Passive acoustic monitoring reveals feeding attempts at close range from soaking demersal longlines by two killer whale ecotypes
Abstract
Odontocetes depredating fish caught on longlines is a serious socio-economic and conservation issue. A good understanding of the underwater depredation behavior by odontocetes is therefore required. Historically, depredation on demersal longlines has always been assumed to occur during the hauling phase. In this study, we have focused on the depredation behavior of two ecotypes of killer whales, Orcinus orca, (Crozet and Type D) from demersal longlines around the Crozet Archipelago (Southern Indian Ocean) using passive acoustic monitoring. We assessed 74 hr of killer whale acoustic presence out of 1,233 hr of recordings. Data were obtained from 29 hydrophone deployments from five fishing vessels between February and March 2018. We monitored killer whale buzzing activity (i.e., echolocation signals) as a proxy for feeding attempts around soaking longlines. These recordings revealed that the two ecotypes were feeding at close range from soaking longlines, even when fishing vessels were not present. Our results suggest that both killer whale ecotypes are likely to depredate soaking longlines, which would imply an underestimation of their depredation rates. The implication of underestimating depredation rates is inaccurate accounting for fish mortality in fisheries' stock assessments.
1 INTRODUCTION
The intensification of fishing activity and resources depletion has led to an increase in competition between fishermen and marine predators worldwide over the last decades (Northridge, 1991; Northridge & Hofman, 1999; Read, 2008; Read et al., 2006). While competing with humans, some marine predators have started to feed directly from fishing gear. This behavior, known as depredation, leads to substantial impacts on fishing productivity, the depredating species, and fish stocks (Donoghue et al., 2002; Gilman et al., 2006; Read, 2008). Depredation behavior has been reported for most fisheries and involves a broad range of large marine vertebrates (Donoghue et al., 2002; Gilman et al., 2006, 2008; Hamer et al., 2012; Northridge & Hofman, 1999; Read, 2008; Werner et al., 2015). Most research efforts have focused on interactions between odontocetes and both pelagic and demersal longlines over the last 15 years (Donoghue et al., 2002; Gilman et al., 2006; Northridge, 1991; Northridge & Hofman, 1999; Read, 2008; Reeves et al., 2013; Werner et al., 2015). Longline fishing consists of snoods (thin cord) connecting unprotected hooks along a mainline. Longline gear increases fishermen's selectivity by catching targeted fish but makes the resource easily accessible for depredators (Gilman et al., 2006; Read, 2008). Longline fishing consists of three steps: (1) setting, when hooks are baited and longlines are deployed at sea, lasting generally from less than an hour to a few hours according to the length of the longline; (2) soaking, when the fish are caught as the longline are left at sea unattended, lasting from a few hours to a few days depending on the fishery; and (3) hauling, when longlines are retrieved by boats, and which, for a given longline, generally lasts longer than the setting phase. The nature of the depredation behavior on longlines is highly variable and depends on the odontocete species involved and the type of longlines used. Pelagic longlines deployed within the water column close to the surface are always easily accessible to odontocetes and depredation appears to occur throughout the whole fishing process (Dalla Rosa & Secchi, 2007; Forney et al., 2011; Gilman et al., 2006; Passadore et al., 2015; Rabearisoa et al., 2012; Read, 2008; Thode et al., 2016; Werner et al., 2015). On the other hand, demersal longlines, which are set on the seafloor, are thought to be mostly depredated during hauling (Gilman et al., 2006; Guinet et al., 2015; Hucke-Gaete et al., 2004; Mathias et al., 2012; Passadore et al., 2015; Read, 2008; Söffker et al., 2015; Thode et al., 2007; Tixier et al., 2015b; Werner et al., 2015) because air-breathing marine mammals are not all deep divers that can easily access benthic resources.
Because depredation on demersal longlines was traditionally assumed to occur during hauling, most studies relied on visually observed surface data of depredating animals around fishing vessels (Gasco et al., 2015; Hucke-Gaete et al., 2004; Janc et al., 2018; Kock et al., 2006; Purves et al., 2004; Roche et al., 2007; Söffker et al., 2015), as is commonly used to study pelagic longline depredation. Other studies count the number of partial fish on hooks where the missing parts would have been consumed by marine mammals (e.g., Rabearisoa et al., 2012, 2018; Passadore et al., 2015). Alternative types of data, such as underwater videos, accelerometers and/or acoustics have shown that odontocetes can depredate bait or remove the whole fish from the hook (Guinet et al., 2015; Mathias et al., 2009; Thode et al., 2014, 2015, 2016). As a result, it is impossible to use only visual surface observations or the number of damaged fish on hauled longlines as a proxy to estimate depredation rates on longlines in general (e.g., Rabearisoa et al., 2012, 2018; Passadore et al., 2015). Therefore, methods using indirect approaches have been implemented to assess the extent of depredation, especially for demersal longlines. A common method used to estimate depredation rates has been the comparison of catch per unit effort (CPUE) obtained between absence of interaction with odontocetes and presence of interaction during hauling at a fine spatial scale, e.g., 0.1° × 0.1° (Gasco et al., 2015; Hucke-Gaete et al., 2004; Purves et al., 2004; Roche et al., 2007).
These estimates are biased if depredation happens during soaking, but cetaceans are not observed depredating during hauling. Longlines are usually left to soak on the seafloor from a few hours to several days while the ship pursues other fishing activities elsewhere. In other words, even if odontocetes were present at the surface above a longline during soaking, surface observations would not be possible during this time, thus missing any interaction between odontocetes and the soaking longline. Consequently, nearly all observations have been carried out during hauling so the conclusion that demersal longline depredation occurs only at hauling might be overestimated.
A recent study revealed that an increase in soaking time was related to higher numbers of sperm whales (Physeter macrocephalus) at hauling, suggesting that individuals arrived around longlines during the soaking phase (Janc et al., 2018). This further supports that videos, passive acoustic monitoring, and bio-loggers with acceleration and pressure measurements are required to better understand the underwater dimension of depredation, notably during soaking. A single study using a bio-logging approach confirmed that sperm whales depredate on demersal soaking longlines, and that killer whales (Orcinus orca) are also likely to do so (Richard et al., 2020). This study was based on a limited data set and the absolute occurrence of this behavior could not be quantified. Overall, the recent findings by Janc et al. (2018) and Richard et al. (2020) strongly suggest that depredation rates are very likely to be underestimated, since they are evaluated without accounting for demersal interactions during soaking. Indeed, in the cases when whales are not present during the hauling phase, the line will be classified as “undepredated” and ultimately the fishing yield of that line will be compared to the yield to the depredated lines to estimate the amount of fish lost. Therefore, a better quantification of this deep-sea interaction is essential for accurate assessment of depredation rates, and therefore better tools for fisheries management and stock assessments.
In this study, performed in the Southern Indian Ocean in the Crozet Archipelago Exclusive Economic Zone (EEZ), we focused on killer whale acoustic behavior during soaking of demersal longlines. The demersal longline fishery we investigated targets the Patagonian toothfish (Dissostichus eleginoides), a highly valuable economic species (Collins et al., 2010; Grilly et al., 2015). Fishermen in the Crozet EEZ experience one of the highest depredation levels observed in the Patagonian toothfish fishery, with about 30% of the total annual catch lost to depredation (Gasco et al., 2015; Guinet et al., 2015; Janc et al., 2018; Roche et al., 2007; Tixier et al., 2010). Two odontocete species, killer whales and sperm whales, are involved in the depredation activity, making fishing around the Crozet islands very difficult (Richard et al., 2017). Killer whales are responsible for most of the depredation experienced by this fishery (Tixier, 2012; Tixier et al., 2010). Two ecotypes of killer whales, the Crozet Type and the Type D, interact with 40% of the longlines hauled in the Crozet EEZ (Tixier, 2012; Tixier et al., 2016, 2010). Although these two ecotypes have similar depredation behavior during hauling (Tixier et al., 2016), only the Crozet Type has been documented as potentially interacting with longlines during soaking (Richard et al., 2020), but this observation was based on a small bio-logging data set (two individuals with satellite relayed time depth recorders).
Understanding feeding activity near soaking longlines may bring new evidence to light of seafloor depredation behavior for the Crozet Type and the Type D killer whales. To do so, we used passive acoustic monitoring (PAM) with autonomous acoustic recorders deployed on longline gear to assess the temporal and the spatial behavior of the killer whales within acoustic detection range. Killer whales notably produce two main types of sounds associated with different behavioral contexts. Their communication signals, which are composed of pulsed-calls and whistles, can be either random or stereotyped (Ford, 1987; Zimmer, 2011). In PAM, these communication signals are relevant clues of social activities or group coordination. Additionally, killer whales (like all odontocetes and bats) produce echolocation signals, called clicks, as an active biosonar to estimate the direction of and distance to objects underwater (Au, 1993; Backus & Schevill, 1966; Griffin, 1958; Simmons et al., 1979; Zimmer, 2011). Odontocetes increase their echolocation click rates and frequencies to more highly resolve their target when approaching a prey, resulting in a “buzz” sound that is usually considered a reliable proxy of a feeding attempt (Fais et al., 2016; Goold & Jones, 1995; Holt et al., 2019; Johnson et al., 2004; L. A. Miller et al., 1995; P. J. O. Miller et al., 2004; Wisniewska et al., 2014). The objective of this study was therefore to monitor for buzzes as a proxy for feeding attempts (Barrett-Lennard et al., 1996; Holt et al., 2019, 2013; Simon et al., 2007) around soaking longlines, especially when the fishing vessel was not in the area to record concurrent visual observations. The motivation for this work was to better understand whether killer whales could be feeding close to soaking longlines, either on freely swimming prey nearby or on toothfish already caught on hooks.
2 MATERIAL AND METHODS
2.1 Data collection
Acoustic recordings were collected from five fishing vessels between February and March 2018 within the Crozet EEZ (46°25′S, 51°59′E; Figure 1). Each vessel used one SoundtrapST300 HF (Ocean Instruments, Auckland, New Zealand) autonomous hydrophone deployed on longline fishing gear (Figure 2). Each hydrophone was clamped on the downline (buoyline) at a depth of 100 m connecting the buoy to the ballast (Figure 2). Hydrophones were programmed to record continuously during an entire longline deployment (from a minimum of 6 hr up to a week) at a sampling rate of 96 kHz. This enabled 13 days of continuous recording until the data storage capacity of 128 GB was full. The SoundtrapST300 HF has a 16-bit resolution and a low self-noise level (~30 dB re 1 μPa.Hz−1). The broadband dynamic range is 90 dB. Clipping occurs at a received level (RL) of around 172 dB re 1 μPa2 with a high preamplification gain.
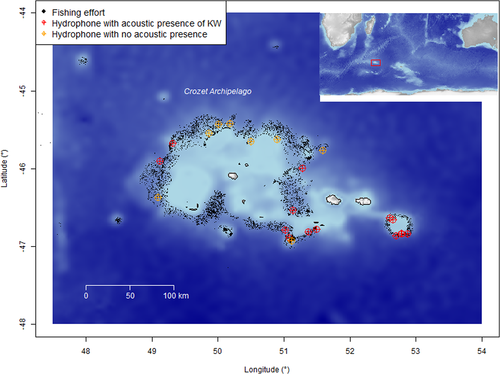
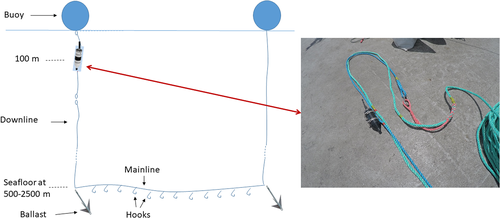
Deployments were performed during the commercial fishing operation, and thus adapted to the fishing routine. Longlines were set at night and primarily hauled during the day, since fishing regulations prohibit setting during daylight to avoid seabird bycatch (Weimerskirch et al., 2000). Longlines stay at sea on average between 1 and 3 days, with a mandatory minimum soaking duration of 6 hr but no maximum duration restriction. All the longline positions (latitude and longitude), seafloor depth at deployments (500–2,500 m in the Crozet EEZ), and the setting and hauling times were recorded. Fishing in waters shallower than 500 m is prohibited to avoid the capture of juvenile toothfish (Collins et al., 2010; Gasco, 2011). The length of the longlines usually varies between 2 and 17 km (Tixier et al., 2010). For each longline hauled, and only at this step of the fishing process, trained fishery observers collected data about interactions with killer whales, such as presence/absence and ecotypes (Tixier et al., 2016). An interaction was defined as when cetaceans were observed making repeated dives within an approximate 500 m range from the vessel hauling the longline (Roche et al., 2007; Tixier et al., 2010). Because longlines are primarily hauled during the day, most of the interactions could be assessed, but when observation was not possible (because of bad weather or poor light) fishery observers reported “unknown” interaction. For each visual observation of killer whales during hauling, ecotypes were recorded based upon phenotype differences. Fishery observers have been trained to distinguish the two killer whale ecotypes based upon photo-identification catalogs (Tixier et al., 2014a, b). There is a slight margin of identification error since Type D killer whales are smaller than the Crozet type and they have distinctive tiny eyepatches (Pitman et al., 2011; Tixier et al., 2016, 2014b). All these data were available through the Pecheker database (accessible from the Natural History Museum of Paris; Martin & Pruvost, 2007).
2.2 Acoustic data processing
No observation data of killer whales around soaking longlines is available, justifying acoustic recording during these phases. Hydrophones were deployed 29 times on longline sets and recorded a total of 1,233 hr, with a mean recording duration of 42.5 ± 24.0 hr (mean ± SD) per deployment. The presence of killer whale sounds was manually assessed by five trained annotators using spectrograms and aural verification through Audacity (1999–2020 Audacity Team). A killer whale detection was defined as when either calls or whistles (Filatova et al., 2012; Ford, 1989, 1991; Riesch et al., 2006; Thomsen et al., 2002) or echolocation signals (Barrett-Lennard et al., 1996; Holt et al., 2019, 2013; Simon et al., 2007) were visually detected on spectrograms.
Once killer whales were detected in the recordings, the sounds were manually labeled using a custom-written interface on MATLAB (R2019b; The MathWorks, Natick, MA). We classified sounds as either calls, whistles, click trains, or buzzes (Figure 3). Buzzes were differentiated from click trains when interclick intervals became shorter than 10 ms (Au, 1993; Goold & Jones, 1995; Griffin, 1958; Johnson et al., 2004; L. A. Miller et al., 1995). All these sounds were used to quantify the presence of killer whales around the hydrophone, but only buzzes were used as the acoustic clue to identify feeding attempts (Backus & Schevill, 1966; Griffin, 1958; P. J. O. Miller et al., 2004; Watwood et al., 2006; Zimmer, 2011).
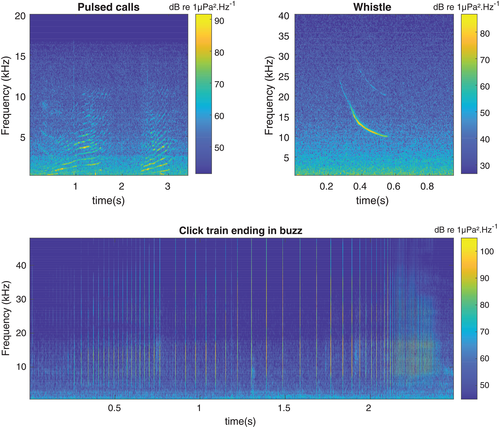
The same analysis was conducted with click trains (estimation of RC as the ratio between the number of minutes with click trains nC and the number of minutes with acoustic detections nA). The results for the click trains are not presented in this paper. However, they are discussed in the supplementary materials.
2.3 Interannotator reliability
Because several annotators classified killer whale sounds, the interannotator reliability was evaluated. To do so, we considered a subset of the acoustic data set with killer whale sounds (140 min, representing 3% of all recordings with killer whale sounds) that was analyzed by all the annotators, and we computed an intraclass correlation, hereafter referred to as ICC (Hallgren, 2012; Koo & Li, 2016; McGraw & Wong, 1996; Shrout & Fleiss, 1979). The ICC estimation consists of an analysis of variance (ANOVA), which assesses the deviation of the number of annotations made by an annotator within a 1 min time scale from the mean number of annotations made by all annotators in this minute. The result of the ANOVA provides the amount of the variance due to the similarity between the annotators and the amount due to the annotator's error. The ICC ranges from 0 (i.e., no annotations were similar between annotators) to 1 (i.e., all annotations were similar). For instance, a value of 0.75 would mean that 75% of killer whale sounds were annotated similarly between all annotators (Koo & Li, 2016; McGraw & Wong, 1996; Shrout & Fleiss, 1979). Koo and Li (2016) provide a scale for a qualitative interpretation of the ICC, with an interannotator reliability being poor for ICC values less than 0.50, fair for values between 0.50 and 0.75, good for values between 0.75 and 0.90, and excellent for values above 0.90.
The ICC was calculated with the icc function from the irr package (developed from McGraw & Wong, 1996; Shrout & Fleiss, 1979) through R software. We first ran the ICC for all killer whale sounds s combined to determine whether we could rely on each annotator's detection of killer whale sounds. We then estimated the ICC for just buzzes to assess how reliable annotators were in analyzing just buzzes.
2.4 Killer whale behavior assessment within detection range
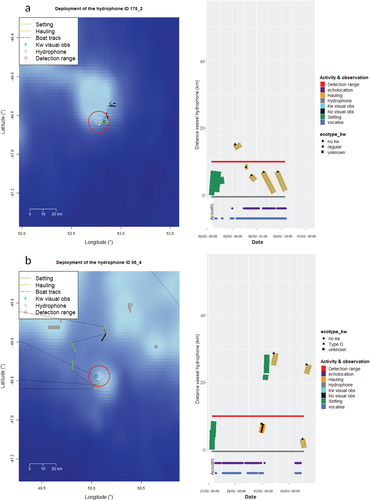
To associate a precise behavior with each detected buzz, our objective was first to determine whether the killer whales detected on the hydrophones were buzzing near the vessels hauling longlines or around the hydrophone set on the soaking longline (Figure 4). This was done by checking if any hauling activity occurred simultaneously with buzzes being detected on the hydrophone (Figure 4). When buzzes cooccurred with a hauling activity, we needed to estimate whether the killer whales could be interacting with the fishing line, as defined for visual observation (Roche et al., 2007; Tixier et al., 2010). We therefore tested two conditions: (1) whether killer whales had been visually observed interacting with a fishing line being hauled and (2) whether this fishing vessel was located at a distance from the hydrophone that was closer than an estimated detection range of killer whale sounds (described below). If both conditions were verified, we categorized the behavior of the killer whales acoustically detected as “interacting with the vessel hauling a longline.” In this case, killer whales were strongly assumed to be depredating on hauled longlines based upon visual observation from fishing vessels (Roche et al., 2007; Tixier et al., 2010). If the first condition was not met (i.e., no visual observation) this meant that the killer whales buzzing were not near the hauling vessel. Thus, individuals acoustically detected (i.e., buzzing) were categorized as “not interacting with a hauling vessel.” If the second condition was not met (i.e., detected killer whale sounds were located at a distance from the hydrophone that was farther than an estimated detection range) this meant that killer whales observed from the fishing vessels were too far from the hydrophone to be the same as the individuals buzzing. Thus, individuals acoustically detected (i.e., buzzing) were also categorized as “not interacting with a hauling vessel.” Finally, when no hauling occurred simultaneously with a buzz detection, we categorized killer whales as “not interacting with a hauling vessel.” In this case, we could not contextualize the buzzing killer whale behavior more precisely. Indeed, buzzes could be associated with social communication in some cases, but a high amount of buzzing activity is more likely to be a proxy of foraging behavior rather than socializing (Barrett-Lennard et al., 1996; Holt et al., 2019). Although we could not distinguish whether killer whales “not interacting with a hauling vessel” were either naturally foraging or depredating on soaking longlines, we counted the number of longlines soaking within the detection range of killer whale sounds to assess whether the latter hypothesis could be true.
To determine the acoustic detection range of killer whales' acoustic sounds and buzzes we assessed the probability of concurrent acoustic detection and visual observation from a fishing vessel at the scale of a hauling longline. Indeed, the acoustic detection range of killer whale sounds is the maximum distance at which killer whales can be detected acoustically on a hydrophone. The detection range of a sound mostly depends on its intensity when emitted, as well as on the environment in which it propagates. Through its propagation, the sound intensity attenuates because of the spreading of the sound wave-front and the absorption loss. Because the source level of killer whale sounds was not available, we used a comparison of concurrent acoustic/visual observations of killer whales to estimate a rough detection range. Therefore, for every acoustic detection, we checked if visual observations were reported from a fishing vessel during the hauling phase. When concurrent acoustic/visual observation occurred, we estimated the distance between the hydrophone and the fishing vessel. The distance between the hydrophone and the fishing vessel/interacting killer whales was estimated on a 1 min time scale (the same as for the acoustic processing), using the coordinates of the hydrophone and the longline being hauled, provided in the Pecheker data set. We then evaluated the probability of concurrent acoustic/visual observations as a function of range by computing a histogram with 2 km bins. A rough estimate of the detection range was then estimated as the distance after which the probability of concurrent acoustic/visual observation becomes negligible. This estimation was calculated for all killer whale sounds and for buzzes alone. In the supplementary materials (see Figure S1), we illustrate that buzzes likely propagate over the same distance as click trains.
Killer whales acoustically detected on the hydrophone could be different from individuals visually observed from hauling vessels. Thus, we also evaluated the probability of having visual observation (outside acoustic detection range) as a function of range by computing a histogram with 2 km bins, as a further verification. The probability of having visual observation alone should be higher than the probability of having concurrent acoustic/visual observations when the distance between the vessel and the hydrophone is larger than the detection range.
2.5 Ecotype association
We sought to monitor the acoustic activity of the two ecotypes. Therefore, we needed to associate acoustic detections to an ecotype. In general, ecotypes have specific acoustic repertoires, so acoustic identification is sometimes possible (Rice et al., 2017). Here, the acoustic repertoires of the considered ecotypes (Crozet Type and Type D) were unknown. We thus used for each detected sound the nearest visual identification around any hauled longline. However, visual observations only occurred during the hauling phase, not allowing for a precise association of every sound to an ecotype. Therefore, we associated acoustic clues to the ecotype observed at the scale of the deployment.
For each deployment, we first measured the time lag (hours) between every sound detected and visual observations from the fishing vessel. We kept the minimum time lag with a sound detection, and we then measured at which distance (kilometers) the visual observation (i.e., longline position) occurred from the hydrophone. We associated an ecotype with a deployment if either one of the following two conditions was met: (1) the nearest visual identification in time occurred within the detection range (estimated in this study) or (2) the nearest visual identification in time occurred outside the detection range but in a given time lag when killer whales could have covered the distance to enter the detection range. We used three different killer whale swimming speeds to estimate the time required to travel the distance between the hydrophone and the nearest visual observation from the fishing vessel (see Table S3): a mean travelling speed of between 1.6 and 1.8 m/s (R. Williams & Noren, 2009; R. Williams et al., 2002), a foraging speed of between 4 and 6 m/s (Ford et al., 2005; Guinet et al., 2007), or their sprinting speed of 12.5 m/s (T. M. Williams, 2009). If at least one of the estimated times was larger than the observed time lag between the acoustic detection and the visual observations, the visual observations were accepted as plausible identifications for the acoustic detections.
As a result, for a whole deployment with killer whale acoustic detection we considered either (1) no ecotype was present (i.e., no visual detection), (2) unidentified ecotype was present, (3) the Crozet Type was present alone, (4) the Type D was present alone, or (5) both ecotypes were present. One sighting at hauling was assumed sufficient to generalize ecotype presence for a whole deployment since recordings were quite brief, i.e., from 0.5 to 4.3 days (Table 1). Additionally, interactions with Crozet Type killer whales are much more common. Only 11% of killer whale depredation is associated with Type D only, and less than 1% of depredation events occurred with both ecotypes present simultaneously at hauling (Tixier et al., 2016).
| Deployment ID (Longline number_Vessel) | Number of hours of recordings (hr) | Cumulative time of acoustic detections (hr) | Proportion of acoustic presence in recordings (RP in %) | Proportion of buzzes within acoustic presence (RB in %) | Proportion of buzzes produced while not interacting with the vessel during hauling (RN in %) | Number of positive minutes with buzzes produced while not interacting with the vessel during hauling (nN in hr) | Ecotype associated | Minimum time lag (hr) between sound detections and visual observations from the surface | Distance (km) between the hydrophone and the surface observation of killer whales for the minimum time lag with a sound detection | Visual observation when hauling the hydrophone | Number of longlines soaking within a 10 km range from the hydrophone |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 268_2 | 11.7 | 0 | 0 | — | — | — | — | — | — | Absence | — |
| 277_2 | 23.7 | 0 | 0 | — | — | — | — | — | — | Absence | — |
| 244_2 | 38.8 | 0 | 0 | — | — | — | — | — | — | Absence | — |
| 232_2 | 41.1 | 0 | 0 | — | — | — | — | — | — | Absence | — |
| 143_1 | 43.2 | 0 | 0 | — | — | — | — | — | — | Absence | — |
| 39_4 | 61.7 | 0 | 0 | — | — | — | — | — | — | Absence | — |
| 249_2 | 103.2 | 0 | 0 | — | — | — | — | — | — | Absence | — |
| 113_3 | 25.8 | 0 | 0 | — | — | — | — | — | — | Absence | — |
| 154_1 | 36.8 | 0.1 | 0.2 | 0 | 0 | 0 | Crozet Type | 0.1 | 4.5 | Absence | 7 |
| 132_1 | 50.1 | 0.1 | 0.2 | 0 | 0 | 0 | No ecotype present | — | — | Absence | 1 |
| 82_4 | 17.2 | 0.2 | 1.4 | 78.6 | 100 | 0.2 | Unknown | 0 | 0 | Presence | 2 |
| 116_1 | 64.6 | 0.4 | 0.7 | 0 | 0 | 0 | Crozet Type | 2.2 | 30.4 | Presence | 1 |
| 143_5 | 24.3 | 0.5 | 2 | 65.5 | 73.7 | 0.2 | No ecotype present | — | — | Absence | 1 |
| 220_2 | 56.6 | 0.7 | 1.3 | 0 | 0 | 0 | Crozet Type | 0 | 4.4 | Absence | 4 |
| 209_1 | 66.5 | 0.9 | 1.4 | 60 | 100 | 0.6 | Unknown | 0 | 0 | Absence | 1 |
| 220_1 | 60.9 | 1.1 | 1.8 | 57.6 | 100 | 0.6 | Type D | 1 | 37 | Presence | 1 |
| 8_5 | 80.1 | 1.2 | 1.5 | 56.3 | 72.5 | 0.5 | Type D | 0.4 | 1 | Absence | 1 |
| 163_1 | 54.7 | 1.2 | 2.2 | 21.9 | 100 | 0.3 | Crozet Type | 0 | 10 | Presence | 2 |
| 161_2 | 19.1 | 1.4 | 7.2 | 35.4 | 0 | 0 | Crozet Type | 0 | 1.9 | Presence | 4 |
| 162_5 | 15.2 | 1.6 | 10.2 | 19.4 | 0 | 0 | Crozet Type | 0 | 0 | Presence | 1 |
| 158_5 | 37.3 | 2 | 5.4 | 0 | 0 | 0 | Crozet Type | 3 | 15 | Absence | 1 |
| 194_1 | 60.6 | 3 | 4.9 | 16.8 | 20 | 0.1 | Crozet Type | 0.2 | 2.4 | Presence | 3 |
| 184_1 | 35.7 | 3.4 | 9.6 | 25.2 | 100 | 0.9 | Crozet Type Type D |
1.0 1.7 |
8.2 8.4 |
Absence | 2 |
| 104_3 | 14.9 | 4.6 | 31 | 8.6 | 58.3 | 0.2 | Crozet Type | 0 | 0.8 | Presence | 6 |
| 190_2 | 12.5 | 5.4 | 42.7 | 15 | 85.4 | 0.7 | Crozet Type | 0 | 0.7 | Presence | 5 |
| 194_2 | 56.2 | 7.5 | 13.3 | 13.1 | 37.3 | 0.4 | Crozet Type | 0 | 0.4 | Presence | 5 |
| 168_2 | 20.5 | 9.4 | 45.8 | 18.7 | 7.6 | 0.1 | Crozet Type | 0 | 0.4 | Presence | 9 |
| 175_2 | 18 | 9.6 | 53.1 | 34 | 8.7 | 0.3 | Crozet Type | 0 | 0.6 | Presence | 7 |
| 55_4 | 82.4 | 19.4 | 23.5 | 59 | 99 | 11.3 | Type D | 1.5 | 0.5 | Presence | 2 |
| Summary of deployments with acoustic detection (mean ± SD) | 42.1 ± 22.9 | 3.5 ± 4.7 | 12.4 ± 16.7 | 27.9 ± 25.2 | 45.8 ± 44.2 | 0.8 ± 2.4 | All killer whales | ||||
| 35.9 ± 20.2 | 3.6 ± 3.4 | 16.7 ± 19.2 | 14.1 ± 12.2 | 24.4 ± 35.2 | 0.2 ± 0.2 | Crozet Type | |||||
| 74.5 ± 11.8 | 7.2 ± 10.5 | 8.9 ± 12.6 | 57.6 ± 1.4 | 90.5 ± 15.6 | 4.1 ± 6.2 | Type D |
3 RESULTS
3.1 Interannotator reliability
The comparison between annotators revealed an ICC of 0.86 for all killer whale sounds (all signals combined) labeled by all five annotators (see Table S1), and an ICC of 0.70 exclusively for buzzes. While the ICC was smaller for buzzes than for all sounds, it was good enough to ensure that annotations of buzzes were consistent between annotators.
3.2 Killer whale acoustic monitoring
Killer whales were acoustically detected for 21 of the 29-hydrophone deployments, and for a total of 74 hr of 1,233 hr of recordings (6% of the total recording time; Table 1). Focusing on the 21 recordings with killer whale acoustic detection, their mean presence duration was 3.5 ± 4.7 hr (mean ± SD) per deployment, corresponding to an average acoustic presence proportion RA = 12.4% ± 16.7% (see Table 1). However, considerable variability was observed between hydrophone deployments: the acoustic presence rate ranged from 0.2% to 53.2% of the recording durations (i.e., from 0.1 to 19.4 hr; see Table 1).
When killer whales were acoustically present, the buzzing proportion was RB = 27.9% ± 25.2% of their presence time (Table 1). Among the 21 deployments with acoustic presence of killer whales, no buzzes were detected on 5 of them, 10 deployments showed that buzzes were produced during less than half of the recordings (range: 8.6%–35.4%) and the last six deployments revealed that buzzes were produced during more than half of the recordings (range: 56.3%–78.6%; Table 1).
3.3 Killer whale behavior assessment within detection range
An acoustic detection range of 10 km was found, given the probability of concurrent acoustic/visual observation became negligible when the hauling vessel with interacting killer whales reached 10 km from a hydrophone (Figure 5). Indeed, we observed that above 10 km the probability of obtaining only visual detections was higher than the probability of concurrent acoustic/visual observation (Figure 5). This implies that the corresponding surface observations were located at a further distance from the hydrophone than the detection range of killer whale sounds could have been.
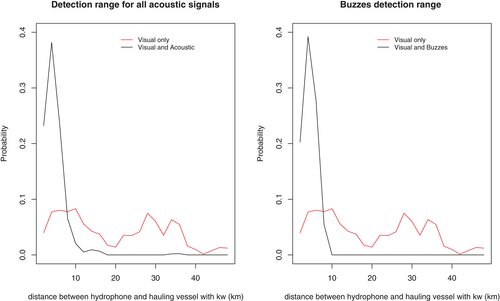
We calculated that RN = 45.8% ± 44.2% of buzzes were produced by killer whales while not interacting with a hauling vessel (and RN = 45.7% ± 44.0% of click trains; see Table S2). Among the 21 deployments with killer whale acoustic presence, we observed that for 33% of these deployments (n = 7), killer whales foraged exclusively during hauling (i.e., RN = 0%; see Table 1). For 38% of these deployments (n = 8), killer whales produced buzzes both while interacting with a hauling vessel and without interacting during hauling (7.6%–85.4% of their buzzes were produced in absence of interactions with a hauling vessel; see Table 1). Finally, 29% of these deployments (n = 6) showed killer whales were foraging almost exclusively in the absence of interactions with a hauling vessel (RN ≥ 99%; Table 1). Thus for 14 out of the 21 deployments, feeding attempts (i.e., buzzes) occurred while killer whales were not interacting with a hauling fishing vessel (either partially, 1 ≤ RN ≤ 99%, or exclusively, RN ≥ 99%). Among these deployments, we observed that 4 longline sets equipped with a hydrophone were hauled in absence of any killer whale visual observation (Table 1). Finally it was noted that among these 14 deployments, around 3.4 ± 2.6 longlines were soaking within a 10 km range from the hydrophones, ranging from 1 longline (the longline that was equipped with the hydrophone) up to 9 longlines (Table 1).
3.4 Killer whale ecotype comparison
Out of 21 deployments with killer whale acoustic detections, ecotype associations were possible on 17 occasions. Three deployments had detections of Type D alone, while 13 deployments had detections of the Crozet Type alone and one deployment was associated with both ecotypes (but the visual observations occurred during different hauled longlines and not at the same time; see Tables 1 and S3). For two deployments, ecotype identification information was missing and so associations were referenced as “unknown,” despite the fact that killer whales were noted as present by observers. Finally, on the last two deployments with killer whale sounds detected, no individual was observed during any of the longline hauling events in the vicinity of the hydrophone (Table 1).
The longest acoustic presence (19.4 hr) was associated with Type D compared to the mean duration of all of the other acoustic presence (3.5 ± 4.7 hr; Table 1 and see Figure 4.). Additionally, during this period, RN = 99% of their buzzes were produced while not interacting with a fishing vessel, which corresponded to 11.4 hr of foraging in the vicinity of the soaking longlines. During the two other deployments with Type D killer whales observed, 72.5% and 100% of buzzes were produced while not interacting with a hauling vessel. The longest acoustic presence associated with Crozet Type lasted 9.6 hr, during which, RN = 8.7% of their buzzes were produced in absence of an interaction with a hauling fishing vessel. When comparing the mean acoustic behavior between the two ecotypes, the Crozet Type killer whales spent significantly less time buzzing (14.1% ± 12.2%) than Type D (57.6% ± 1.4%, Table 1; t-test: t = −12.25, p < .001). Additionally, the proportion of buzzes produced by the Crozet Type while not interacting with a hauling vessel (24.4% ± 35.2%) was also significantly lower than for the Type D (90.5% ± 15.6% Table 1; t-test: t = −4.95, p < .001).
4 DISCUSSION
4.1 Feeding attempts of two killer whale ecotypes at close range from soaking longlines
Results show a high degree of buzzing activity near soaking longlines in the absence of concurrent hauling activity. Although buzzes could be part of killer whale pulsed calls (Ford 1989, 1991), similar results were obtained when considering only click trains (Tables 1 and S2) implying a high echolocation activity around soaking longlines. Such echolocation activity could also indicate navigation or socializing activities (Simon et al., 2007) but here the high buzzing activity is more likely to be associated with feeding activities (Au, 1993; Barrett-Lennard et al., 1996; Holt et al., 2013, 2019; Simon et al., 2007). As a result, killer whales in our study were very likely either naturally foraging on a resource found around longline sets and/or were depredating from the soaking longlines. Whether killer whales use buzzes while depredating remains unknown. However, similar studies of sperm whale interactions with sablefish fisheries in Alaska revealed that buzzes were a good indication of depredation behavior (Mathias et al., 2012; Thode et al., 2014, 2015), since the sperm whales were observed with underwater cameras to use buzzes in line depredation. It is thus likely that killer whales also produce buzzes if depredating from soaking longlines, especially at depths below 500 m where the absence of light prevents them from hunting by sight (Holt et al., 2019). Our results did not enable us to distinguish between these two activities, as well as whether natural foraging occurred on pelagic or on benthic prey. However, optimal foraging theory, hereafter OFT (Charnov, 1976; Charnov & Orians, 2006; Pyke, 1984), suggests that the animals are more likely to use the easiest resource. In our context, it means that killer whales are likely to depredate on soaking longlines, providing a better energetic balance (Faure et al., 2021; Tixier et al., 2015a), rather than hunting for free swimming toothfish, which are known to be active swimmers and thus able to make a fast escape from their predators (Brown et al., 2013; Collins et al., 2010). Such depredation behavior on an easy resource, fitting the OFT, has notably been observed for southern elephant seals (Mirounga leonina), since individuals have been caught on video while removing a toothfish from a hook (van den Hoff et al., 2017).
Crozet Type killer whales rely naturally on demersal toothfish, marine mammals, and penguins (Guinet, 1991a; Guinet et al., 2000, 2015; Tixier et al., 2019). In our recordings it is unlikely that Crozet Type killer whales were foraging on marine mammals or penguins since they are known to remain silent when hunting such prey (Barrett-Lennard et al., 1996; Deecke et al., 2005; Guinet, 1991b). Also, it is unlikely that Crozet Type killer whales were foraging within the water column near soaking longlines since no pelagic fish nor cephalopods have been previously described as part of their diet (Tixier et al., 2019). Additionally, a recent bio-logging study revealed that this ecotype performs foraging dives close to the seafloor (Richard et al., 2020). Diet and foraging strategies of Type D killer whales are still poorly understood. However, this ecotype has been described as potential deep divers, suggesting that this ecotype may also feed on benthic prey (Pitman et al., 2011; Tixier et al., 2016). Interactions described between Type D killer whales and toothfish longline fisheries during hauling strongly suggests that this ecotype also naturally feeds on toothfish (Tixier et al., 2016). Overall, the literature indicates that both ecotypes are more likely to forage on benthic toothfish than on pelagic prey.
A 10 km detection range of buzzes and killer whale sound was calculated, which matched the average propagation range of killer whale sounds estimated at around 11.0 ± 4.7 km by P. J. O. Miller (2006). The 10 km detection range allowed us to refine the spatial presence of killer whales near the hydrophones, especially around several soaking longlines (Table 1). The ecology of the two ecotypes described above paired with the OFT suggests that killer whales would be more likely to target toothfish already caught on these soaking longlines. A similar assumption has been made through the description of killer whales acoustically active around soaking demersal longlines of the blue-eye trevalla (Hyperoglyphe antarctica) fishery in Australia (Cieslak et al., 2021). However, the significantly higher buzz rate observed in Type D killer whales compared to Crozet Type killer whales suggests some differences in the foraging ecology of those two ecotypes. This suggests that Type D killer whales might be more specialized foragers by devoting more time foraging on fish and possibly squids as compared to the more generalist Crozet Type killer whale (Barrett-Lennard et al., 1996). This may be a by-product of Type D killer whales having greater ecophysiological abilities for repeated long deep dives compared to the Crozet Type killer whales.
Our study relies on a relatively limited data set that does not allow an accurate quantification of foraging activity around soaking longlines and seafloor depredation behavior. However, it clearly reveals that the Type D and Crozet Type commonly feed around soaking longlines. Within the assumptions made above, both ecotypes might depredate on soaking longlines more commonly than previously expected.
4.2 Implication for fish stock management
The passive acoustic monitoring of killer whales around longlines proves to be a complementary approach to visual observation from longlines to assess interaction behaviors. Indeed, our study shows a larger number of acoustic detections of the Crozet Type than of Type D, which is consistent with the 89% of killer whale encounters that are visually associated with the Crozet Type (Tixier et al., 2016). However, we also noticed that Type D killer whales may feed more than Crozet Type killer whales around soaking longlines. Although more data for Type D would be required to draw a robust conclusion, our results suggest that their interaction rate with longlines, previously described from surface observation (Tixier et al., 2016), may be underestimated. Indeed, a third of longlines set with a hydrophone were hauled in visual absence of killer whales even though acoustical activities were detected. Considering that feeding attempts could be depredation events, thus depredation rates (i.e., the difference of CPUEs in the absence and presence of odontocetes) would be underestimated. For instance, the depredation rate estimated at around 30% at Crozet (Gasco et al., 2015; Guinet et al., 2015; Janc et al., 2018; Roche et al., 2007; Tixier et al., 2010) implies that 1,570 tons of fish need to be caught in order to reach the 1,100 ton quota per year allocated to Crozet. These extra 470 tons of fish caught by fishers are taken into account in the fish stock management for quota allocation (Gasco et al., 2015; Roche et al., 2007). Considering potential depredation during the soaking phase, fishermen may actually need to catch more than the 470 extra tons to reach their quota. This missing part in the quota allocation is a serious bias that affects fisheries management. Moreover, seafloor depredation may increase predation pressure by killer whales on toothfish, even though the Patagonian toothfish is part of the natural diet of the Crozet Type killer whales in addition to marine mammals and penguins (Richard et al., 2020; Tixier et al., 2019). The question is thus whether the amount of fish depredated by killer whales on soaking longlines supplants the amount of fish that they would have naturally predated otherwise. Because a resource on a longline is easier to catch, killer whales might depredate more fish than they naturally predate (Faure et al., 2021). This could increase the proportion of toothfish in their overall diets and reduce the predation stress on penguins and marine mammals. However, as the Crozet type population is subject to an abnormally high mortality rate likely related to illegal and undeclared fishing vessels resulting in a net loss of individuals (Tixier et al., 2021) this is unlikely to be the case. This issue would require further investigation to improve stock assessment and to maintain a sustainable fishery.
Within a mitigation effort, this study strengthens the idea that fishing techniques should consider the development of protection systems of the longline/hooks during the soaking period. Conversely, previous efforts to minimize odontocete depredation on demersal longline fisheries have primarily relied on the assumption that fish were removed from hooks only during the hauling (Gilman et al., 2006; Werner et al., 2015). For instance, the “Cachalotera” is a floating net sleeve which slides down over individual caught fish when the longline is hauled to avoid sperm whale depredation (Moreno et al., 2008). Similarly, the “Sago” is a catching pod designed to descend along the longline to collect the fish during hauling (Arangio, 2012). Our results suggest that such an approach may not address the problem that caught fish are not protected during soaking with these devices. An alternative fishing system protecting the fish as soon as it is caught, such as fishing pots, might be favorable. Modification of fishing methods is nevertheless difficult to implement since they may result in a loss of efficiency. A preliminary trial performed as part of the ORCASAV program in 2010 around the Crozet Archipelago was not conclusive as to the economic sustainability of fishing pots (Bavouzet et al., 2011; Gasco, 2013).
Another option to reduce depredation is to understand how odontocetes locate fishing activities to reduce the probability of encounters. As odontocetes rely mostly on acoustic signals, the vessel acoustic signature is a good candidate to attract odontocetes. Decoy hauling acoustic signatures have been tested in Alaska to lure sperm whales away from the halibut fisheries, producing promising results until sperm whales figured the illusion (Thode et al., 2015, 2007; Wild et al., 2017). Besides, other preliminary results suggest that settings have specific acoustic signature which could attract attention of odontocetes (Richard et al., 2021). This insight would explain how the killer whales in our study were aware of the longline positions and could interact with them during the soaking phase.
4.3 Conclusion and perspectives
This study has provided additional insights into potential seafloor depredation on demersal longlines by two killer whale ecotypes. Depredation on soaking longlines may be more common than expected. However, a precise quantification remains difficult and additional PAM data are required. Indeed, this study mostly focused on the acoustic presence of killer whales around longlines based upon vocalization and echolocation signals (buzzes in this case). Using hydrophone arrays would enable a more accurate localization of where killer whale buzzes occurred over longlines (Gassmann et al., 2013; Mathias et al., 2013a, b; Roy et al., 2010) to more clearly associate buzzes with depredation events (Mathias et al., 2012; Thode et al., 2014, 2015).
Characterizing the acoustical difference between the two ecotypes would be useful to avoid relying on visual observations and propagation likelihoods. However, the acoustic repertoires of both killer whale ecotypes found within the Crozet EEZ have still not been described. Since matriarchal units for both ecotypes are relatively well known (Busson et al., 2019; Tixier et al., 2014a, b), an association between stereotyped calls and units present near the hydrophone (when photo-ID is possible like at hauling) should enable the determination of whether these populations possess different repertoires and present matriarchal unit acoustic signatures (Deecke et al., 1999, 2000; Filatova et al., 2012, 2016; Knight, 2014; P. J. O. Miller & Bain, 2000; Strager, 1995). The identification of individuals by acoustic signatures would be an interesting tool to determine whether the units acoustically detected are from the same ecotype as those seen by fishing vessels. This would improve the quantification of interaction rates, but further studies should also focus on methods to determine killer whale depredation behaviors from longlines.
For future work, a refined quantification of interaction rates is of great interest to keep this fishery sustainable. It would also be useful to assess the time it takes killer whales to arrive at soaking longlines. Such information could improve the understanding of the natural distribution of killer whales within the waters of Crozet. Knowing the areas favored by killer whales could help fishermen better target their fishing areas to avoid interaction.
ACKNOWLEDGMENTS
We warmly thank the captains, their crew and the fishery observers for their help in the data collection. We also thank the Natural History Museum (Musée National d'Histoire Naturelle) of Paris for providing access to the Pecheker Database. This study was partly funded by the ANR program OrcaDepred and by ENSTA Bretagne. We are also very grateful for the support of the Fondation d'Entreprise des Mers Australes, the Syndicat des Armements Réunionais des Palangriers Congélateurs, fishing companies, the Direction des Pêches Maritimes et de l'Aquaculture, Terres Australes et Antarctiques Françaises (Natural Reserve and Fishery units). We also thank Dr. Simon Northridge for providing very useful comments on the manuscript and Dr. Viviane David for reviewing the writing. We thank Andrea Northan for proofreading the English of the article. Finally, we thank the anonymous reviewers for their insightful comments to improve the manuscript.
The authors declare they have no conflict of interest.




