134 (44.82%) | 49 (34.30%) | 85 (54.84%) | <0.001 |
| Female | 165 (55.18%) | 95 (65.97%) | 70 (45.16%) | |
| Age at diagnosis | ||||
| Mean ± SD | 61.54 ± 13.67 | 63.49 ± 13.59 | 59.72 ± 13.53 | 0.02 |
| Range | 26.93–98.67 | 32.35–98.66 | 26.93–90.15 | |
| Smoking status | ||||
| No | 162 (54.18%) | 96 (66.67%) | 66 (42.58%) | <0.001 |
| Yes | 98 (32.78%) | 27 (18.75%) | 71 (45.81%) | |
| Missing | 39 (13.04%) | 21 (14.58%) | 18 (11.61%) | |
| Alcohol consumption | ||||
| No | 122 (40.80%) | 69 (47.92%) | 53 (34.19%) | 0.04 |
| Yes | 124 (41.47%) | 50 (34.72%) | 74 (47.74%) | |
| Missing | 53 (17.73%) | 25 (17.36%) | 28 (18.06%) | |
| Primary site of the index OED | ||||
| Non-cancer pronea | 169 (56.52%) | 91 (63.19%) | 78 (50.32%) | 0.03 |
| Cancer proneb | 130 (43.48%) | 53 (36.81%) | 77 (49.68%) | |
| Grade of the index OED | ||||
| Low-risk OED | ||||
| Mild dysplasia | 180 (60.20%) | 89 (61.81%) | 91 (58.71%) | 0.46 |
| High-risk OED | ||||
| Moderate dysplasia | 67 (22.41%) | 34 (23.61%) | 33 (21.29%) | |
| Severe dysplasia | 52 (17.39%) | 21 (14.58%) | 31 (20.00%) | |
| Treatment of the index OED (n = 299) | ||||
| Low-risk OED (n = 180) | ||||
| Surgical removal/laser ablation | 15 (8.33%) | 8/89 (8.99%) | 7/91 (7.69%) | 0.33 |
| High-risk OED (n = 119) | ||||
| Surgical removal/laser ablation | 81 (68.07%) | 40/55 (72.73%) | 41/64 (64.06%) | 0.32 |
| Treatment of subsequent OED (n = 117) | ||||
| Low-risk OED (n = 33) | ||||
| Surgical removal/laser ablation | 7 (21.21%) | 4/11 (18.18%) | 3/22 (27.27%) | 0.55 |
| High-risk OED (n = 84) | ||||
| Surgical removal/laser ablation | 65 (77.38%) | 36/48 (75.00%) | 29/36 (80.56%) | 0.31 |
| Length of follow-up | ||||
| Median years of follow-up (range) | 3.93 (1.84–6.62) | 4.54 (1.97–6.87) | 3.77 (1.61–5.95) | 0.08 |
| Time to subsequent new primary OED (n = 62) | ||||
| Median (range) | 1.46 (0.51–2.40) | 2.96 (0.79–4.74) | 0.04 | |
| Time to progression of OSCC (n = 42) | ||||
| Median (range) | 3.21 (1.20–5.81) | 2.23 (0.71–5.04) | 0.44 | |
- Bold values indicate statistically significant (p < 0.05).
- a Buccal mucosa, gingivae, alveolar mucosa, labial mucosa and palate.
- b Floor of mouth and tongue.
3.2 Development of new primary OED episodes following the index OED and time to onset
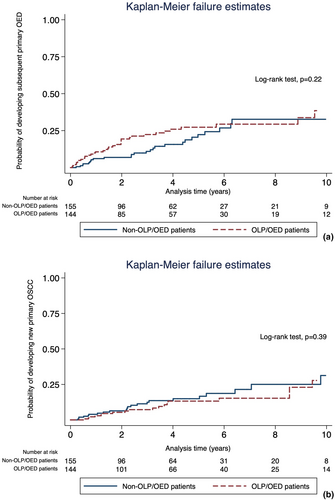
A total of 62 of 299 patients (20.74%) experienced at least one additional new primary OED episode after the index OED: 35 in the OLP/OED group (56.45%) and 27 (43.55%) in the non-OLP/OED group (p = 0.14). The incidence rates of developing new primary OED episodes after the index OED were 5.75 and 4.38 per 100 person-years for OLP/OED and non-OLP/OED individuals respectively. Kaplan–Meier curves showed no significant difference between the groups in the risk of developing additional primary OEDs after the index OED (p = 0.22; Figure 1a). With respect to the Cox regression models, the proportional hazard assumption was not satisfied for OLP indicating that the effect of OLP on the occurrence of subsequent new primary OED was not constant over time. We, therefore, carried out separate univariate and multivariate Cox regression analyses for the periods before and after 3 years after the index OED, as in these the proportional hazard assumption was valid. This was further confirmed by fitting the Cox regression model with time-varying covariate to visualize how the HR associated with OLP changes over the study period (Table S4). In univariate analysis, OLP was found to be significantly associated with a higher risk of developing additional new primary OEDs within the first 3 years following the diagnosis of the index OED (HR = 2.20, 95%CI 1.16–4.18, p = 0.02). After controlling for age, gender, smoking and alcohol usage, site and grade of the index OED in multivariate analysis, the presence of OLP remained significantly associated with a 2.02-fold increased risk of developing subsequent primary OED events (HR = 2.02, 95%CI 1.02–3.98, p = 0.04). However, no significant association between OLP and the development of subsequent new primary OED was observed behind the 3-year time point on univariate and multivariate analyses (HR = 0.45, 95%CI 0.16–1.23, p = 0.12; Table 2). Regarding the median time from the index OED to subsequent new primary OED, there was a significant difference observed between groups, with OLP/OED group being associated with earlier development of new primary OED episodes (1.46 years vs. 2.96 years in non-OLP/EOD, p = 0.04).
| Parameters | Patients who developed new primary OEDs (n = 62), No. (%) | Patients who did not develop further OEDs (n = 237), No. (%) | Length of follow-up after the index OED | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Up to 3 years (n = 299) | After 3 years (n = 152) | |||||||||
| Univariate analysis | Multivariate analysisa | Univariate analysis | Multivariate analysisa | |||||||
| Unadjusted HR (95%CI) | p-value | Adjusted HR* (95% CI) | p-value | Unadjusted HR (95%CI) | p-value | Adjusted HRa (95% CI) | p-value | |||
| OLP status | ||||||||||
| Non-OLP | 27 (17.42%) | 128 (82.58%) | 1 | 1 | 1 | 1 | ||||
| OLP | 35 (24.31%) | 109 (75.69%) | 2.20 (1.16–4.18) | 0.02 | 2.02 (1.02–3.98) | 0.043 | 0.52 (0.21–1.32) | 0.17 | 0.45 (0.16–1.23) | 0.12 |
| Age, mean (SD) | 62.12 (16.09) | 61.38 (12.99) | 1.03 (1.00–1.05) | 0.05 | 1.01 (0.99–1.04) | 0.27 | 0.98 (0.94–1.01) | 0.24 | 2.31 (0.86–6.21) | 0.09 |
| Gender | ||||||||||
| Male | 24 (17.91%) | 110 (82.09%) | 1 | 1 | 1 | 1 | ||||
| Female | 38 (23.03%) | 127 (76.97%) | 1.38 (0.74–2.57) | 0.32 | 1.24 (0.65–2.38) | 0.52 | 1.47 (0.60–3.61) | 0.40 | 0.98 (0.94–1.01) | 0.16 |
| Smoking status | ||||||||||
| No | 36 (22.22%) | 126 (77.78%) | 1 | 1 | 1 | 1 | ||||
| Yes | 16 (16.33%) | 82 (83.67%) | 0.52 (0.24–1.13) | 0.10 | 0.77 (0.34–1.75) | 0.53 | 1.40 (0.55–3.53) | 0.48 | 1.34 (0.48–3.71) | 0.58 |
| Unknown | 10 (25.64%) | 29 (74.36%) | ||||||||
| Alcohol intake | ||||||||||
| No | 26 (21.31%) | 96 (78.69%) | 1 | 1 | 1 | |||||
| Yes | 22 (17.74%) | 102 (82.26%) | 0.88 (0.45–1.71) | 0.71 | 1.02 (0.51–2.03) | 0.96 | 0.87 (0.31–2.41) | 0.79 | 0.92 (0.31–2.68) | 0.88 |
| Unknown | 14 (26.42%) | 39 (73.58%) | ||||||||
| Site of index OED | ||||||||||
| Non-cancer prone | 28 (16.57%) | 141 (83.43%) | 1 | 1 | 1 | 1 | ||||
| Cancer prone | 34 (26.15%) | 96 (73.85%) | 1.38 (0.75–2.53) | 0.30 | 0.95 (0.50–1.81) | 0.87 | 1.46 (0.59–3.61) | 0.41 | 0.90 (0.33–2.42) | 0.83 |
| Degree of index OED | ||||||||||
| Low-risk OED | 19 (10.56%) | 161 (89.44%) | 1 | 1 | 1 | 1 | ||||
| High-risk OED | 43 (36.13%) | 76 (63.87%) | 4.04 (2.07–7.89) | <0.001 | 4.05 (1.98–8.25) | <0.001 | 3.11 (1.24–7.81) | 0.02 | 3.77 (1.44–9.88) | 0.007 |
- Bold values indicate statistically significant (p < 0.05).
- a Adjusted for age, gender, smoking and alcohol consumption, site and degree of the index OED.
Of note, the degree of the index OED (high-risk vs. low-risk OED) was also found to be a significant predictor of the development of new primary OED events in the univariate (HR = 4.04, 95%CI 2.07–7.98, p < 0.001 in the first 3 years; HR = 3.11, 95%CI 1.24–7.81, p = 0.02 afterwards) and multivariate analyses (HR = 4.05, 95%CI 1.98–8.25, p < 0.001 within the first 3 years; HR = 3.77, 95%CI 1.44–9.88, p = 0.007 afterwards) in both the OLP/OED and non-OLP/OED groups.
3.3 Number, site and degree of subsequent OED lesions after the index OED
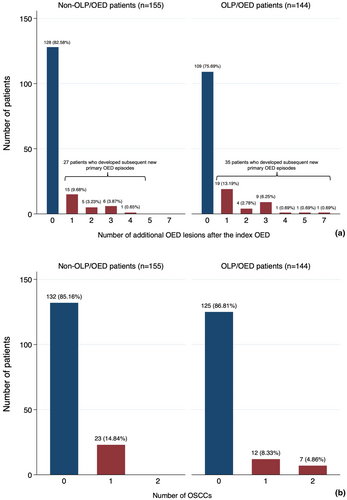
Study patients developed a total of 117 subsequent new primary OED episodes. Forty-seven (40.1%) new primary OED episodes were noted in 27 non-OLP/OED patients and seventy (59.8%) new primary OED episodes were identified among 35 OLP/OED individuals. Of the 27 non-OLP/OED patients, 15 (9.68%), 5 (3.23%), 6 (3.87%) and 1 (0.65%) developed 1, 2, 3 and 4 new primary OED events after the index OED lesion respectively. In the group of 35 OLP/OED individuals, 19 (13.19%), 4 (2.78%), 9 (6.25%), 1 (0.69%), 1 (0.69%) and 1 (0.69%) developed 1, 2, 3, 4, 5 and 7 new primary OED events after the index OED respectively (Figure 2a). The presence of OLP was associated with no significant difference in the number of subsequent primary OED events in both the univariate (IRR = 1.37, 95%CI 0.95–1.99, p = 0.09). and multivariate models (IRR = 1.21, 95%CI 0.70–2.08, p = 0.49; Table S2). Regarding the topographic relationship between the index OED and subsequent OED episodes, 60% (21/35) of OLP/OED patients and 40.74% (11/27) of non-OLP/OED patients developed multifocal subsequent OED lesions distant to the previous sites, whereas the remaining patients developed further primary OED events in the same location as the index OED. However, the difference was not statistically significant (p = 0.13). Considering the anatomical sites of subsequent new primary OED lesions, non-cancer-prone sites were more frequently affected in the OLP/OED group (43/70, 61.43%) than in non-OLP/OED patients (14/47, 29.79%; p = 0.001). No statistically significant difference was seen between the OLP/OED and non-OLP/OED patients with respect to the degree of subsequent new primary OEDs (p = 0.34), the majority being high-risk OED lesions in both groups (48/70, 68.57% in OLP/OED and 36/47, 76.60% in non-OLP/OED). Of note, the degree of the index OED (high-risk vs. low-risk OED) was a statistically significant independent predictor for having a high number of subsequent multiple OED lesions in both the univariate (IRR = 2.76, 95%CI 1.86–4.09, p < 0.001) and multivariate analyses (IRR = 2.88, 95%CI 1.57–5.29, p < 0.001). Age was significantly associated with a higher number of new primary OED events (IRR = 1.02, 95%CI 1.00–1.04, p = 0.001) in the unadjusted model, but became null in the fully adjusted model (IRR = 1.02, 95%CI 0.99–1.04, p = 0.16).
3.4 Progression to OSCC after the index OED and time to onset
A total of 42 of 299 patients (14.05%) progressed to OSCC following the diagnosis of the index OED, of whom 19 (45.23%) and 23 (54.76%) were in the OLP/OED and non-OLP/OED group respectively (p = 0.68). The incidence rates of developing new primary OSCCs following the first diagnosis of OED were 2.77 and 3.72 per 100 person-years for OLP/OED and non-OLP/OED individuals respectively. Overall, the prevalence of OSCC development was 13.19% (19/144) and 14.8% (23/155) in the OLP/OED and non-OLP/OED groups respectively. Kaplan–Meier curves showed no significant difference in the probability of progressing to OSCC between OLP/OED and non-OLP/OED individuals (p = 0.39; Figure 1b).
With respect to the Cox hazard regression models, both the univariate (HR = 0.76, 95%CI 0.42–1.41, p = 0.39) and multivariate models (HR = 0.84, 95%CI 0.42–1.66, p = 0.61) demonstrated that OLP was not significantly associated with an increased risk of progression to oral cancer after the index OED (Table 3). Of note, the degree of index OED (high-risk vs low-risk OED) was found to be a significant predictor of progression to OSCC in both the univariate (HR = 5.11, 95%CI 2.44–10.71, p < 0.001) and multivariate models (HR = 4.18, 95%CI 1.91–9.12, p < 0.001). The site of index OED (cancer-prone vs other sites) was associated with a higher risk of progression to OSCC in the univariate analysis (HR = 2.52, 95%CI 1.31–4.88, p = 0.006) but became not significant in the multivariate model (HR = 1.63, 95%CI 0.81–3.30, p = 0.17).
| Parameters | Developed OSCC (n = 42), No. (%) | Did not develop OSCC (n = 257), No. (%) | Univariate analysis | Multivariate analysisa | ||
|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | p-value | Adjusted HR (95% CI) | p-value | |||
| OLP status | ||||||
| Non-OLP | 23 (14.84%) | 132 (85.16%) | 1 | 1 | ||
| OLP | 19 (13.19%) | 125 (86.81%) | 0.76 (0.42–1.41) | 0.39 | 0.84 (0.42–1.66) | 0.61 |
| Age, mean (SD) | 63.44 (15.94) | 61.22 (13.26) | 1.02 (0.99–1.05) | 0.07 | 1.02 (0.99–1.04) | 0.14 |
| Gender | ||||||
| Male | 22 (16.42%) | 112 (83.58%) | 1 | 1 | ||
| Female | 20 (12.12%) | 145 (87.88%) | 0.88 (0.48–1.64) | 0.70 | 0.99 (0.50–1.94) | 0.97 |
| Smoking status | ||||||
| No | 17 (10.49%) | 145 (89.51%) | 1 | 1 | ||
| Yes | 9 (9.18%) | 89 (90.82%) | 0.96 (0.44–2.10) | 0.92 | 1.21 (0.51–2.87) | 0.66 |
| Unknown | 16 (41.03%) | 23 (58.97%) | ||||
| Alcohol consumption | ||||||
| No | 11 (9.02%) | 111 (90.98%) | 1 | 1 | ||
| Yes | 14 (11.29%) | 110 (88.71%) | 1.24 (0.57–2.72) | 0.58 | 1.10 (0.51–2.40) | 0.80 |
| Unknown | 17 (32.08%) | 36 (67.62%) | ||||
| Site of primary OED | ||||||
| Non-cancer prone | 13 (7.69%) | 156 (92.31%) | 1 | 1 | ||
| Cancer prone | 29 (22.31%) | 101 (77.69%) | 2.52 (1.31–4.88) | 0.006 | 1.63 (0.81–3.30) | 0.17 |
| Degree of primary OED | ||||||
| Low-risk OED | 9 (5%) | 171 (95%) | 1 | 1 | ||
| High-risk OED | 33 (27.73%) | 86 (72.27%) | 5.11 (2.44–10.71) | <0.001 | 4.18 (1.91–9.12) | <0.001 |
- Bold values indicate statistically significant (p < 0.05).
- a Adjusted for age, gender, smoking and alcohol consumption, site of primary OED and degree of primary OED.
With respect to time to onset, the median time of progression to OSCC was not significantly different between the two groups (3.21 years in OLP/OED vs. 2.23 years in non-OLP/OED, p = 0.44; Table 1).
3.5 Number of OSCCs after the index OED
A total of 49 primary OSCCs developed following the index OED in 42 individuals. Of these, 26 (53.06%) were observed in 19 OLP/OED patients, whereas 23 (46.94%) developed in 23 non-OLP/OED individuals (Figure 2b). Of the 19 OLP/OED patients, 12 developed one OSCC after their index OED and 7 developed a further new primary OSCC. All 23 non-OLP/OED patients developed one single OSCC after their index OED, with no further new primary OSCC. In both the univariate (IRR = 1.05, 95%CI 0.59–1.83, p = 0.87) and multivariate analyses (IRR = 1.05, 95%CI 0.55–1.99, p = 0.89) (Table S3), the total number of primary OSCCs developed after the index OED were not significantly different between groups. Of note, age at diagnosis (IRR = 1.03, 95%CI 1.01–1.05, p = 0.006), site (IRR = 1.98, 95%CI 1.09–3.60, p = 0.03) and grade of the index OED (IRR = 4.59, 95%CI 2.29–9.19) were significant predictors of the number of primary OSCCs in the univariate analysis. However, only age at OSCC diagnosis (IRR = 1.03, 95%CI 1.01–1.05, p = 0.02) and the degree of index OED (IRR = 3.67, 95%CI 1.75–7.73, p = 0.001) were found to be significantly positively associated with a higher number of subsequent new primary OSCCs in the multivariate model.
Among the 26 primary OSCC events recorded in OLP/OED individuals, 19 (73.08%) were early stages (TNM stages I and II) and the other 7 (26.92%) OSCC tumours were advanced stages (TNM stages III and IV). Twenty-two of the 23 OSCCs (95.65%) in non-OLP/OED individuals were early stage (TNM stages I and II). The difference in the stages of primary OSCCs between the two groups was not statistically significant (p = 0.05).
3.6 Mortality related to OSCC and other causes
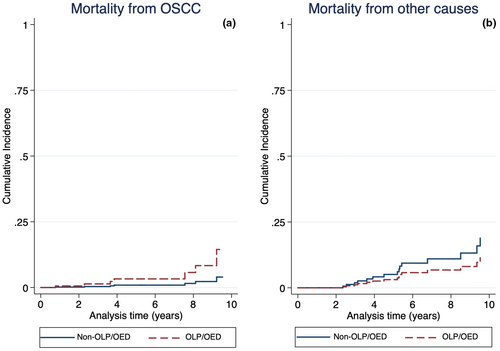
Twenty-seven of 299 studied patients (9.03%) died by the end of data collection period (28th February 2019). Of these, 10 individuals (37.04%) died from OSCC, including 8 OLP/OED and 2 non-OLP/OED individuals, and 17 (62.96%) died due to other causes. The 5-year cumulative OSCC-related mortality was 5.78% and 1.64% for OLP/OED and non-OLP/OED patients respectively (Figure 3a; p = 0.31). The 5-year cumulative mortality related to other causes was 4.55% and 8.02% for OLP/OED and non-OLP/OED patients respectively (Figure 3b; p = 0.36).
Regarding cause-specific Cox regression models (Table 4), OLP was not significantly associated with OSCC-related mortality in both univariate (HR = 3.64, 95%CI 0.77–17.16, p = 0.10) and multivariate analyses (HR = 0.84, 95%CI 0.29–2.48, p = 0.76). Of note, age at index OED diagnosis was a statistically significant predictor of OSCC-related mortality in both the univariate (HR = 1.07, 95%CI 1.02–1.13, p = 0.004) and multivariate analyses (HR = 1.05, 95%CI 1.02–1.09, p = 0.003), but not for mortality due to other causes.
| Parameters | Mortality related to OSCC | Mortality related to other causes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Death (10) | Unadjusted HR (95% CI) | p-value | Adjusted HRa | p-value | Death (17) | Unadjusted HR (95% CI) | p-value | Adjusted HRa | p-value | |
| OLP status | ||||||||||
| Non-OLP | 2 | 1 | 1 | 10 | 1 | 1 | ||||
| OLP | 8 | 3.64 (0.77–17.16) | 0.10 | 0.84 (0.29–2.48) | 0.76 | 7 | 0.61 (0.23–1.61) | 0.32 | 0.63 (0.18–2.14) | 0.46 |
| Age, mean (SD) | 71.98 (12.39) | 1.07 (1.02–1.13) | 0.004 | 1.05 (1.02–1.09) | 0.003 | 66.48 (16.46) | 1.05 (1.01–1.08) | 0.02 | 1.05 (1.01–1.11) | 0.01 |
| Gender | ||||||||||
| Male | 5 | 1 | 1 | 12 | 1 | 1 | ||||
| Female | 5 | 1.01 (0.29–3.50) | 0.99 | 0.77 (0.30–1.98) | 0.59 | 5 | 0.43 (0.15–1.29) | 0.12 | 0.36 (0.10–1.31) | 0.12 |
| Smoking status | ||||||||||
| No | 4 | 1 | 1 | 8 | 1 | 1 | ||||
| Yes | 3 | .01 (0.24–4.17) | 0.99 | 1.87 (0.56–6.23) | 0.31 | 5 | 1.09 (0.38–3.12) | 0.87 | 1.09 (0.27–4.40) | 0.91 |
| Unknown | 3 | 4 | ||||||||
| Alcohol consumption | ||||||||||
| No | 4 | 1 | 1 | 5 | 1 | 1 | ||||
| Yes | 3 | 0.54 (0.12–2.50) | 0.43 | 0.60 (0.19–1.87) | 0.38 | 6 | 0.99 (0.31–3.18) | 0.99 | 0.71 (0.16–3.14) | 0.65 |
| Unknown | 3 | 6 | ||||||||
| Site of primary OED | ||||||||||
| Non-cancer prone | 5 | 1 | 1 | 9 | 1 | 1 | ||||
| Cancer prone | 5 | 0.63 (0.18–2.28) | 0.49 | 0.51 (0.20–1.29) | 0.15 | 8 | 0.69 (0.26–1.83) | 0.45 | 0.53 (0.17–1.67) | 0.28 |
| Degree of primary OED | ||||||||||
| Low-risk OED | 2 | 1 | 1 | 6 | 1 | 1 | ||||
| High-risk OED | 8 | 3.96 (0.83–18.85) | 0.08 | 2.20 (0.82–5.92) | 0.12 | 11 | 1.82 (0.67–4.99) | 0.24 | 1.97 (0.62–6.28) | 0.25 |
| Number of OSCC | ||||||||||
| ≤1 OSCC episode | 4 | 1 | 1 | 2 | 1 | 1 | ||||
| >1 OSCC episodes | 6 | 4.35 (1.80–10.50) | <0.001 | 2.32 (0.71–7.58) | 0.17 | 1 | 0.82 (0.11–6.26) | 0.85 | 0.31 (0.03–3.16) | 0.32 |
- Bold values indicate statistically significant (p < 0.05).
- a Adjusted for age, gender, smoking and alcohol consumption, site of primary OED, degree of primary OED and number of OSCC.
4 DISCUSSION
The results of the present study show that, in individuals with OED, a pre-existing diagnosis of OLP is associated with a two times greater risk of developing additional new primary episodes of OED as compared to individuals without OLP. This risk was independent of other prognostic factors and was mostly evident during the first 3 years following the diagnosis of the index OED. The lack of association beyond 3 years may be explained by the smaller number of patients with follow-up time longer than 3 years, which reduced the study power. Interestingly, the additional episodes of OED following the index OED occurred significantly earlier in the OLP/OED than in the non-OLP/OED group (median of 1.46 vs. 2.96 years respectively). These findings are in keeping with previous studies reporting that chronic inflammation of OLP may provide a cytokine-based microenvironment affecting cell survival, growth, proliferation and differentiation (Bascones et al., 2005; González-Moles et al., 2006; Mignogna et al., 2004), hence, contributing to a higher risk of cancer initiation and progression at different time points.
Of note, the higher risk of developing new primary OED does not seem to translate into a statistically significant higher total number of OED episodes, although we did observe a tendency towards multiple OED lesions in the OLP/OED group (up to seven metachronous episodes of primary OED as opposed to a maximum of four episodes). It is possible that the limited duration of follow-up and the relatively small number of OLP/OED individuals developing multiple OED lesions may have led to insufficient statistical power to detect a significant difference.
With respect to the sites of subsequent OED episodes, the OLP/OED group showed a statistically significant higher involvement of non-cancer-prone sites. This is in keeping with the findings reported by Mignogna et al. (2007) and further confirms the possibility of field cancerization (Brinkmann & Wong, 2011; Mohan & Jagannathan, 2014) in patients with OLP.
The present study found no convincing evidence of a greater risk of progression of OED to OSCC in individuals with underlying OLP. This is consistent with the findings of other studies (Rock et al., 2018). Interestingly, the prevalence of oral cancer development in the OLP/OED subgroup was 13.19% (19/144), which is notably higher than the 8% (6/73) reported by Rock et al. (2018). This discrepancy is, however, not unexpected as the present study included all degrees of dysplasia, whereas Rock et al. (2018) only included patients with mild and moderate OED at baseline. Furthermore, the present study found no significant difference in the total number of OSCCs or in the time to OSCC onset between groups. However, we observed that none of the 155 individuals in the non-OLP/OED experienced more than one OSCC episode during their follow-up, whereas 7 of 144 patients with OLP-associated OED (4.86%) experienced up to two OSCC events after the index OED, which may suggest a higher tendency towards multiple OSSCs in the OLP/OED group. Of note, our multivariate analyses showed that the degree of index OED was a statistically significant predictor of both subsequent progression to OSCC and the total number of OSCCs, which is in keeping with previous literature (Liu et al., 2011; Mehanna et al., 2009; Warnakulasuriya et al., 2011). This is the first study assessing the impact of OLP up mortality rates in a sample of patients with OED. Although not statistically significant, our data suggest a trend of nearly fourfold increased mortality related to OSCC in OLP/OED patients (8/10) as compared to the non-OLP/OED group (2/10), as well as a notable difference in 5-year cumulative OSCC-related mortality (5.78% vs. 1.64%). As expected, older age was a significant predictor of OSCC-related mortality and mortality due to other causes (Extermann, 2000; Søgaard et al., 2013). Of note, the mortality outcomes reported in the present study are unlikely to be affected by OSCC stage, as the OLP/OED and non-OLP/OED groups were well balanced and not statistically different in terms of TNM staging. This may explain the contrasting results of a recent systematic review and meta-analysis (González-Moles et al., 2020). The authors reported that the 5-year mortality of OSCC in OLP patients was notably lower than in individuals with no background OLP, however, their conclusions were mostly based on studies at moderate-to-high risk of bias and likely to be affected by stage at diagnosis as confounding factor, considering that 81.51% of OSCC in OLP patients were stage I/II, as opposed to 50% in the control group. We suggest that further prognostic studies with a larger sample size are needed in order to clarify the role of OLP in OSCC mortality.
The results of this study should be considered in light of its limitations. The data were collected in a secondary/tertiary care oral medicine and head and neck cancer unit where the majority of OED patients are on long-term surveillance by specialists in both disciplines with a research interest in OED. This may not reflect the real-life set-up of most centres and could increase the likelihood of metachronous OED being detected as well as OSCC being diagnosed at an early stage due to close monitoring and multidisciplinary expertise. Therefore, the figures reported in the present study may overestimate the true prevalence of the index OED in OLP patients and secondary OED episodes compared to the general population due to increased surveillance. On the other side, they may underestimate the natural history and long-term prognosis of OED, as patients under close monitoring are more likely to be diagnosed and treated at an early stage should they develop metachronous disease or progression to malignancy, which may in turn translate into overall reduced mortality (ascertainment bias).
Furthermore, cohort studies, especially with respect to uncommon conditions, typically require a large sample size in order to obtain adequate statistical power (Mitani & Haneuse, 2020; Suresh & Chandrashekara, 2012). Although the number of patients included in this study is one of the largest within the OLP/OED literature, there were only a small number of OED patients who developed further OEDs or progressed to OSCC. Therefore, there is a possibility of type II error (false negative) as a result of underpowered statistics, especially when the confidence interval was wide.
5 CONCLUSION
The results of the present single-centre study differ from previous studies as they suggest that the prognosis of OED in patients with background OLP differs from that of OED patients with no underlying OLP. We found convincing evidence of a higher risk of developing new episodes of primary OED in the OLP/OED group occurring at non-cancer-prone sites. The trend towards a high number of multifocal metachronous OEDs, multiple OSCCs and increased OSCC-related mortality in the OLP/OED group was not statistically significant and therefore not fully convincing. We suggest that further long-term prospective studies would be required in order to clarify the effects of underlying OLP upon OED prognosis, in particular progression to OSCC and mortality. Should future studies confirm the hypothesis of a worse prognosis of OED in patients with background OLP, pragmatic changes in the treatment of this sub-group of OED patients may be considered, including a more extensive surgery where feasible, and possibly the use of systemic chemopreventative agents where available (McCarthy, Fedele, et al., 2021; McCarthy, Sacco, et al., 2021).
AUTHOR CONTRIBUTIONS
Kununya Pimolbutr: Conceptualization; data curation; formal analysis; methodology; writing – original draft; writing – review and editing. Woei Tatt Lim: Data curation; formal analysis; writing – review and editing. Rachel Lesson: Writing – review and editing. Colin Hopper: Writing – review and editing. Nicholas Kalavrezos: Writing – review and editing. Colin Liew: Writing – review and editing. Clare Schilling: Writing – review and editing. Deepti Sinha: Writing – review and editing. Amrita Jay: Data curation; writing – review and editing. Reshma Agrawal: Data curation; writing – review and editing. Stephen Porter: Supervision; writing – review and editing. Stefano Fedele: Conceptualization; methodology; supervision; writing – review and editing.
ACKNOWLEDGEMENTS
The authors would like to thank Dr Nat Na-Ek for technical assistance with statistical analysis and valuable advice and thank Dr Lujain Alsulimani for assistance with data collection.
FUNDING INFORMATION
Kununya Pimolbutr received a PhD Scholarship from the Faculty of Dentistry, Mahidol University, Bangkok, Thailand. Stefano Fedele received salary support from the National Institute for the Health Research University College London Hospitals Biomedical Research Centre.
CONFLICT OF INTEREST
None disclosed.
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.




