Effects of exercise timing on metabolic health
Almudena Ortega-Gómez and Mora Murri contributed equally to this work and share last authorship.
Summary
The increasing prevalence of metabolic syndrome is associated with major health and socioeconomic consequences. Currently, physical exercise, together with dietary interventions, is the mainstay of the treatment of obesity and related metabolic complications. Although exercise training includes different modalities, with variable intensity, duration, volume, or frequency, which may have a distinct impact on several characteristics related to metabolic syndrome, the potential effects of exercise timing on metabolic health are yet to be fully elucidated. Remarkably, promising results with regard to this topic have been reported in the last few years. Similar to other time-based interventions, including nutritional therapy or drug administration, time-of-day-based exercise may become a useful approach for the management of metabolic disorders. In this article, we review the role of exercise timing in metabolic health and discuss the potential mechanisms that could drive the metabolic-related benefits of physical exercise performed in a time-dependent manner.
Abbreviations
-
- AMPK
-
- adenosine monophosphate-activated kinase
-
- ATP
-
- adenosine triphosphate
-
- BMAL1
-
- brain and muscle ARNT-like 1
-
- BMI
-
- body mass index
-
- CGM
-
- continuous glucose monitoring
-
- CLOCK
-
- circadian locomotor output cycles kaput
-
- CRY
-
- cryptochrome
-
- HbA1c
-
- glycated hemoglobin
-
- HDL-C
-
- high-density lipoprotein cholesterol
-
- HIIT
-
- high-intensity interval training
-
- IL-6
-
- interleukin 6
-
- LDL-C
-
- low-density lipoprotein cholesterol
-
- PER
-
- period
-
- PGC-1α
-
- peroxisome proliferator-activated receptor-gamma coactivator 1-alpha
-
- T2DM
-
- type 2 diabetes mellitus
-
- TG
-
- triglycerides
1 INTRODUCTION
In the last decades, the dramatic increase in the global prevalence of obesity and related metabolic complications, such as type 2 diabetes mellitus (T2DM), dyslipidemia, and hypertension, encompassed within metabolic syndrome, has led to a major health, social, and economic burden.1, 2 Importantly, the imbalance between energy intake and expenditure, including unhealthy dietary habits and a sedentary lifestyle, has been demonstrated to be a decisive factor in the pathophysiology of metabolic diseases.3 Accordingly, nutritional therapy and physical exercise have become the mainstay of treatment and prevention of these conditions.4 In this regard, physical exercise acts as a key modifier in the development and progression of several chronic diseases, including obesity and related metabolic disorders.5, 6
Circadian rhythms are essential processes in human physiology and play a central role in a wide range of molecular mechanisms involved in metabolism and energy homeostasis.7 In fact, the disruption of circadian rhythms may lead to the development of metabolic diseases.8 Moreover, mounting evidence suggests that different time-based interventions may be associated with distinct metabolic effects and health-related outcomes. Therefore, the timing of food intake, as well as the distribution of calorie consumption through the day, can result in different effects on weight loss and distinct metabolic parameters.9-11 It is also noteworthy that scheduling some pharmacological agents within a specific time of day may entail significant benefits.12, 13 However, despite the well-known health-related benefits of physical exercise and the characterization of the impact of exercise modality, duration, volume, or intensity on metabolic health, little is known about the optimum timing of exercise.
In this review, we summarize the current knowledge on the role of exercise timing in metabolic health and discuss the potential mechanisms involved in time-dependent metabolic outcomes related to physical exercise.
2 PHYSICAL EXERCISE AND METABOLIC HEALTH
Physical exercise is a key modulator of metabolism and exerts protective effects against the development and progression of metabolic diseases. One of the central mechanisms involved in these effects is the positive remodeling of skeletal muscle through the stimulation of several exercise-induced pathways.14 Chronic adaptations of skeletal muscle to exercise include enhanced lipid oxidation and reduced intramuscular lipid content, increased insulin sensitivity and glucose uptake, or improved mitochondrial oxidative capacity and health, which drive the metabolic amelioration associated with physical exercise.15, 16 However, it is important to take into account that exercise improves metabolic disease through the modulation of other tissues beyond the skeletal muscle.17 Indeed, acute and chronic multi-tissue adaptations, including the liver, adipose tissue, pancreas, and endothelium/cardiovascular system, have been postulated to occur after exercise. Moreover, the systemic response to physical exercise involves a complex crosstalk among the skeletal muscle and these organs, via the release of exercise-induced hormonal mediators, immune-related factors, and cytokines (exerkines) into circulation, which also orchestrate the metabolic changes triggered by physical exercise.17, 18
On the other hand, it should be noted that different training modalities are associated with distinct metabolic adaptations and clinical outcomes.14, 19-21 For example, aerobic training results in greater reductions in body weight and body fat than resistance training, while the latter is more effective in reducing lean mass loss during weight loss.19, 22 Additionally, other determinants, such as the intensity, frequency, or duration of training sessions, also have an impact on metabolic health.19, 21, 23-25 In this regard, it has been reported that high-intensity aerobic exercise is effective in improving lipid profile, while an increased number of repetitions may have a greater impact upon lipid profile than increased intensity during resistance training.20, 26, 27 Moreover, in elderly men with T2DM, multiple short-duration bicycle sessions led to better glycemic control than single long-duration bicycle sessions.28 It is also noteworthy that several international organizations have taken these findings into consideration in order to recommend physical activity/exercise in the treatment and/or prevention of metabolic diseases, and the combination of different training strategies is often considered.19, 29 As an example, the American Diabetes Association recommends at least 150 min/week of moderate-to-vigorous (aerobic) physical activity and 2–3 sessions/week of resistance training in patients with prediabetes or T2DM.30 Nevertheless, despite the growing interest observed in this field, the potential role of exercise timing in metabolic health remains unexplored.
3 THE ROLE OF EXERCISE TIMING IN METABOLIC HEALTH: CLINICAL IMPLICATIONS
Physical exercise performed at different times of the day may result in diverse health outcomes. In the following subsections, we explore the impact of exercise timing on the prevention and treatment of the main components of metabolic syndrome, including obesity, insulin resistance/T2DM, dyslipidemia, and hypertension (Table 1). It also should be noted that several of the selected studies did not evaluate the role of the timing of exercise (i.e., planned, structured, and repetitive physical activity to improve/maintain physical fitness),51 but the role of the timing of moderate-to-vigorous physical activity (i.e., any bodily movement produced by skeletal muscles that results in energy expenditure)51 regarding the aforementioned results.
| Study | Design | Participants | Training program | Physical activity/exercise time | Main results |
|---|---|---|---|---|---|
| Di Blasio et al. (2010)31 | Parallel-group trial | Postmenopausal women with a BMI ≥ 25 kg/m2 (n = 42) | 50-min moderate-intensity walking for 3 months (4 times/week) | Morning (8–10 a.m., after breakfast)/evening (6–8 p.m., before dinner) | Evening group achieved greater reductions in fat mass than morning group. |
| Chomistek et al. (2016)32 | Cross-sectional study | Adult women (mean BMI of 26.1 kg/m2) (n = 7157) | Physical activity assessed by accelerometry during 2 years | Before/after 12 p.m. | The lowest quartile of percent accelerometer counts before 12 p.m. (based on total accelerometer counts per day) had higher odds for obesity than the highest quartile. |
| Alizadeh et al. (2017)33 | Parallel-group randomized trial | Adult women with overweight (n = 48) | 6-week aerobic exercise (30 min of moderate- to high-intensity treadmill running, 3 sessions/week) |
Morning (8–10 a.m.)/afternoon (2–4 p.m.) Participants were requested to eat a light meal 2 h before exercise |
Morning exercise led to greater reductions in body weight, BMI, and abdominal circumference than evening exercise. |
| Willis et al. (2020)34 | Retrospective analysis of data from a randomized trial | Adults with overweight/obesity (n = 88) | 10-month intervention (5 sessions/week: 15 min of moderate- to high-intensity treadmill walking/jogging)/control group | Morning (7–11:59 a.m.)/afternoon (3–7 p.m.) | Exercise training between 7 and 11:59 a.m. resulted in significantly greater weight loss than exercise training between 3 and 7 p.m. |
| Teo et al. (2020, 2021)35, 36 | Parallel-group randomized trial | Adults with overweight, with and without T2DM (n = 40) | 12-week multimodal program (3 sessions/week: 30 min of moderate-intensity walking and 4 resistance-based exercises, 3 sets, 12–18 repetitions) |
Morning (8–10 a.m.)/evening (5–7 p.m.) Participants were requested to eat a light meal at least 1 h before exercise |
No differences in weight loss/body composition between groups. Improved HbA1c, fasting/postprandial venous glucose, regardless of morning/afternoon group. Morning exercise showed greater benefits on HbA1c, fasting/postprandial venous glucose (without reaching statistical significance) in subjects with T2DM (n = 20). |
| Arciero et al. (2022)37 | Parallel-group randomized trial | Healthy adults (n = 56; 30 women and 26 men) | 12-week multimodal program: 4 sessions/week: resistance/interval sprints/stretching/endurance exercise lasting less than 1 h, except endurance exercise (1 h or longer) |
Morning (6–8 a.m.)/evening (6:30–8:30 p.m.) Participants consumed a small snack <1 h prior to resistance and interval training, and stretching and endurance exercise were performed pre-meal |
Morning exercise reduced total body fat mass, abdominal fat percentage, and BP in women. Evening exercise reduced BP in men. |
| Brooker et al. (2023)38 | Parallel-group randomized trial | Adults with overweight/obesity (n = 100) | 12-week self-paced aerobic exercise (250 min/week) | Morning (6–9 a.m.)/evening (4–7 p.m.)/control | Morning and evening exercises reduced weight. A greater proportion of participants in the evening group achieved clinically meaningful weight loss (>5%). |
| Savikj et al. (2019)39 | Randomized crossover trial | Adult men with T2DM (n = 11) | 2-week HIIT (6 × 1 min of pulses; 3 sessions/week) |
Morning (8 a.m.)/afternoon (4 p.m.) Participants had a light breakfast 1 h before morning exercise, and afternoon session was performed 3 h after lunch |
Afternoon HIIT, but not morning HIIT, reduced CGM-based glucose concentrations. Morning HIIT increased CGM-based glucose concentrations in the first week. |
| Munan et al. (2020)40 | Randomized crossover trial | Adults with T2DM (n = 14) | 50 min of walking at 5 km/h for 12 days/no exercise |
Morning (ending 20 min before breakfast)/afternoon (3–4 h after lunch)/evening (20 min after evening meal) |
CGM-based glucose concentrations did not differ among groups. |
| Moholdt et al. (2021, 2023)41, 42 | Parallel-group randomized trial | Adult men with overweight/obesity and no T2DM (n = 25) receiving an 11-day HFD | 5-day intervention program (3 days of HIIT × 10 min and 2 days of moderate-intensity cycling × 40/60 min)/no exercise |
Morning (6:30 a.m.)/evening (6:30 p.m.) Participants followed a high-fat diet 6 days prior and during the intervention. Exercise was performed before meals |
Fasting and nocturnal blood glucose, and fasting and postprandial insulin significantly decreased only in the evening group. Significant reductions in total cholesterol, LDL-C, and TG in the evening group. |
| Mancilla et al. (2021)43 |
Retrospective analysis of data from a non-randomized trial |
Adult men with overweight/obesity, with or without T2DM (n = 32) |
12-week exercise program (2 sessions/week of moderate-intensity cycling, 30 min; 1 session/week of resistance training [3 series of 10 repetitions]) |
Morning (8–10 a.m.)/afternoon (3–6 p.m.) Exercise was performed 2–3 h after breakfast/lunch |
Improved fasting glucose and peripheral insulin sensitivity in the afternoon group, compared with the morning group. |
|
Hetherington-Rauth et al. (2022)44 |
Cross-sectional study |
Adults with T2DM (n = 74) |
Moderate-to-vigorous physical activity assessed by accelerometry during 7 days |
Morning (waking hour to 12 p.m.)/afternoon (12–8 p.m.) |
Moderate-to-vigorous physical activity in the afternoon, but not in the morning, was inversely related to HbA1c in the multivariate model. |
|
Kim et al. (2022)45 |
Randomized crossover trial |
Healthy adult men (n = 12) |
1-week moderate-intensity endurance exercise (3 sessions/week, 60 min of treadmill walking) |
Morning (9–11 a.m.)/afternoon (4–6 p.m.) |
Afternoon exercise significantly decreased postprandial glucose and improved 24-h glucose after the intervention, compared with morning exercise. Significant reductions in TG and TG/HDL-C after afternoon intervention. |
|
Van der Velde et al. (2022)46 |
Cross-sectional study |
Adults without T2DM, mean BMI of 26.2 kg/m2 (n = 775) | Physical activity assessed by accelerometry during 7 days |
Morning (6 a.m.–12 p.m.)/afternoon (12 p.m.–6 p.m.)/evening (6 p.m.–12 a.m.) |
Moderate-to-vigorous physical activity in the afternoon or evening, but not in the morning, was associated with a reduction up to 25% in HOMA-IR, compared with an even distribution of physical activity during the day. |
| Lian et al. (2014)47 | Randomized controlled trial | Adults with coronary artery disease (n = 330) | Walking of 30 min/day or more on at least 5 days/week (12 weeks) |
Morning/evening |
Evening intervention resulted in significantly greater reductions in LDL-C than morning intervention. |
|
Park et al. (2005)48 |
Crossover trial |
Adults with hypertension (n = 14) | Single bout of walking (30 min; 50% peak oxygen uptake) |
Morning (6–8 a.m.)/evening (5–7 p.m.) |
Evening intervention achieved greater reductions in night-time systolic BP in non-dippers. The effects of morning intervention were similar in dippers and non-dippers. |
| Jones et al. (2008)49 | Crossover trial | Healthy adult men (n = 12) | Single bout of exercise (30 min of cycling at 70% peak oxygen uptake); 24 h of ABPM | Morning (8 a.m.)/afternoon (4 p.m.) |
Reduced mean ± SE BP following exercise at 4 p.m., but increased after exercise at 8 a.m. |
|
Brito et al. (2019)50 |
Parallel-group randomized trial |
Adult men with hypertension (n = 50) |
10-week training program (3 sessions/week of 45-min cycling at moderate intensity)/no training |
Morning (7–9 a.m.)/evening (6–8 p.m.) |
Only evening training reduced clinic systolic BP and 24-h diastolic BP. |
- Abbreviations: ABPM, ambulatory blood pressure monitoring; BMI, body mass index; BP, blood pressure; CGM, continuous glucose monitoring; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; HFD, high-fat diet; HIIT, high-intensity interval training; HOMA-IR, homeostatic model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; SE, standard error; T2DM, type 2 diabetes mellitus; TG, triglycerides.
3.1 Obesity
Time-of-day-based exercise could be useful for the management of obesity. In this regard, in a 6-week randomized control trial including 48 adult women with overweight, 30 min of moderate- to high-intensity morning treadmill running (3 sessions/week) resulted in greater reductions in body weight, body mass index (BMI), and abdominal circumference than the evening group.33 Despite the fact that no differences related to overall appetite scores between groups were found, the morning group presented a higher satiety and a decrease in calorie intake during the intervention, which might explain, in part, these results.33 In the 10-month Midwest Exercise Trial 2, including 88 physically inactive young adults with overweight/obesity, the predominant completion of aerobic training sessions between 7:00 and 11:59 a.m. was associated with greater weight loss than 3:00 and 7:00 p.m. (−7.2 ± 1.2% vs. −2.1 ± 1.0%, respectively), although no significant differences in terms of energy balance assessed via the doubly labeled water method were detected.34 In line with these findings, morning exercise was significantly superior to evening exercise in terms of reduction of total body fat mass and abdominal fat percentage in exercise-trained women, but not in men, with no changes in the self-reported dietary intake during a 12-week intervention of multimodal training regimen (resistance, high-intensity interval training [HIIT], stretching, and endurance exercise).37 These results might be due to sex differences within physiological systems involved in exercise response, including different fuel utilization and hormonal release, as previously reported.52, 53 In a cross-sectional analysis including more than 7000 older women from the Women's Health Study, the lowest quartile of physical activity in the morning (measured by percent accelerometer counts before 12 p.m. according to total accelerometer counts per day) was associated with a higher risk of obesity.32 In contrast, in an interventional study conducted in 42 sedentary postmenopausal women with BMI ≥ 25 kg/m2, evening walking led to greater reductions in fat mass than morning walking.31 Conversely, a 12-week randomized controlled trial including 40 sedentary participants with overweight/obesity that completed a supervised multimodal program (moderate-intensity walking and resistance-based training) did not find differences in weight loss or changes in body composition between morning/evening exercise.35 Although Brooker et al. did not find significant differences in weight loss in a 12-week randomized trial (100 inactive adults with overweight/obesity, 76% women) between morning/evening self-paced aerobic exercises (250 min/week), a greater proportion of participants in the evening group achieved clinically meaningful weight loss (>5%).38
It should be noted that weight loss and weight loss maintenance are two different concepts: In general, a reduction in dietary intake is considered as a key element in weight loss, whereas exercise may facilitate weight loss maintenance,54 a fact that should be considered in the aforementioned studies. In fact, in a cross-sectional study, Creasy et al. observed that weight loss maintainers accumulated higher amounts of moderate-to-vigorous-intensity physical activity in the morning than controls.55 Also, behavioral adaptations (e.g., consistency of exercise) could play a role in these results. As an example, early morning exercise might relate to a greater exercise routine stability and exercise habit, as previously reported.56
Overall, a number of studies have revealed that exercise timing might lead to differences in weight loss. However, it should be pointed out that an important number of the available studies only included women, and mixed results have also been reported in this regard. The inconsistency of some of these findings may be related to the heterogeneity of the available studies. On the other hand, although endurance exercise seems to induce time-dependent effects related to weight loss, it should be mentioned that only a few studies have evaluated the effects of exercise timing on weight loss/changes in body composition in other potentially effective modalities, such as HIIT or resistance training. Therefore, long-term, well-designed randomized trials are needed to confirm these results. Besides, the underlying mechanisms implicated in the association between exercise timing and weight loss are yet to be fully elucidated. Thus, it has been postulated that timing of exercise may involve a different release of signals and appetite-related hormones, which might have an influence on appetite regulation and energy intake.57 In line with this, morning exercise, particularly when it is performed close to lunch, has been reported to have a greater impact on the reduction of calorie intake, although the evidence is still limited.33, 57 Additionally, a recent trial showed that acute evening exercise prompted greater post-exercise ad libitum energy intake than morning exercise.58 Conversely, a 30-min bout of aerobic exercise performed in the evening was associated with a greater decrease in the orexigenic hormone asprosin and other obesity-related hormones (e.g., serum lipocalin-2) than morning exercise.59 Also, exercise performed in fasted (vs. fed) state has been reported to reduce energy intake and induce a higher fat oxidation.60, 61 However, differences in meal timing in relation to exercise were not assessed in most studies evaluated in this section.
3.2 Type 2 diabetes mellitus
Timing of exercise/physical activity might also have an impact on glycemic response, as some studies have shown (Table 1). Therefore, modulating the time at which physical exercise is performed could become a useful tool for the prevention and treatment of T2DM. A randomized crossover trial of 11 men with T2DM that underwent 2 weeks of morning/afternoon HIIT (3 sessions/week) demonstrated that afternoon HIIT resulted in the improvement of glucose levels measured by continuous glucose monitoring (CGM) compared with morning HIIT.39 In addition, Mancilla et al. retrospectively analyzed data from 32 men with T2DM or at risk of T2DM and reported that 12 weeks of multimodal training program (moderate-intensity cycling and resistance training) in the afternoon was associated with improved insulin sensitivity, glucose levels, and fat mass, compared with morning training.43 In a recent cross-sectional study, the performance of moderate-to-vigorous physical activity in the afternoon was related to improved glycated hemoglobin (HbA1c) in patients with T2DM.44 Additionally, moderate-to-vigorous physical activity in the afternoon/evening, but not in the morning, was associated with higher insulin sensitivity in 775 middle-aged adults without T2DM (mean BMI of 26.2 kg/m2) than an even distribution of physical activity during the day in a cross-sectional study.46 One possible explanation to these findings may relate to differences in the patterns of fuel utilization between morning and afternoon/evening exercises. Accordingly, afternoon/evening exercise may result in an increased oxidative capacity, compared with morning exercise, along with a more efficient utilization of substrates (e.g., glucose) by the skeletal muscle.62 Moreover, as glucose tolerance and insulin sensitivity exhibit circadian oscillations, the alignment of circadian clocks with specific time-of-day-based exercise bouts may induce greater effects on glucose metabolism.63
On the other hand, some studies did not find substantial effects of exercise timing on glycemic response. Thus, a randomized trial evaluating the effects of 12-week multimodal training program (3 sessions/week; 30 min of moderate-intensity walking and 3 sets of resistance-based exercises [12–18 repetitions]) conducted in 40 adults with overweight with and without T2DM showed no significant differences between morning/evening exercises with regard to glycemic outcomes (HbA1c, fasting glucose, and postprandial glucose).36 Indeed, no differences in the circadian rhythm of wrist skin temperature (which could be used as proxy for the rhythmicity in the central clock),64 in response to training (evaluated >24 h after the final training session, compared with pre-intervention), were observed, although the authors argued that a higher exercise intensity/duration might have resulted in greater shifts in the circadian rhythms and glucose metabolism.36 Similarly, Munan et al. also found no differences between 50 min of walking at 3 different times of day in 24-h glucose concentrations in patients with T2DM.40
In line with some of the findings reported in patients with T2DM, a randomized trial including 25 men with overweight and obesity and no T2DM found that 5-day evening training (HIIT and moderate-intensity cycling), but not morning training, improved nocturnal glycemic control (assessed by CGM) after a short-term high-fat diet.41 Similarly, other studies have described the benefits of afternoon exercise in glycemic outcomes in healthy subjects. Accordingly, a randomized crossover trial (12 healthy men assigned to morning or late-afternoon endurance exercise for 1 week) revealed that afternoon exercise was associated with significantly lower postprandial glucose and 24-h glucose levels after the intervention.45 Importantly, due to the small sample size of the majority of the studies in patients with and without T2DM, and their different durations, type of training programs, or glycemic outcomes, these results should be cautiously interpreted. Although afternoon/evening exercise seems to be more effective than morning training with regard to glycemic outcomes, large-scale, long-term devoted trials are needed to confirm these findings.
It is noteworthy that timing of exercise in relation to meals could involve different glycemic responses. In fact, a growing body of evidence suggests that the interaction between exercise timing and the timing of food intake may play an important role in the modulation of the circadian clock and the prevention of metabolic diseases, such as T2DM.65 In line with this, a single bout of postprandial treadmill walking, compared with treadmill walking in a fasted state, further reduced glycemic excursions and glycemic variability, measured by CGM, over the next 22 h in 12 patients with T2DM.66 A randomized crossover trial including 77 healthy individuals revealed that different exercise bouts performed early after meal, but not in the fasted state, led to significant reductions in postprandial glycemia.67 Also, a trial conducted in 54 healthy postmenopausal women demonstrated that 2-h moderate-intensity acute exercise performed 1 h after meals was more effective in reducing insulin resistance than exercise performed 1 h before meals.68 On the other hand, a randomized crossover trial including 10 patients with T2DM using CGM showed that HIIT/moderate-intensity continuous exercise performed in the fasted state was more effective than post-breakfast exercise in reducing postprandial glycemia.69 Additionally, a crossover study (nine healthy postmenopausal women who performed twice a day 2 h of treadmill moderate exercise) showed that exercise before meals, but not after meals, lowered blood glucose over a 16-h period, a finding that could be related to a reduced carbohydrate availability during exercise.70 Therefore, due to the reported conflicting results, the topic is to be investigated further.
3.3 Dyslipidemia
Several studies have evaluated the influence of timing of physical activity/exercise on lipid profile. In a randomized controlled trial that enrolled 330 patients with coronary artery disease, walking ≥ 30 min/day in the evening at least 5 days/week over a period of 12 weeks resulted in greater reductions in low-density lipoprotein cholesterol (LDL-C) and some inflammatory markers (i.e., fibrinogen and high-sensitivity C-reactive protein) than morning walking.47 Although the underlying mechanisms related to the reported differences are poorly understood, the authors inferred that the circadian rhythms of some hormones, including the response to cortisol to exercise in a time-dependent manner, could be implicated.47, 71 Furthermore, lipid metabolism (including triglyceride and cholesterol synthesis) is under the influence of circadian rhythms, with a peak at night-time,72 and the potential lipid-lowering effects of exercise might be time dependent, similar to other time-based therapeutic strategies (e.g., the use of statins).13 Another trial, conducted in patients with overweight/obesity, concluded that only evening combined HIIT/moderate-intensity cycling performed pre-meal was able to decrease cholesterol, LDL-C, and triglycerides (TG) during an 11-day high-fat diet.41 Besides, a secondary analysis of this trial revealed that evening exercise reduced LDL-C in three subfractions by ~30%, whereas morning exercise only lowered cholesterol in the largest LDL particles by 19%.42 Additionally, a significant decrease in TG and TG:high-density lipoprotein cholesterol (HDL-C) was also reported after 1 week of pre-meal late-afternoon endurance training compared with morning endurance training in healthy subjects.45 Accordingly, evening/late-afternoon physical activity/exercise could be more effective to improve lipid profile, although further research is needed.
Similar to the glycemic response, serum lipid levels may be affected by exercise timing according to prandial state. Thus, Zhang et al. showed that pre-meal exercise could have a greater benefit on TG and HDL-C than post-meal exercise,73 although opposite results have also been reported74; therefore, further research is warranted.
3.4 Hypertension
Even though physical activity/exercise has been proven to exert benefits in the prevention and treatment of hypertension,75 a greater decrease in blood pressure has been shown after evening interventions. In this regard, Brito et al. showed that only evening aerobic training (45 min of cycling at moderate intensity, 3 sessions/week during 10 weeks) reduced clinic and ambulatory blood pressure in men with treated hypertension in a randomized controlled trial.50 These results were related to a higher decrease in systemic vascular resistance and vasomotor sympathetic modulation in the evening group.50 In line with this, it is known that endogenous circadian clocks have a major influence on blood pressure regulation,76, 77 and the modulation of clock-mediated target genes by time-of-day-based exercise might play a role in these results.78, 79 On the other hand, acute hypotensive effects of evening exercise may be greater when compared with morning exercise, as reported in a crossover trial,49 and some authors argue that these findings may be due to a greater boost in intravascular shear stress after morning exercise,80 although additional mechanisms are yet to be elucidated. Besides, sex differences may play a role in the potential effects of timing of physical exercise on blood pressure. Therefore, a recent randomized trial including healthy volunteers found that 12 weeks of multimodal exercise in the morning led to a stronger decrease in blood pressure in women, whereas a decrease in blood pressure levels was observed in men following evening exercise.37 Also, dipper and non-dipper status of patients with hypertension (i.e., the adequate nocturnal decrease in blood pressure ≥ 10% or <10% reduction in average nocturnal blood pressure, respectively)81 may lead to different responses to exercise timing. Thus, Park et al. demonstrated in a crossover trial conducted in 14 adults with hypertension (dippers and non-dippers) that a single bout of evening walking (30 min, moderate intensity) led to greater reductions in night-time systolic blood pressure in non-dippers than in dippers, whereas the effects of morning walking on blood pressure were similar in both groups.48
4 POTENTIAL VARIABLES EXPLAINING DIVERGENT RESULTS IN CLINICAL STUDIES
Even though physical activity/exercise might elicit time-dependent benefits regarding different outcomes related to metabolic health, a non-negligible degree of variability may be observed in the results from clinical studies evaluating this topic. Accordingly, several clinical variables, such as sex, age, health status, training status prior to the intervention, exercise modality, or chronotype (discussed in following sections), may explain some of these results (Figure 1). Indeed, in a large-scale cohort study (92,139 UK Biobank participants with valid accelerometer data, followed up over a median period of 7 years), although moderate-to-vigorous physical activity at any time of day was associated with lower risks of overall or cardiovascular mortality, afternoon and mixed (but not evening) groups had even lower risks than morning groups, and these associations were stronger in the male sex, older participants, less physically active participants, or those with stablished cardiovascular disease.82 Therefore, these and other clinical variables might be considered as important modulators of time-dependent effects of physical activity/exercise regarding metabolic health.
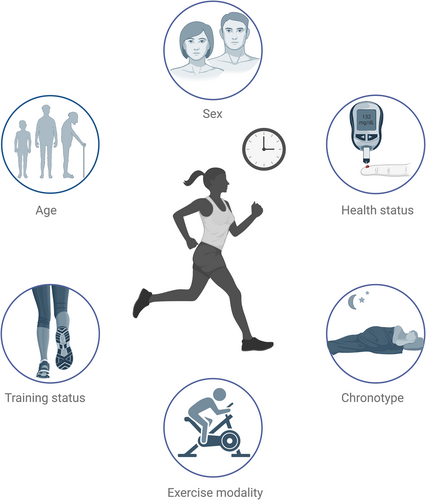
4.1 Sex
Previous works have revealed that sex may be a decisive biological variable in the response to physical activity/exercise.52 Thus, substrate metabolism and utilization, together with hormonal fluctuations during/after training, may be different between women and men.52, 83 Noteworthy, Arciero et al. evaluated the effects of a multimodal training program in a separate cohort of healthy women and men, showing sex-based differences in response to exercise timing (women exercising in the morning experienced greater reductions in body fat and blood pressure, whereas men exercising in the evening achieved greater reductions in blood pressure, as well as a significant increase in fat oxidation).37 Furthermore, timing of moderate-to-vigorous physical activity has been associated with cardiorespiratory fitness and cardiovascular risk in men (but not in women) with T2DM.84 Therefore, interactions between sex and exercise timing may also account for different outcomes related to metabolic health. However, a significant number of previous studies only enrolled men, but not women (or vice versa), regarding specific metabolic outcomes,31-33, 39, 41, 43, 45, 49, 50 and others did not explore potential differences in sex.34, 36, 38, 40, 47, 48 Hence, further research is required in this area.
4.2 Age
Age might be another potential clinical factor involved in the variability of response to the timing of physical activity/exercise. Remarkably, most studies assessing the role of exercise timing in metabolic health included middle-aged subjects, and only a few studies have been specifically conducted in older participants (i.e., subjects aged ≥65–70). Thus, in a cross-sectional and prospective analysis of 207 sedentary older participants, higher physical activity in the morning (measured by accelerometry) was related to lower fasting glucose and insulin resistance85 (as opposed to a significant number of studies).39, 41, 43, 44, 46 Moreover, divergent results might be observed in older adults according to other clinical variables, such as BMI, depending on sex.32, 85 As particular characteristics and different responses to physical activity/exercise may be found in the elderly, further research in this population is needed. On the other hand, similar outcomes may be found among those studies performed in younger adults (e.g., higher effectiveness of morning exercise in weight loss).33, 34 Of note, different results might be expected in the pediatric population.86, 87
4.3 Training status
Previous research has suggested that training status might have an influence on energy and substrate utilization, as well as other clinical characteristics (e.g., hypotensive response to exercise).88, 89 It is important to note that the majority of the studies that evaluated the potential benefits of exercise timing in metabolic health enrolled sedentary participants (prior to the intervention), and only some trials were conducted in healthy, physically active individuals.37, 49 Indeed, studies including both physically active and inactive participants are lacking and did not evaluate this factor due to the small sample size.48 As a consequence, there are no available studies considering training status as a potential modulator of the effects of the timing of physical activity/exercise on metabolic health. Given the findings observed in studies evaluating cardiovascular mortality in relation to the timing of physical activity,82 the influence of training status on time-of-day-based physical activity/exercise should be further investigated.
4.4 Health status
Some metabolic disorders, such as obesity and T2DM, might influence metabolic response to exercise, and states of severe metabolic dysregulation may be predictors of non-response.90, 91 Most studies evaluating glycemic response to exercise timing included patients with well-controlled T2DM or healthy volunteers, but not both groups, and showed, in general, greater benefits when exercise was performed in the afternoon/evening. Interestingly, Teo et al. enrolled participants with overweight with and without T2DM and reported overall improvements in glycemic response regardless of the timing of exercise, although a trend towards the superiority of morning training was observed only among participants with T2DM.36 On the other hand, differences in weight loss/body composition after time-of-day-based exercise might be less evident in trained, healthy subjects (particularly in men).37 Also, appetite regulation and hormonal release induced by exercise timing might be different between healthy individuals and subjects with overweight/obesity.59 Nevertheless, studies including matched subjects with different metabolic characteristics are needed to shed light on the potential role of this variable.
4.5 Exercise modality
Type of exercise (i.e., endurance vs. resistance training) has been suggested to be differentially affected by time of day. Accordingly, resistance training/HIIT may be more susceptible to circadian variations than aerobic exercise, and exercise performance may peak in the afternoon/evening in the former.92-94 Remarkably, trials evaluating the role of timing of HIIT (or multimodal programs including HIIT) in glycemic response showed greater effects of afternoon/evening exercise,39, 41 whereas studies including moderate-intensity aerobic physical activity/exercise in training programs showed conflicting findings.36, 40, 44, 45 On the other hand, the heterogeneity of results found in some studies evaluating the role of timing of physical activity/exercise in weight loss might be related to the fact that most of them only included aerobic workouts. Hence, the impact of the timing of HIIT/resistance training on body composition should be considered in future research.
5 MECHANISMS BEHIND THE EFFECTS OF TIME-BASED EXERCISE ON METABOLISM
Although there are still many gaps in the knowledge of the determinants that may drive the variance in metabolic-related outcomes according to time-of-day-based exercise, recent research has shed light on the potential mechanisms involved in this relationship, including the circadian clock, metabolic signatures imprinted by timely exercise, and mitochondrial fitness (Figure 2).
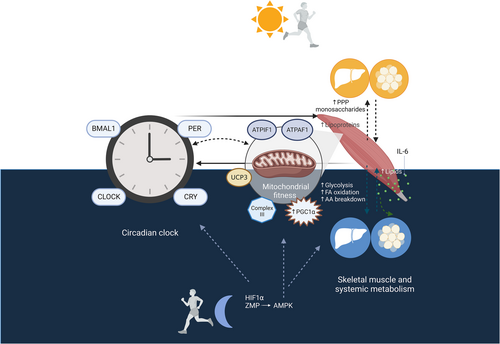
5.1 The circadian clock
Mammalian circadian clocks are endogenous oscillators that control 24-h physiological and behavioral processes. Cell-autonomous sets of protein-coding genes oscillate within a 24-h cycle to control human physiological rhythms.95 On the one hand, the central clock, located in the hypothalamic suprachiasmatic nucleus, has light as its main zeitgeber (time cue) and orchestrates the synchronization of external clocks in peripheral tissues (including the skeletal muscle), which are also capable of functioning with independent rhythmicity.7, 95 The core clock is a master regulator of metabolism and energy homeostasis through several interconnected transcriptional autoregulatory feedback loops, which encompass activators (i.e., circadian locomotor output cycles kaput [CLOCK] and brain and muscle ARNT-like 1 [BMAL1]) and target genes (i.e., period [PER], cryptochrome [CRY], and nuclear receptor subfamily 1 group D member 1 [NR1D1]), which repress CLOCK/BMAL1.7, 95 Indeed, the alterations of circadian systems may lead to metabolic disease.96 Thus, circadian disruption has been postulated to be involved in alterations in glucose metabolism/insulin sensitivity and the development of T2DM, obesity, and dyslipidemia.97, 98 Also, other physiological processes, such as appetite/food intake, and the oscillating levels of hormones involved in their control, are closely regulated by circadian clocks.99
Notably, physical exercise is known to be a key zeitgeber and acts as an essential regulator of circadian clock machinery in the skeletal muscle, with important implications in metabolic health.100, 101 Besides this, exercise can differently regulate several circadian clock genes in the skeletal muscle and other tissues in a time-dependent manner. In this regard, a single bout of exercise in the morning and afternoon increased the expression of BMAL1 in human leukocytes, while the expression of CRY1 increased only after morning exercise.102 Moreover, it has been hypothesized that time-of-day-based exercise exerts different metabolic effects via the modulation of the core clock. In the following section, we provide an integrative view of specific exercise timing-dependent metabolic signatures and the connection between these metabolic pathways and the circadian clock.
5.2 Time-dependent metabolic signatures in exercise
In the last few years, emerging evidence has revealed that exercise exerts different effects on skeletal muscle gene expression and a number of metabolic pathways depending on the time of day. Remarkably, some of these mechanisms may be orchestrated by the skeletal muscle core clock and, at the same time, exercise-induced metabolites and metabolic sensors have a direct influence on the circadian clock.103, 104 In addition, the complex crosstalk between the skeletal muscle and other tissues may be also modulated by exercise in a time-dependent manner.62, 105
Exercise performed at different times of the day has been postulated to provoke distinct metabolic responses in the skeletal muscle. In a mouse model, Sato et al. showed a time-dependent effect of exercise on the daily skeletal muscle transcriptome and metabolome with a different pattern of rhythmic genes and metabolites.103 Furthermore, in a complementary experimental approach, Ezagouri et al. observed distinct specific time-of-day changes in substrate utilization and transcriptomic/metabolic signatures in skeletal muscle in mice and humans.104 Of note, the results suggested a better exercise efficiency in humans in the evening hours than the morning hours.104
Beyond the skeletal muscle, exercise timing can elicit time-dependent effects on additional tissues, such as adipose tissue.106 Moreover, timing of exercise has been postulated to lead to a wide activation of systemic metabolism. It should be pointed out that Sato et al. provided for the first time a comprehensive insight into multi-tissue individual and coordinated metabolic response to acute exercise at different times of the day in a systems biology approach.105 Nevertheless, clinical studies have also shown differences in the human serum metabolome specific to time-of-day-based exercise. Therefore, in subjects with overweight/obesity, short-term combined training (HIIT and moderate-intensity cycling) in the evening, as opposed to morning exercise, partially reversed high-fat diet-induced alterations in the circulating metabolite profile, including changes in sphingolipid and amino acid metabolism,41 with potential implications in the pathophysiology of obesity and related metabolic disorders.107, 108 More recently, Savikj et al. showed that exercise timing influences multi-tissue metabolome and skeletal muscle proteome profile in a randomized crossover trial including 15 patients with T2DM.62 In this regard, the authors observed an increase in skeletal muscle lipids and mitochondrial content after 2 weeks of afternoon HIIT compared with morning HIIT, while the later intervention was associated with higher skeletal muscle lipoproteins and plasma carbohydrates via the pentose–phosphate pathway.62 Accordingly, although the clinical relevance of these findings warrants further research, they might be in line with some of the aforementioned clinical studies that have shown the potential benefits of afternoon exercise with regard to glycemic response.39, 41, 43, 44
The systemic response to exercise also involves the activation of the immune system, which plays an important role in the modulation of metabolism.109, 110 A study conducted in 14 healthy adult men showed that acute endurance exercise in the evening resulted in higher serum levels of interleukin 6 (IL-6) than morning exercise, and IL-6 levels correlated with post-exercise free fatty acid levels.111 These results reinforce the influence of time-of-day-based exercise on human metabolism, because IL-6 is an exerkine with several systemic effects (e.g., white adipose tissue lipolysis, fatty acid oxidation, glucose homeostasis, or anti-inflammatory response).18 However, despite the fact that some studies have evaluated the role of immune/inflammatory response after different types of exercise,112, 113 the effects of time-of-day-based exercise on the immune system remain poorly explored.
5.3 Mitochondrial fitness
Mitochondria, the powerhouse of eukaryotic cells, integrate fuel metabolites (i.e., carbohydrates, fatty acids, and amino acids) to produce energy in the form of adenosine triphosphate (ATP).114 Furthermore, beyond their bioenergetic function, mitochondria are a hub for the generation of biosynthetic precursors, maintenance of redox balance, and management of metabolic waste.114 As mitochondria play an essential role in human metabolism and energy homeostasis, mitochondrial dysfunction is associated with the pathophysiology of metabolic diseases.115-117 It is noteworthy that exercise training is able to induce beneficial mitochondrial adaptations, including enhanced mitochondrial content, protein synthesis, oxidative capacity, or biogenesis, as well as the more efficient removal of dysfunctional/damaged mitochondria, with derived benefits in metabolic health.118, 119 Indeed, these adaptations appear to be exercise modality dependent120 and may also be affected by other factors, such as training volume or intensity.119 However, less attention has been paid to the potential effects of exercise timing on mitochondrial fitness, although it is important to keep in mind that mitochondrial activity and dynamics follow a circadian pattern,79, 121 and disrupted circadian oscillations in different metabolic diseases, such as T2DM, are related to an altered mitochondrial metabolism.122 Interestingly, a recent randomized crossover trial showed that afternoon HIIT increased mitochondrial complex III to a greater extent than morning HIIT in patients with T2DM, suggesting an enhanced mitochondrial oxidative function after this time-based intervention.62 As mitochondrial oxidative capacity peaks at the end of the day,121 the synchronization between exercise timing and physiological rhythms of the circadian clock may explain these results. On the other hand, peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1α) may become an important target for time-of-day-based exercise.115, 123 PGC-1α is considered the main regulator of mitochondrial biogenesis and also modulates clock gene expression.124 Accordingly, the stimulation of PGC-1α via time-of-day-based exercise may help to improve mitochondrial fitness in metabolic syndrome.115 Furthermore, enhanced PGC-1α activation through the adenosine monophosphate-activated kinase (AMPK) signaling pathway, which presents a time-dependent exercise activation, could also contribute to the amelioration of metabolic diseases.115
6 EXERCISE TIMING AND CHRONOTYPE: POTENTIAL IMPLICATIONS IN METABOLIC HEALTH
Despite the fact that exercise timing may have a key role in metabolic health via the regulation of the circadian clock, it is important to take into account that inter-individual differences in the preferred circadian pattern of activity and sleep–wake cycles (i.e., different chronotypes) could imply distinct responses to morning/evening exercise. It is noteworthy that a growing body of evidence suggests that the evening chronotype (i.e., the preference for a later activity schedule and sleep/wake cycle) may be especially prone to the development of circadian misalignment and metabolic disease.125-128 Indeed, later chronotypes are associated with sedentary behavior129, 130; consequently, physical exercise could be particularly useful in subjects with an evening chronotype. In line with this, a randomized trial including 52 young sedentary adults with early/late chronotypes demonstrated that both morning and evening exercises induced phase advances in the internal circadian rhythm (calculated by differences in dim light melatonin onset) in subjects with a late chronotype, which may improve circadian misalignment in this population.131 In contrast, phase advances were detected in earlier chronotypes only after morning exercise.131
On the other hand, other authors have proposed that the performance of physical exercise in accordance with each individual's chronotype might entail important benefits. Accordingly, a recent crossover trial conducted in 30 patients with T2DM showed significant improvements in several glycemic parameters and lipid profile when the timing of physical exercise was synchronized with the chronotype.132 Of note, chronotype might also have an influence on appetite in response to acute bouts of exercise. Thus, Beaulieu et al. reported a higher suppression of appetite after moderate-intensity cycling in the morning in early chronotypes and after this intervention in the evening in late chronotypes.133
Nevertheless, it should be noted that most clinical studies assessing the effects of exercise timing on metabolic health did not evaluate the potential effects of chronotype among their participants. Therefore, this point might have an impact on the divergent results observed in some of these studies. Although further investigation is required in this field, chronotype-adjusted exercise might become an attractive approach in the management of metabolic diseases, as might also occur in other disciplines (e.g., nutritional interventions).134-136
7 CONCLUDING REMARKS
Physical activity/exercise is recognized as an effective therapy for the prevention and treatment of metabolic syndrome. As different exercise modalities, as well as distinct frequency, intensity, or duration of exercise, may lead to different metabolic effects, specific training programs should be prescribed. Likewise, exercise timing may play an important role in the management of different metabolic conditions, such as obesity, T2DM, dyslipidemia, or hypertension. In this regard, a considerable number of studies support that afternoon/evening exercise may be more effective than morning exercise to improve several metabolic outcomes, such as glycemic control, blood pressure, or lipid profile. Interestingly, some of these results might be explained by important differences in the activation of skeletal muscle and systemic metabolism, along with oscillations in the circadian clock genes induced by time-of-day-based exercise. On the other hand, several studies evaluating the effects of exercise timing on weight loss found that morning training could entail additional benefits compared with evening exercise, and these differences could be related to changes in energy balance and appetite regulation, among other factors, although mixed results have also been reported. However, despite these promising findings, it is important to take into account that we are still at the early stages of understanding the complex relationship between exercise timing and metabolic health, and large-scale, long-term randomized trials are needed to confirm the potential impact of chrono-exercise on metabolic diseases. Future perspectives in this area may include the combination of exercise and nutrient timing (e.g., manipulating feeding–fasting cycles, or the distribution of macronutrient consumption over the day, along with specific time-of-day-based exercise) to optimize the metabolic effects of these interventions. Indeed, recent research has shown that the combination of time-restricted eating and HIIT may have additive effects on glycemic control and fat mass reduction in women with overweight/obesity.137 Furthermore, inter-individual differences should be taken into consideration in future research, as they might result in different responses to exercise timing. Therefore, clinical variables such as sex, age, health status, training status, exercise type, or chronotype may have a role in the effects of timing of physical activity/exercise. Also, the potential differences between afternoon and evening exercises need to be evaluated in future clinical studies, because light/dark cycles in these periods may have an influence on metabolic health. Despite the fact that the mechanisms that might drive exercise timing-related metabolic effects are not fully understood, exercise training appears to modulate the circadian clock in a time-dependent manner, which may trigger different oscillations of systemic metabolites and energy homeostasis. Furthermore, physical exercise might induce direct changes in skeletal muscle/systemic metabolism, and mitochondrial biogenesis and/or function, depending upon the time of day when it is performed, with potential implications in metabolic health. Nevertheless, further investigation is needed to evaluate the coexistence of additional factors in this complex crosstalk, as well as the chronic effects of physical exercise on different signaling pathways, which may help to attain a better understanding of the impact of exercise timing on metabolic health, and the development of personalized exercise-based therapeutic strategies.
FUNDING INFORMATION
J.I.M.-M. was supported by a Río Hortega grant from Instituto de Salud Carlos III (ISCIII), Madrid, Spain (CM22/00217). J.B.-P. was supported by University of Málaga (Program D2) and by EQC2019-005901-P from Spanish Ministry of Science, Innovation and Universities. F.J.T. was supported by PI21/01667 from ISCIII. Further funding supporting this work are Principal Investigator grants to A.O.-G.: Miguel Servet Program (CP20/0060) and PI22/01813 from ISCIII, as well as ProyExcel_00962 from Consejería de Universidad, Investigación e Innovación, Junta de Andalucía. This work was also supported by Principal Investigator grants to M.M. from the Miguel Servet II Program (CPII22/00013) from ISCIII, Nicolás Monardes Program from Consejería de Salud de Andalucía, Spain (C10002-2022), UMA18-FEDERJA-285 co-funded by University of Málaga, Junta de Andalucía, and FEDER funds, CB06/03/0018 and PI-0297-2018 co-funded by FEDER funds and Consejería de Salud y Familias, Junta de Andalucía, Spain, and by PI19/00507 from ISCIII and co-funded by FEDER funds. Funding for open access charge: Universidad de Málaga/CBUA.” was incorrect as some missing information was needed. This should have read as follows: “J.I.M.-M. was supported by a Río Hortega grant from Instituto de Salud Carlos III (ISCIII), Madrid, Spain (CM22/00217) and co-funded by the European Union. J.B.-P. was supported by the University of Málaga (Program D2) and by EQC2019-005901-P from Spanish Ministry of Science, Innovation and Universities. F.J.T. was supported by PI21/01667 from ISCIII and co-funded by the European Union. Further funding supporting this work are Principal Investigator grants to A.O.-G.: Miguel Servet Program (CP20/0060) and PI22/01813 from ISCIII and co-funded by the European Union, as well as ProyExcel_00962 from Consejería de Universidad, Investigación e Innovación, Junta de Andalucía. This work was also supported by Principal Investigator grants to M.M. from the Miguel Servet II Program (CPII22/00013) from ISCIII and co-funded by the European Union, Nicolás Monardes Program from Consejería de Salud de Andalucía, Spain (C10002-2022), UMA18-FEDERJA-285 co-funded by University of Málaga, Junta de Andalucía, and FEDER funds, CB06/03/0018 and PI-0297-2018 co-funded by FEDER funds and Consejería de Salud y Familias, Junta de Andalucía, Spain, and by PI19/00507 from ISCIII and co-funded by the European Union. Funding for open access charge: Universidad de Málaga/CBUA. [Correction added on 2 July 2025, after first online publication: The Funding information section has been updated in this version.]
ACKNOWLEDGMENTS
The authors acknowledge Richard Carlsson for the English language editing of this article. Figures 1 and 2 were created with BioRender.com.
CONFLICT OF INTEREST STATEMENT
The authors have nothing to disclose.




