Low-carbohydrate ketogenic diets in body weight control: A recurrent plaguing issue of fad diets?
Summary
The most appropriate type of diets to maintain or lose body weight over the medium to long term has been a matter of controversy and debates for more than half a century. Both voluntarily and coercive food restriction, resulting in negative energy and macronutrient balance and hence weight loss, have not been designed to be maintained for the long term. By contrast, when a classical and traditional type of alimentation is consumed in ad lib conditions (e.g., the Mediterranean “diet”), it generally provides an appropriate nutritional density of essential macronutrients and micronutrients; it is hence appropriate for long-term use, and it provides several benefits for health if the compliance of the individuals is maintained over time. In this short review, we focus on four specific aspects: first, the need to agree on a clear definition of what is “low” versus “high” in terms of total carbohydrate intake and total fat intakes, both generally inversely related, in a representative individual with a certain lifestyle and a certain body morphology; second, the importance of discussing the duration over which it could be prescribed, that is, acute versus chronic conditions, focusing on the comparison between the fashion and often ephemeral low-carbohydrate diet (acute) with the well-recognized traditional Mediterranean type of alimentation (chronic), which includes lifestyle changes; third, the particular metabolic characteristics induced by the low-carbohydrate (high fat) diet, namely, the scramble up of ketone bodies production. The recent debate on ketogenic diets concern whether or not, in iso-energetic conditions, low-carbohydrate diets would significantly enhance energy expenditure. This is an issue that is more “academic” than practical, on the ground that the putative difference of 100–150 kcal/day or so (in the recent studies) is not negligible but within the inherent error of the methodology used to track total energy expenditure in free living conditions by the doubly labeled water technique. Fourth, the potential medical risks and shortcomings of ingesting (over the long term) low-carbohydrate ketogenic diets could exacerbate underlying renal dysfunction, consecutive to the joint combination of high-fat, high-protein diets, particularly in individuals with obesity. This particular diet promotes metabolic acidosis and renal hyperfiltration, which ultimately may contribute to a significant reduction in life expectancy in middle-age people.
1 INTRODUCTION
The issue of imposing an imbalance in macronutrient composition—low-carbohydrate (CHO) versus high-fat diets or vice versa—with varying levels of energy intake (i.e., the degree of energy deficit), together with good subjects adherence as a mean of obtaining the more efficient quantitative and qualitative weight loss, has plagued nutritionists for more than one century. What is the most optimal diet composition to maintain lean tissue and improve health during slimming? The answer is that we do not know. In this respect, the low-CHO diet, which historically was promoted by Dr Atkins in the United States half a century ago, is coming back on the quackery scene again. What are the potential shortcomings and reservations about this extreme diet, as compared with, for example, the virtues of the much acclaimed traditional Mediterranean diet?
2 DEFINITION: LOW-CHO (HIGH FAT) DIETS, WHAT IS MEANT BY “LOW”?
In our literature review of this topic, we came across the inherent difficulty of finding an appropriate definition of what is “low” and what is “high” when diets are confronted. Logically, a “low” fraction of one given macronutrient mathematically means a concomitant high fraction of another macronutrient (e.g., high fat or high protein, respectively) to make up to 100% (Figure 1). As a result, the denomination of a “low-CHO diet,” as encountered in almost all publications, lacks sufficient descriptive information. Therefore, we propose that the level of the accompanying macronutrient should be specified, for example, a “low-CHO/high-fat” diet or a “low-fat/high-CHO diet” or a “low-fat/moderate-CHO/high-protein diet.” The classification of “low” versus “high” could be different using this expression of data! There are two complementary issues that also need consideration:
- First, when the dietary CHO is judged to be “low,” is that the case when CHO is expressed in absolute energy value (kcal or kJ), in mass value (total g) or as a percentage of the total (metabolizable) energy intake? Because there is an inverse correlation between fat% and CHO% (Figure 1), the total of both, with protein% added, will be clamped at 100%. In contrast, in absolute value, the two substrates may be more independent from each other because the difference in energy density (4 vs. 9 kcal/g for CHO and fat, respectively) could be largely compensated by their difference in energy value. Take a simple example: if the absolute amount of CHO in a diet (expressed in g/day) is 2.2 higher than that of fat, they will both have the same energy contribution. Hence, if protein energy is assumed to represent 10% of the total calories, they will both share the remaining gap, that is, 45% energy each (iso-energetic).
- Second, the assumption is generally made that when feeding a low-CHO diet, its effect is totally attributed to the depleted macronutrient (CHO) and not to the alteration of the proportion of the remaining macronutrients (high fat and/or high protein). In fact, the global effect that is measured could partly stem from the low-CHO feeding per se and partly to the accompanying high-fat content or a high-protein level and their respective contributions. In terms of N (protein) balance (for high-protein diets) and fat balance (for high-fat diets), we definitely expect a different global effect with the same amount of CHO.
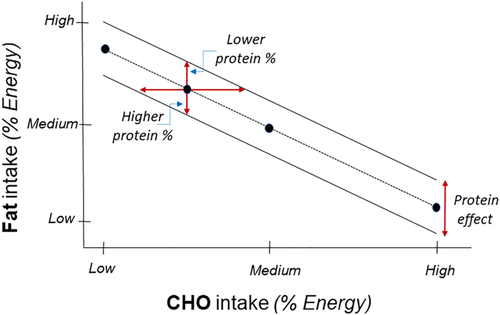
Furthermore, the magnitude of energy dislocation, that is, the gap between energy intake and energy requirement (based on total energy expenditure), dictates (over the medium term) the total weight loss and the total weight gain; the latter, for a given energy imbalance, depends less upon the macronutrient composition, assuming that the level of protein intake remains adequate. A fundamental concept is that the adequacy of protein intake to maintain N balance largely depends upon the concomitant energy level, particularly in the state negative energy balance, in which endogenous macronutrients are mobilized. As a result, the labeling of “low” or “high” CHO (respectively fat) diets, should not be based on their relative level of intake of both because these are negatively interrelated (Figure 1). It also depends upon the level of physiological/nutritional recommendations for each macronutrient. In other words, for the same relative level in the diet (say a proportion contributing to 45% energy for both), this would be considered as a “normal” value for CHO but a high value for fat.
The teleological important question that arises is why feeding humans with a marked departure (up or down) of the evidence-based nutritional recommendations, set out by multiple international institutions from the middle of the 20th century, could be favorable for health over the long term. In the last part of this paper, we attempt to demonstrate, at least in terms of longevity, that it is not the case. For example, a very low-CHO diet limited to say below 50 g/day (i.e., 5% to 10% of total energy intake) is about five times lower than the daily 250–350 g CHO per day allowances (50% to 55% energy) that are recommended by many international dietary recommendations for an average individual.
3 COMPARISON WITH OTHER DIETS
Today, we still tend to overlook if a low-CHO (high fat) diet does indeed provide substantial health benefits or not over the long term, in terms of maintenance (or improvement) of body functions. Yet, one can claim that certain specific populations in the world (Inuits in Arctic regions) are chronically eating low-CHO high-fat diets (containing virtually no vegetable and fruits) and having, as staple foods, fatty fishes rich in omega-3 fatty acids. The latter are known to protect against coronary heart disease. These populations seem to cope well with this high-fatty/protein diet almost devoid of CHO. Two hypotheses can be considered:
- These populations have been adapted (from several generations) to that particular high-fat/low-CHO diet during their life cycle, and
- A high prevalent genetic mutation (CPT1A, known as “Artic variant”) appears to circumvent the effect of high ketones production consecutive to consuming the very low-CHO diet.1
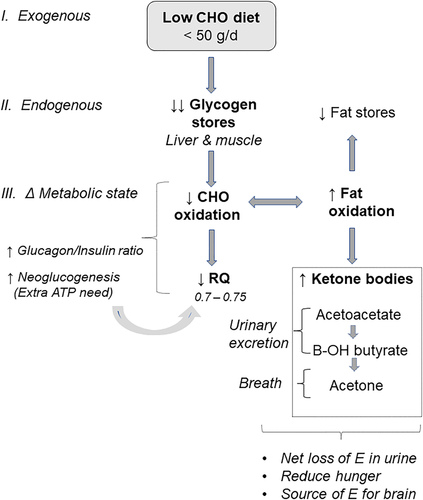
The low-CHO diets, by inducing a ketogenic state, can under noncoercive conditions lead to a decreased appetite,2 which can significantly contribute to acute weight loss. Furthermore, rapid regain in body weight occurs as the person switches back to a more “balanced” diet, although this is not specific to this type of diet. For a better understanding of the numerous changes incurred by low-CHO/high-fat ketogenic diets, it seems useful to present an overall comprehensive scheme (Figure 2) on its physiological and metabolic effects that, in hypocaloric conditions, lead to rapid weight loss during the first few weeks.
3.1 Mediterranean “diet” is not a diet
The comparison of the low-CHO/high-fat diets with the traditional Mediterranean diet is fragile because the latter it is not a “diet” stricto sensu destined for losing weight, at least over short term. The Mediterranean “diet” as it is called, is partly a way of life that goes much beyond the dietary factors and includes behavioral aspects such as enhanced physical activity, limited stress, and psycho-social factors (positiveness, joy of life, etc.). Furthermore, this way of eating has several advantages over a “diet”—including organoleptic aspects and the possibility to follow this mode of feeding virtually for life! To summarize the basic differences between the two types of alimentation, we have presented their comparison in tabular form (Table 1).
| Low-CHO diets | Mediterranean alimentation | |
|---|---|---|
| Data available | No long-term data | Long-term studies |
| Utilization for | Slimming | Preventive nutrition for limiting morbidity factors (CVD) |
| Degree of energy imbalance | Very restrictive (CHO) | Equilibrated eating pattern |
| Body weight loss | Quick initially | Slow, long term (overweight subjects) |
| Energy imbalance | Yes, energy restriction | No, ad libitum |
| Confounding factors | Type + composition | Not a fixed eating pattern of other macronutrients |
| Types of food eaten | Mostly commercial diets | Natural food products |
| Fat intake | Very high (saturated FA) | Moderate |
| CHO + sugar intake | Extremely low | Not restricted |
| Nutritional density | Low | High (grams of nutrients per 1,000 kcal) |
| Adherence/compliance | Rather good initially (ketogenic diet) | Not designed to be hypoenergetic |
| Medical evidence to resolve diseases | Epilepsy | Preventive approach (CVD) |
- Abbreviations: CHO, carbohydrates; CVD, cardiovascular diseases; FA, fatty acids.
4 METABOLIC CHARACTERISTICS: IS ENERGY EXPENDITURE “BOOSTED” BY LOW-CHO KETOGENIC DIET?
The origin of ketone bodies is the accelerated breakdown of adipocyte triglycerides releasing glycerol and fatty acids, the latter being preferential oxidized given its high flux and ultimately forming ketone bodies in the liver, that is, 3-hydroxybutyrate, acetoacetate, and acetone (Figure 2). The evidence that a very low-CHO (high fat) ketogenic diet has a statistically significant effect on energy expenditure remains questionable. Repeated controversy in the recent scientific literature3-6 can be attributed to several factors, as indicated below.
4.1 Methodological
The tendency is to neglect the real imprecision (and inaccuracy) of the methods used, in particular the doubly labeled water (DLW) technique. The DLW method measures VCO2 (and not VO2), which makes the error of calculating energy expenditure five times higher than with the measurement of VO2 due to the much larger range in energy equivalent of CO2 (kcal/L), as compared with O2, over the span of physiological respiration quotient (RQ). Furthermore, neglecting the effect of both low-CHO diet and negative energy imbalance did result in a substantial overestimation of energy expenditure in all conditions because of an inappropriate selection of an average un-tailored RQ.7 Finally, the low-CHO ketogenic state also biases downward the RQ consecutive to the enhanced net gluconeogenesis, which has a very low RQ not explained by an increase in fat oxidation.8
4.2 Design of the study
Nonrandomization of the groups studied3 and duration of the steady state under isocaloric conditions compared with nonketogenic high-CHO low/fat diets.
4.3 Statistical and data analysis
Outliers in DLW methods, frequently occurring in virtually all human DLW studies, could be included in the data analysis or could not.3, 4
4.4 Interpretation of results
Intra-individual variability has been neglected. Comparison of energy expenditure by one method (which has not the same duration of measurement) with the other method, namely, 7–10 days for DLW versus 3 days for respiratory chamber, yielding different conclusions.
4.5 Lack of convincing mechanisms involved
If energy expenditure was indeed significantly higher with the ketogenic diet, it is not known: (i) the extent to which the different components of total energy expenditure (resting and spontaneous physical activity) are altered, (ii) whether or not muscle net work efficiency remains unchanged and if dietary-induced thermogenesis is significantly affected, and (iii) what is the nature of the different organs and tissues involved in this adaptive metabolic process.
4.6 Limits of the carbohydrate-insulin model
The carbohydrate-insulin model of obesity, which often forms the basis for limiting dietary CHO and hence for promoting low-CHO diets,9 considers the insulin released by CHO-containing meals to exert only “anabolic” effects—by diverting fuel substrates to storage in adipose tissues thereby leading to a state of cellular starvation in metabolically active tissues that would trigger increased appetite and diminished thermogenesis. Yet, the postprandial insulin release after a CHO-containing meal can also be involved in “catabolism,” namely, through its central actions in meal-induced satiety10 and in sympathetically mediated thermogenesis that contributes to the thermic effect of the meal.11 Furthermore, insulin also interact synergistically with other “thermogenic” hormones (e.g., leptin, catecholamines, and thyroid hormones) in peripheral tissues to promote thermogenesis, in particular through a multitude of substrate cycles that include glycolytic substrate cycles,12 protein turnover,13 the triglyceride hydrolysis/re-esterification cycle,14 and lipid synthesis/oxidation cycle.15
4.7 Energy losses beyond energy expenditure
The residual energy contained in these three water-soluble ketone bodies constitutes an additional source of energy losses because the first two are either excreted in urine or eliminated in breath, considering that acetone is a volatile organic compound. These small energy losses have rarely been taken into account in the global energy balance in humans. However, in their study,6 Hall et al. have calculated that it accounted for less than 15 kcal/day (neglecting acetone). Yet in the original Atkins diet, the argument was given that ketone bodies excretion (between 4.7 kcal/g for acetone to 7.4 kcal/g for beta-hydroxybutyrate) was an elegant physiological way of getting rid of stored energy by transforming endogenous fatty acids into ketone bodies for subsequent excretion. Initially, it was estimated to be as much as seven to 10 times higher than the values derived in Hall et al.'s recent study,6 namely, 100 to 150 kcal/day.16
Taken together, we must admit that in energy balance studies, there are greater potential errors than 15 kcal/day considering that just the DLW method has an accuracy within 5% to 7% of total daily energy expenditure17 and a precision (within-subject variability) of about 8%–15% of total daily energy expenditure,18, 19 thus generating an inherent error much greater than the small amount of energy “exported” into ketone bodies in urine.
5 WHAT ARE THE LONG-TERM RISKS OF A LOW-CHO KETOGENIC DIET?
The increased popularity of a ketogenic diet due to its effectiveness in helping lose weight raises the following question: do the benefits of losing weight outweigh the diet's potential complications, particularly if one continues the diet over a period of years to maintain the weight loss? Numerous diet-related discomforts and complications in the first few days, weeks, or months of a ketogenic diet have been reported in the literature. These usually include relatively moderate complaints such as chronic fatigue, nausea, headaches, hair loss, reduced tolerance to alcohol, reduced physical performance, heart palpitations, leg cramps, dry mouth, bad taste, bad breath, gout, or constipation.20 Many of these complications can be directly related to ketosis and its associated metabolic acidosis, worsened by some degree of dehydration, lack of fiber intake, increased uric acid, or loss of minerals, particularly of magnesium. Patients with diabetes and/or hypertension also need to carefully monitor their blood sugar and blood pressure to adjust their medicine, as the diet tends to lower blood pressure and the need for insulin. Sometimes, more serious complications can arise with life-threatening metabolic acidosis,21, 22 particularly in a context of pregnancy or lactation,23, 24 as both pregnancy25 and lactation26 may promote metabolic acidosis.
However, if one aims to successfully maintain a weight loss over a longer period of time, the diet must be continued with great discipline to avoid regaining weight. In such a case, one should look at the long-term complications of a ketogenic diet. A recently published observational study that followed more than 15,000 American men and women over 25 years suggested that a low-CHO intake, as assessed by a dietary survey, may have an impact on longevity.27 The authors reported that the lower the CHO intake (as a percentage of total energy intake), the higher the overall mortality, leading to a 2.3-year reduction of projected mean survival age when CHO intake was between 30% and 40% of energy intake and to a 4-year reduction when CHO intake was less than 30% (Figure 3). Other studies analyzed comparatively by the same authors tend to corroborate their conclusions.27
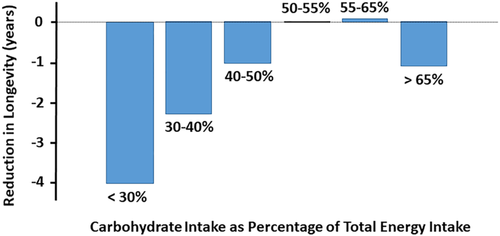
What could be the reasons for this reduction in projected longevity? Interestingly, the increase in overall mortality was mainly observed in individuals complementing the low-CHO intake with fats and proteins of animal origin, whereas individuals supplementing their diet with fats and proteins of plant origin seemed to be spared. In this context, several mechanisms can be proposed that need further studies for a better assessment of the risks.
First, chronic metabolic acidosis due to persistent ketosis and worsened by the higher acid load from products of animal origin28 has several consequences. It favors the progression of chronic kidney disease (CKD) in people with underlying renal dysfunction,29 whereas alkaline treatment attenuates the progression of CKD. As obesity per se is associated with an increased risk of renal dysfunction,30, 31 great care should be given to the monitoring of renal function. In addition, chronic acidosis leads to bone demineralization, particularly in subjects with underlying kidney diseases. Although bone mineral density has been shown to be preserved after 2 years on a low-CHO diet in otherwise healthy adult subjects with obesity,32 there are no carefully conducted studies over a longer period of time or in a context of reduced renal function. Renal dysfunction is a clear risk factor for bone demineralization, and additional metabolic acidosis may only worsen bone demineralization. Finally, chronic acidosis may promote muscle wasting, which in turn may lead to muscular inactivity and placidity, resulting in a sedentary lifestyle. This, associated with the chronic fatigue, may be counterproductive for the maintenance of weight loss.
Second, a low-CHO diet is compensated by an increase in fat intake and often also by an increase in protein intake. Both increases, particularly if they are of animal origin, are considered to be cardiovascular and renal risk factors. Although observational studies in low-CHO diets often show an improvement of the lipid profile,32 switching from a baseline diet to an isocaloric ketogenic diet is associated with increased inflammatory markers.33 Furthermore, the potential increase in protein intake puts an additional burden on the kidney by promoting glomerular hyperfiltration.34, 35 Indeed, glomerular filtration rate, either estimated from serum creatinine36 or measured directly by 24-h creatinine clearance,37 is increased during a low-CHO diet. This hyperfiltration, if maintained over many years, could lead in the long term to progressive renal dysfunction.34 In addition, animal products, which are rich in phospholipids and sulfur-containing amino acids, further worsen the acid load, whereas plant-derived products, rich in organic anions, tend to minimize the acid load.28
Finally, a low-CHO diet, due to the choice of the macronutrients, is often associated with a low fiber intake. This may not only cause constipation, a commonly described side-effect, but also promote diverticular disease of the colon and colon cancer and alter the microbiome. In addition, the diet lacks a well-balanced intake of fruits and vegetables, a source of antioxidants.
In summary, a low-CHO diet may reduce longevity through a constellation of risk factors. A key unfavorable element could be the presence of an underlying renal dysfunction, which could over years be worsened by the combination of high-fat, high-protein, and metabolic acidosis and lead to a vicious cycle. Subjects with renal dysfunction and/or propensity to metabolic acidosis should avoid a low-CHO diet and rather aim for healthier diets such as the Mediterranean diet described above.
6 CONCLUSION
The concept that “one given diet cannot reasonably fit all individuals” due to the huge interindividual variability of physiological and metabolic responses is particularly true for (very) low-CHO diets considering that both exogenous factors (degree of restriction/deficiency in CHO; types and nature of fat provided in surplus, quantity, and quality of associated protein; and duration of “dieting”) as well as the degree of endogenous adaptation to the extreme unbalanced diet (ketogenesis and acid–base imbalance) should be kept in mind before promoting this particular diet to untailored population. Depriving the body of the sole and unique substrate (glucose), which can be utilized by all the cells and tissues of the organism with a high net efficiency of energy utilization and the highest energy equivalent of oxygen (6% higher than fat), should make the investigators rethink about their judgment. The long-term problem of chronic feeding such a diet should be considered carefully.
CONFLICT OF INTEREST
None.




