Glycopatterns of the foregut in the striped dolphin Stenella coeruleoalba, Meyen 1833 from the Mediterranean Sea
Received: 12 December 2020 | Accepted: 4 July 2021
Funding information: Universita degli Studi di Bari Aldo Moro
Abstract
The glycopatterns of the glycans secreted by the mucosa of stomach and duodenal ampulla of the striped dolphin, Stenella coeruleoalba were studied by histochemical (Periodic acid-Schiff, Alcian Blue pH 2.5, High Iron Diamine) and lectin-binding (SBA, DBA, PNA, WGA, MAA-II, SNA, ConA, UEA-I, AAA, LTA) techniques. The stomach can be divided into four compartments: main stomach, two connecting chambers and pylorus. The pylorus is followed by the duodenal ampulla. Mucins are secreted by surface cells and intramucosal glands specific for each compartment. In the main stomach glands, neck cells were weakly sulphated, with prevailing glycosaminylated, glycosylated/mannosylated, and fucosylated residuals. Parietal and chief cells in general were scarcely reactive. In the connecting chambers glands, there were high levels of sulphation, glycosaminylation, glycosylation/mannosylation, and fucosylation, the latter with more complex patterns than those observed in the main stomach glands. In the pyloric glands sulphated, glycosaminylated and fucosylated residuals decreased, whereas the opposite was observed for galactosyl/galactosaminylated residuals. Glycosylation patterns in the glands of the duodenal ampulla differed from those of the pyloric ones, with similar levels of sulphation, lower levels of galactosyl/galactosaminylation and glycosaminylation, and higher level of fucosylation. The results are compared with those available in literature.
1 INTRODUCTION
The stomach of Cetacea is plurilocular: it is subdivided into a series of compartments, the number of which varies among species and families (Cozzi et al., 2017; Harrison et al., 1970; Hosokawa & Kamiya, 1971; Langer, 1996; Mead, 2007; Slijper, 1962). Three main chambers are observed, e.g., the forestomach, the main stomach, and the pylorus. Each of these can be further subdivided and, in many species, there are some lesser chambers connecting the main stomach to the pylorus. In Odontoceti, the number of connecting chambers varies among species and up to 11 are observed in Ziphiidae (Mead, 2007). In addition, the duodenal ampulla can be present between the pylorus and the duodenum. In comparison to the anatomical studies, the histological analyses of the digestive tract, particularly the composition of the gastric secretions, are scarce (Cornaglia et al., 2000; Cozzi et al., 2017; Harrison et al., 1970; Hosokawa & Kamiya, 1971; Shimokawa et al., 2012; Smith, 1972; Tarpley et al., 1987; Yamasaki & Takahashi, 1971; Yamasaki et al., 1974). The mucosa of the forestomach is composed of a stratified squamous epithelium generally cornified and lacks glands. In the main stomach the mucosa is lined by a simple, cylindrical muciparous epithelium with gastric glands. These are of the tubular branched type and show three main cells involved in gastric juice production e.g., parietal, chief, and mucous neck cells. Sometimes, in the fore part of the main stomach a cardiac region with muciparous glands can be found. The parietal and the chief cells in the gastric glands secrete hydrochloric acid and pepsinogen, respectively, whereas neck cells are involved in the secretion of mucus, which forms a viscoelastic barrier lining the lumen of the stomach and has several functions, such as protection against physicochemical agents (including the gastric juice), lubrication to facilitate food passage, interactions with the microbiota (both symbiotes and pathogens), immunoregulation, coordination of cell proliferation, differentiation, and apoptosis (Bansil & Turner, 2018; Celli et al., 2009; Phillipson et al., 2008). The connecting chambers, the pylorus and the duodenal ampulla have a mucosa with a lining epithelium like that of the main stomach and muciparous glands.
One of the main components of mucus are mucins. These proteins are expressed by the Muc gene family and present several O-linked saccharidic chains whose patterns can change according to physiological and pathological conditions (Gomez-Santos et al., 2018; Wagner et al., 2018). Thus, knowledge about gastrointestinal mucin composition and variation is important in understanding physiological and pathological processes of the digestive tract. Lectin histochemistry is a useful tool in investigating mucin diversity in tissues (Brooks, 2017). In recent years, several studies appeared around gastric mucins characterization by lectin histochemistry in mammals (Fayed & Makita, 1997; Gomez-Santos et al., 2018; Lueth et al., 2005; Schumacher et al., 2004; Scillitani et al., 2007; Sommer et al., 2001). By contrast, only one paper deals with gastric glycoconjugates in a cetacean species, the Pacific white-sided dolphin, Lagenorhynchus obliquidens (Shimokawa, et al., 2012).
The cetacean stranding monitoring system set in the Northern Ionian Sea (Carlucci et al., 2020) and Eastern Aegean Sea (Milani et al., 2017) (Mediterranean Sea) has offered the opportunity to examin carcasses of striped dolphins, Stenella coeruleoalba Meyen, 1833 and to collect samples to analyze the glycopatterns of their gastric mucosa and duodenal ampulla. This study increases knowledge about glycan variation and composition, which is almost unknown in cetacean species, representing a scientific reference for further comparative, functional, and pathological studies about S. coeruleoalba, which is the most abundant cetacean species in the Mediterranean Sea, although recently ranked as Vulnerable by the IUCN Red List (Braulik, 2019).
2 MATERIAL AND METHODS
Five stranded females of S. coeruleoalba were collected on the coasts of the Gulf of Taranto (northern Ionian Sea) and Samos Island (eastern Aegean Sea) (Table 1). These areas host suitable habitats for the striped dolphin, which is widely distributed in offshore zones exploiting mainly benthopelagic prey (Carlucci et al., 2018a, b, 2021; Milani et al., 2018). The necropsy was carried out applying best practice on cetacean postmortem investigation and tissue sampling, collecting photo-video documentation and basic morphometric data (IJsseldijk et al., 2019). Stages of growth and physical maturity were estimated following Calzada & Aguilar (1996) and Calzada et al. (1997).
| Location | Sea | Date | GPS coordinates (decimal degrees) | Total length (cm) | Age class |
|---|---|---|---|---|---|
| Marina di Chiatona | NI | March 6, 2016 | 40.514489, 17.054043 | 150 | Subadult |
| San Pietro in Bevagna | NI | November 5, 2018 | 40.304853, 17.680967 | 180 | Adult |
| A. Konstantinos | EA | May 26, 2018 | 37.80621, 26.82541 | 200 | Adult |
| A. Konstantinos | EA | March 1, 2019 | 37.808099, 26.808970 | 196 | Adult |
| Karlovasi | EA | June 3, 2018 | 37.80262, 26.71240 | 201 | Adult |
Samples of main stomach, connecting chambers, pylorus, and duodenal ampulla were removed from each individual and washed in phosphate-buffered saline (PBS) pH 7.4, 0.1 M, fixed in 10% neutral buffered formalin, dehydrated in a graded series of ethanol and embedded in paraffin wax. Sections were serially cut at 5 μm and mounted on slides for processing with conventional and lectin histochemistry.
2.1 Conventional histochemistry
For conventional histochemistry, sections were rehydrated and stained with three methods: (1) Periodic-Acid Schiff (PAS) to demonstrate the presence of carbohydrates with 1,2 glycols; (2) Alcian Blue (AB) at pH 2.5, to demonstrate acidic glycans, both sulphated and sialylated; (3) high iron diamine (HID), to demonstrate acidic glycans with sulphated residuals. For each stain, counterstain with Mayer's hematoxylin (HE) was also performed. Protocol details have been reported previously (Petraccioli et al., 2013). All chemicals used in these procedures were from Sigma (St. Louis, MO). Photographs in bright light were taken by an Eclipse E600 photomicroscope equipped with a DMX1200 digital camera (Nikon Instruments SpA, Calenzano, Italy), keeping the same conditions for all the stains, tracts, and samples. Stain intensity was compared among tracts by computing optical density (OD) values from RGB photographs processed by the color deconvolution, a method that allows to separate color channels for the stain, the counterstain, and the background (Ruifrok & Johnston, 2001). For each stain and tract, three fields per individual were selected (size: 340 × 270 μm) and 10–20 secreting cells of each type having the best orientation were chosen for the analysis. Stain vectors for reference were created from single-stained slides as previously reported. Single cell ODs were computed from mean intensities of stain channels (Mastrodonato et al., 2017). Image analysis was performed with the ImageJ package (Rasband, 2016) implemented with the color deconvolution plugin (Landini et al., 2020).
OD values were analyzed by statistical methods to detect significant differences (Mastrodonato et al., 2017). Mean OD values from each staining and cell type were compared by one-way analysis of variance (ANOVA) followed by resampling through a 10,000-iteration bootstrap of the F-values. All tests for statistical significance were set at p < .01. Statistical computations were generated by the Real Statistics Resource Pack plugin for Excel (Release 4.3; Zaiontz, 2019).
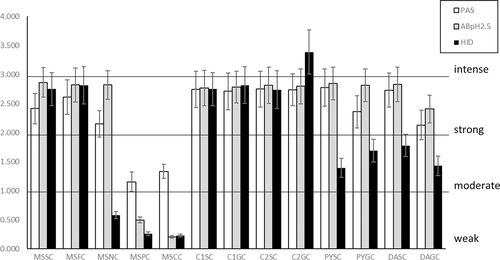
For a verbal, semiquantitative evaluation of the stain intensity in the histochemical analyses, the range of OD values was divided into four intervals, labeled as follows: “weak,” OD <1,000; “moderate,” 1,000 < OD < 2,000; “strong,” 2,000 < OD < 3,000; “intense,” OD > 3,000.
2.2 Lectin histochemistry
Binding experiments with 10 FITC-labelled lectins (SBA, DBA, PNA, WGA, MAA-II, SNA, ConA, UEA-I, AAA, LTA) from Vector Laboratories (Burlingame, CA) were performed to detect the main residuals in the oligosaccharidic chains. Details for the lectins employed, their concentrations, and sugar specificities are in Table 2. Lectin protocols followed manufacturer's instructions and were reported elsewhere (Scillitani et al., 2011). Sections were incubated in the dark for 1 hr at room temperature in the appropriate lectin solution in HEPES, then rinsed in the same buffer, and finally mounted in Fluoromount (Sigma-Aldrich) for observation. Controls for lectin labelling were performed as follows: (1) incubation with lectin-free HEPES; (2) incubation with lectin in presence of an inhibitory sugar (types and concentrations given in Table 2); (3) binding to secreting epithelia of the duodenum of the house mouse, Mus musculus, or Guinea-pig, Cavia porcellus, as well as to the egg extracellular matrix of the toad, Bufo bufo, the mucins of which have been previously demonstrated to be labeled by the tested lectins (Mentino et al., 2014; Scillitani & Mentino, 2015).
| Lectin | Source | Binding specificitiy | Lectin concentration (mg/ml) | Inhibitory sugar |
|---|---|---|---|---|
| SBA | Glycine max | GalNAc/Gal | 0.02 | 0.2 M GalNAc |
| DBA | Dolichos biflorus | α-GalNAc | 0.02 | 0.2 M GalNAc |
| PNA | Arachis hypogaea | Galβ1,3GalNAc | 0.06 | 0.2 M Gal |
| WGA | Triticum vulgaris | (GlcNAcβ1,4)*n | 0.02 | 0.01 M TACT |
| MAA-II | Maackia amurensis | Neu5Acα2,3Galβ1,3GalNAc | 0.02 | 0.02 M Neu5Ac |
| SNA | Sambucus nigra | Neu5Acα2,6Gal/GalNAc | 0.02 | 0.2 M Neu5Ac |
| Con A | Canavalia ensiformis | D-Man, D-Glc | 0.05 | 0.1 M MαM |
| UEA-I | Ulex europaeus | Fucα1,2 | 0.10 | 0.2 M L-Fuc |
| AAA | Aleuria aurantia | Fucα1,6GlcNAcβNAsn Fucα1,3, Fucα1,4 | 0.10 | 0.2 M L-Fuc |
| LTA | Tetragonolobus purpureus | L-Fucα1,6GlcNAc L-Fucα1,2Galβ1,4[L-Fuc1,3] GlcNAcβ1,6R |
0.10 | 0.2 M L-Fuc |
Except for lectins, all the chemicals used here were from Sigma-Aldrich (St. Louis, MO).
Images were captured under 495 nm fluorescence light using the same microscopic and photographic system used for conventional histochemistry, equipped with an epifluorescence device set.
For each binding experiment three fields per tract and specimen were considered and 10–20 cells for each type were selected for the analysis. For each cell, the corrected total cell fluorescence, CTFC (McCloy et al., 2014) was computed. Mean CTFC values were then processed for statistical comparisons among tracts and species by ANOVA with resampling, as previous, setting significant p-values at p < .01. Image analyses and statistical computations were conducted using the same software packages as above (Rasband, 2016; Zaiontz, 2019).
A verbal, semiquantitative evaluation of the lectin-binding intensity was obtained by dividing the range of CTFC values into four intervals, labeled as follows: “weak,” CTFC <40,000; “moderate,” 40,000 < CTFC <80,000; “strong,” 80,000 < CTFC <120,000; “intense,” CTFC >120,000.
3 RESULTS
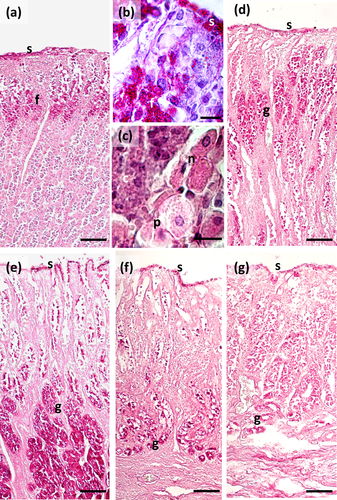
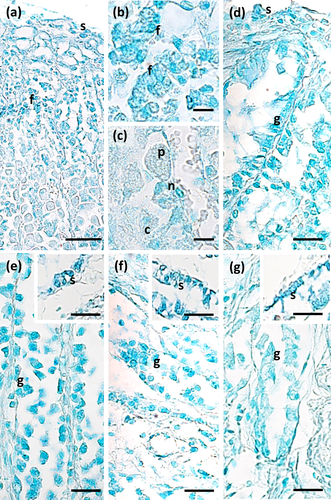
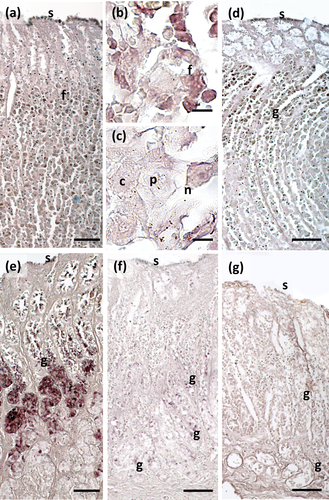
The main stomach of S. coeruleoalba presents a mucosa lined by surface cells of cubic or cylindrical shape (Figure 1a, b). Gastric glands open at the bottom of gastric pits, lined by foveolar cells similar to the surface cells. In the gastric gland three cell types are observed, i.e., chief, parietal, and neck (Figure 1c). Chief cells are cuboidal with cytoplasmic granules. Parietal cells are larger, ovoidal in shape and neck cells are small, polygonal and are scattered among the two previous described cell types. Surface, foveolar, and neck cells stain strongly with PAS (Figure 1a–c) and AB pH 2.5 (Figure 2a–c), whereas chief and parietal cells are weakly or not stained (Figures 1c and 2c). High Iron Diamine (HID) stain is strong in surface and foveolar cells and weak or absent in the neck, parietal, and chief cells. (Figure 3a–c). As far as lectin binding (Figures 4-8), surface, foveolar, and neck cells show similar binding patterns, with SBA (Figure 4a) and PNA (Figure 5a) reacting weakly or moderately, WGA (Figure 6a) and UEA-I (Figure 9a) reacting strongly or intensely, ConA (Figure 8a) moderately, and SNA (Figure 7a) and AAA (Figure 10a) weakly.
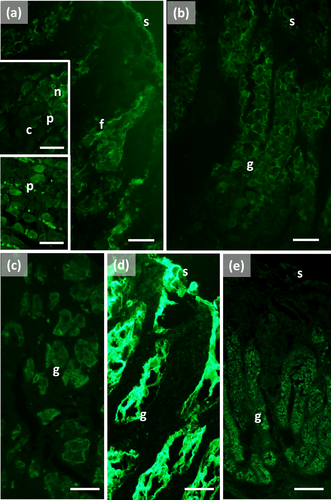
Chief and parietal cells in general stained weakly. DBA binds only to parietal cells (Figure 4a). MAA-II and LTA do not bind to any cell type (Figure 11).
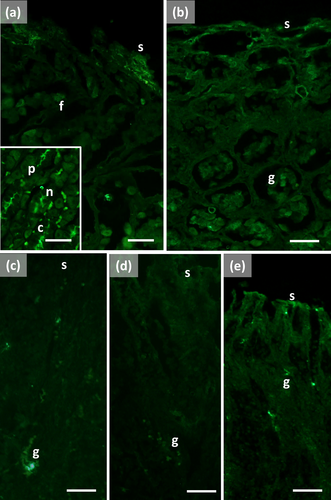
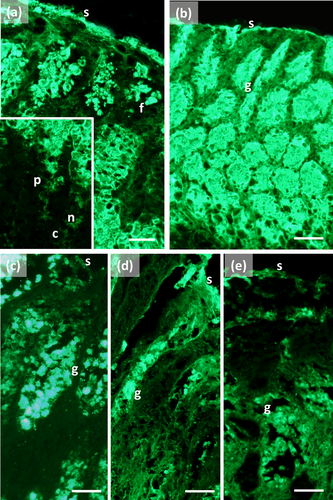
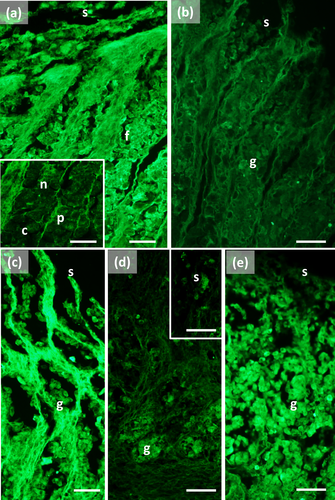
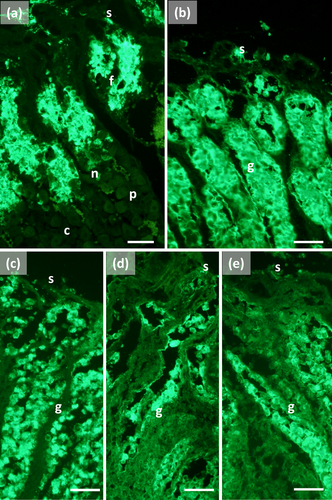
Two consecutive connective chambers, indicated as oral and aboral, respectively, are found between the main stomach and the pylorus. The mucosa of both is lined by surface cells and several glands are observed, made by one type of secreting cells. Histochemical stains are similar, with surface and gland cells of both chambers staining strongly with PAS (Figure 1d, e), AB pH 2.5 (Figure 2d, e), and HID (Figure 3d, e). Gland cells in the aboral connecting chamber are particularly rich in HID-positive residuals (Figure 3e). Surface and glandular cells in the oral chamber bind intensely to WGA (Figure 6b), UEA-I (Figure 9b), and AAA (Figure 10b) and weak to moderately to SBA, PNA, SNA, and ConA (Figures 4b, 5b, 7b, 8b). Similar patterns were observed in the cells of the aboral chambers for SBA, PNA, WGA and AAA (Figures 4c, 5c, 6c, and 10c); SNA and ConA link with higher intensity (Figures 7c and 8c) and UEA-I binds only moderately to the surface cells (Figure 9c). DBA, MAA-II and LTA do not bind to any cell type in both the connecting chambers.
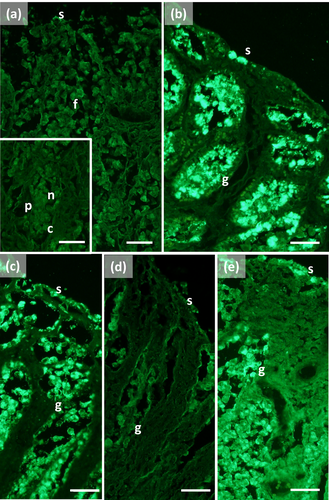
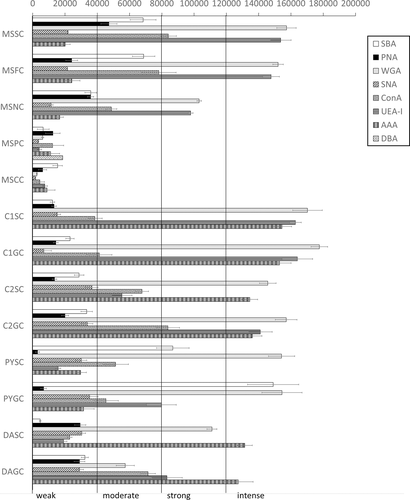
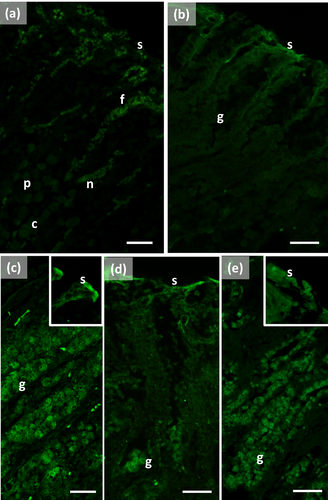
The pylorus has surface cells lining the lumen and mucous glands with a single type of secreting cells, like those of the connecting chambers (Figure 1f). Surface and gland cells are positive to PAS (Figure 1f) and AB pH 2.5 (Figure 2f), with the same intensity observed for mucous cells in the connecting chambers, whereas HID stain is moderate (Figure 3f). Positive cells are observed mainly in the base of the glands. Lectin-binding experiments show a strong to intense linking of SBA (Figure 4d) and WGA (Figure 6d) in both surface and glandular cells and of UEA-I in the glandular cells (Figure 9d). A weak binding is observed for PNA, SNA, and AAA (Figures 5d, 7d, 10d), whereas ConA stains moderately (Figure 8d). DBA, MAA-II, and LTA do not bind to any cell type.
The duodenal ampulla has surface and glandular cells like the previous tracts and villi are not observed. The histochemical staining patterns are similar to those of the pylorus (Figures 1g, 2g, and 3g). Lectin histochemical staining indicates strong to intense affinity of surface cells for WGA (Figure 6e) and AAA (Figure 10e), whereas glandular cells bind strongly or intensely to UEA-I (Figure 9e) and AAA (Figure 10e). SBA-linking is faintly visible in the surface cells and moderate in glandular cells (Figure 4e). PNA and SNA bind moderately to both surface and glandular cells (Figures 5e and 7e), whereas Con A binding is stronger in the glandular than in the surface cells (Figure 8e). WGA-linking is strong in the surface cells and moderate in the glandular ones (Figure 6e). DBA, MAA-II, and LTA do not bind to any cell type.
The mean values of OD computed for each cell type from histochemical stains are in Figure 12. PAS and AB pH 2.5 values are similar among the tracts, except for main stomach parietal and chief cells in which they are significantly lower, as confirmed by the ANOVA analysis (resulting in each test with p < .0001). HID values were also significantly lower in the pyloric and duodenal ampullar tracts in respect to the main stomach and connecting chambers.
The mean values of CTFC computed for each cell type from lectin-binding experiments are in Figure 11. In the chief and parietal cells of the main stomach low-intensity binding was generally observed. In surface, foveolar, and neck cells high intensities are observed for WGA and UEA-I. High values are also observed in surface and glandular cells of the subsequent tracts. AAA binding increases towards the connecting chambers, pylorus, and duodenal ampulla. High values of SBA characterize the pyloric cells. The low values in the chief and parietal cells and the higher values in the other glandular cells renders all the ANOVA test significant (resulting in each test with p < .0001).
All controls for lectin labeling were negative.
4 DISCUSSION
The gastric mucosa of S. coeruleoalba has several types of secreting cells with variable distribution and secretion among compartments. Strong PAS-positivity in most cell types indicates secretion of glycoconjugates, mostly acidic, as indicated by AB- and HID-stains, as well as by WGA- and SNA-binding. Differences in lectin-binding reveals that glycosylation patterns differ among cell types. Glycan profiling was not considered for the esophagus and forestomach mucosae because they are not secreting tracts (Harrison et al., 1970).
Galactosylated and galactosaminylated residuals, as detected by PNA- and SBA-binding, respectively, are weakly or moderately represented, except for the pyloric gland cells, strongly stained by SBA. DBA also binds to galactosaminylated residuals, but only the parietal cells in the main stomach are positive to the lectin. This confirms the general affinity of this lectin to the HCl-secreting cells (both parietal and oxyticopeptic) in vertebrates (Liquori et al., 2005), probably due to residuals bound to the β-subunit of the integral glycoprotein H+,K+-ATPase in the tubule-vesicular system (Mastrodonato et al., 2009).
Both glycosaminylated and sialylated residuals are detected by the WGA lectin. Strong or intense binding indicates that these residuals are abundant along the stomach compartments. The SNA lectin detects sialylated residuals only: its lower affinity in respect to WGA suggests that the positivity of the latter is partly due to galactosaminylated residuals.
Mannosylated/glycosylated residuals are detected by ConA and they are moderately present in all the compartments. Fucosylated residuals link UEA-I (1,2-Fuc) and AAA (1,3-1,4- and 1,6-Fuc) lectins. Residuals fucosylated in 1,2 prevail in the main stomach, whereas those bound by AAA increase towards the connecting chambers. Glycans in the pyloric mucosa present a general lower fucosylation, increasing in the duodenal ampulla due to a raise in AAA-positive residuals.
A comparison of these results with the few available references on Odontoceti confirms that the mucosae of the various gastric tracts secrete sialosulphomucins, as revealed by AB-positivity at both pH 2.5 and 1.0 (Harrison et al., 1970; Shimokawa et al., 2012). The only available study about glycan profiling of mucins deals with the gastric mucosa of the Pacific white-sided dolphin, Lagenorhynchus obliquidens (Shimokawa et al., 2012). Similar to S. coeruleoalba, the mucins secreted by both the main stomach and the pylorus have galactosyl/galactosaminylated, glycosyl/glycosaminylated, mannosylated and fucosylated residuals. As far as the parietal and the chief cells of the gastric glands in the main stomach, in L. obliquidens there are neutral carbohydrates and glycan profiling is similar to the mucous cells. Shimokawa et al. (2012) attribute the stain of the parietal cells to the oligosaccharidic chains of the glycoproteins bound to the membranes of the tubule-vesicular system and the positivity of the chief cells to the pepsinogen granules. In S. coeruleoalba both parietal and chief cells resulted AB- and HID-negative, confirming the presence of neutral glycans, but they generally bind weakly to the lectins. These differences could be explained in terms of differences in the techniques used for the lectin-binding (we used FITC-labeled lectins instead of biotinylated lectins like Shimokawa et al. 2012 did) or by interspecific differences between the two distantly related species (McGowen, 2011).
Dolphins swallow their prey whole and salivary glands are absent or reduced (Cozzi et al., 2017), with the main mechanical and chemical treatments of food occurring in the stomach. The forestomach is involved in grinding and squeezing prey and has no secreting activity (Cozzi et al., 2017; Smith, 1972); the gastric juice can reflux from the main stomach and thus begin the chemical treatment, that takes place mostly in the main stomach (Smith, 1972). This is confirmed by the functional interpretation of results from this study. In fact, mucins in the main stomach are involved in the protection of the mucosa from mechanical insults, pathogens, oxidating agents, and gastric juice (Bansil & Turner, 2018). Acidic residuals like the sialylated and sulphated ones modulate most of these functions by forming a polyanionic barrier that protects the underlying cell layer. Other saccharidic residuals in the mucins probably coadiuvate in the protecting functions: as an example, glucosamine is known to prevent peptic ulcers (Santhosh et al., 2007), while fucose affects mucus viscosity and reduces bacterial motility (Becker & Lowe, 2003).
Posterior to the main stomach there are two connecting chambers with pyloric-like glands. The presence of valves suggest that they regulate the flow of chyme from the main stomach to the pylorus (Smith, 1972). Mucus secreted by the connecting chambers is rich in sulphates, in particular the aboral portion, and fucose links in more complex patterns than those of the main stomach (indicated by the strong binding of both AAA and UEA-I). Since both sulphates and fucose are involved in the regulation of the interactions with the microbiota (Ouwerkerk et al., 2013), we hypothesize that the connecting chambers could be important in protecting the posterior tracts from ingested pathogens.
The secretion of the pyloric glands differs from those of the connecting chambers. In the pyloric glands a higher amount of galactosaminylated residuals is observed in respect to the connecting chambers, whereas a lower amount of sulphated and fucosylated residuals is found. According to Smith (1972) in the harbor porpoise, Phocoena phocoena, the pylorus has the main function to adjust the pH of the chyme and regulate its passage to the duodenum by the pyloric sphincter. It is not clear whether galactosaminylated residuals could be involved in these functions, but the low amount of fucose and sulphates suggest that the pyloric mucus has the main function to lubricate the luminal surface for chyme passage.
The duodenal ampulla is regarded as the first tract of the small intestine, and it appears similar to the pylorus with the same type of mucous glands and lack of villi, but this could be a postmortem artifact (Cozzi et al., 2017). The composition of mucins anyway differs from those of the pyloric glands, with lower levels of galactosamine and higher fucosylation, maybe related to absorption and forming a barrier against bacteria (Mastrodonato et al., 2013). It is worthwhile noting that the duodenum of Cetacea lacks true Brunner's glands (Langer, 2017). However, Zhou & Li (1981) speculated that the lower part of the glands in the duodenal ampulla of the baiji, Lipotes vexillifer, could represent rudimentary Brunner's glands. In general, pyloric and Brunner's glands show very similar glycopatterns (Schumacher et al., 2004), but in S. coeruleoalba the differences we observed between the two gland types do not support the identification of the duodenal ampullar glands with Brunner's ones.
In conclusion, the present study showed that the mucin composition in the stomach of S. coeruleoalba varies among cells and compartments and that this is probably related to the different functions of mucus in the gastric chambers. Many aspects of functionality need clarification, requiring further research on a wide array of species to better understand mucin diversity and function in odontocetes.
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Bari Aldo Moro within the CRUI-CARE Agreement.
AUTHOR CONTRIBUTIONS
Roberto Carlucci: Conceptualization; investigation; project administration; writing - original draft; writing-review & editing. Giulia Cipriano: Investigation. Carmelo Fanizza: Conceptualization; funding acquisition; investigation; resources. Tommaso Gerussi: Investigation. Rosalia Maglietta: Investigation. Antonio Petrella: Investigation. Guido Pietroluongo: Funding acquisition; resources. Pasquale Ricci: Investigation. Daniela Semeraro: Formal analysis; investigation. Marco Guglielmi: Formal analysis. Giovanni Scillitani: Conceptualization; formal analysis; investigation; project administration; resources; writing - original draft; writing-review & editing. Donatella Mentino: Formal analysis; investigation; writing - original draft; writing-review & editing.




