Age-related changes to the speckle patterns on wild Indo-Pacific bottlenose dolphins
Funding information: Japan Society for the Promotion of Science, Grant/Award Number: 20K12584; Kyoto University, Grant/Award Number: 2020-A-4
Abstract
Age estimating wild dolphins using noninvasive methods will improve our understanding of their ontogeny. Speckles appear on the ventral through to the lateral areas of Indo-Pacific bottlenose dolphins (Tursiops aduncus) as they age. Age-related changes in the speckle density and shape were examined using type classification. Speckles first appeared on the ventral area of the genital area at approximately 6.5 years. They increased in two specific directions, from the genital area to the jaw and from the ventral side to the lateral side. The speckle shape changed with aging from a dot-shape to oblong. Individuals aged over 35 years possessed more speckles than those aged 24–26 years. The oldest age category of dolphins in this study was over 35 years. The speckling area similarly increased with age for all individuals. Therefore, the results suggest that speckle condition can be used to estimate age. The relationship between speckling area and age showed no significant sexual dimorphism except near the genital slit area. There was no speckle area around the genital slit which differed in shape between the sexes. Therefore, speckle patch shape may be a useful sex confirmation tool.
1 INTRODUCTION
Understanding age structure of populations aids conservation and allows us to study the life history of these mammals. Teeth (Best, 1976), ear plugs (Purves, 1955), and eye lenses (Nishiwaki, 1950) are currently used to estimate the age of cetaceans. However, collecting the data requires using postmortem techniques, which are inadequate for species that have slow reproductive rates or small population sizes. Few cetacean species require noninvasive methods for age estimation. Longitudinal observation of identified individuals is the main method used to obtain individual ages and the age composition of these population. For example, Indo-Pacific bottlenose dolphin (Tursiops aduncus) surveys have been conducted for more than 26 years around Mikura Island, Japan (Kogi, 2013), and have continued for more than 35 years in Shark Bay, Australia (Connor et al., 2019). However, acquiring the age composition of a population is expensive and time consuming because cetaceans have a long lifespan. Therefore, a noninvasive, low-cost technique for age estimation needs to be developed. Krzyszczyk and Mann (2012) suggested that speckling patterns in Indo-Pacific bottlenose dolphins in Shark Bay could be used for estimating age. We focused on age-related changes in dolphin coloration to confirm whether speckling can be used as an indicator of age in Indo-Pacific bottlenose dolphins around Mikura Island.
Various cetacean species show growth-related changes in skin color patterns, and the color patterns of some cetacean species continuously change throughout their life span. The Indo-Pacific bottlenose dolphin has dark spots on the ventral side to the lateral area (Wang et al., 2000). Krzyszczyk and Mann (2012) described speckling on Tursiops sp. in Shark Bay, Australia. Their study showed that speckles increased in number and density throughout their life span, allowing speckling to be used to estimate dolphin ages in this population. The body coloration of narwhals (Monodon monoceros) decreases with age, allowing the proportion of the white area to be used for age estimation (Auger-Méthé et al., 2010; Hay & Mansfield, 1989). Pan-tropical spotted dolphins (Stenella attenuata) also change coloration as they age. Dark ventral spots and light dorsal spots continue to increase with maturity until both spots finally overlap (Kasuya et al., 1974; Perrin, 1970; Perrin et al., 1976). The same ontogeny is observed in the Atlantic spotted dolphin (S. frontalis; Herzing, 1997). Age groups can be estimated by observing speckles in S. attenuata (Kasuya et al., 1974; Perrin, 1970; Perrin et al., 1976) and S. frontalis (Herzing, 1997). Some cetacean species show variations in coloration among geographically and/or genetically distinct populations, such as the pantropical spotted dolphins (Stenella attenuata; Perrin 1975), killer whales (Orcinus orca; Evans et al., 1982), and Pacific white-sided dolphins (Lagenorhynchus obliquidens; Brownell, 1965; Hayano et al., 2004; Sekiguchi et al., 2014). Therefore, the speckling ontogeny of the Indo-Pacific bottlenose dolphin should be independently verified for each population. In addition, Krzyszczyk and Mann (2012) did not define the speckle density with descriptions and only provided a simple figure define different densities. Occasionally, ventral regions were not adequately observed in the study. They also did not describe speckle shapes. Due to the potential geographical differences in coloration among populations and some ambiguities in the previous study, additional research is required on the relationship between age and speckling in other populations of Indo-Pacific bottlenose dolphins.
We conducted underwater observations of the Indo-Pacific bottlenose dolphins around Mikura Island, Japan. This study aimed to investigate speckling in further detail and determine whether speckling patterns can be used for age estimation in this population. We also assessed whether age groups can be estimated by detailed speckle observations in this species, similar to previous studies on other species.
2 MATERIALS AND METHODS
2.1 Study area and species
We observed Indo-Pacific bottlenose dolphins around Mikura Island, Japan (33°90′N, 139°60′E). Mikura Island is a small (approximately 20.54 km2) volcanic island. An underwater video recording survey was conducted within 300 m of the coastline at water depths of 2–45 m. Mikura Island Tursiops was confirmed as an Indo-Pacific bottlenose dolphin (T. aduncus) using DNA analysis (Kakuda et al., 2002; Wang et al., 1999).
2.2 Data collection
Speckling data were collected using underwater video recording between 2003 and 2019. Since 1994, over 225 individuals have been identified, and the current population size is approximately 110–160 individuals (Kogi, 2013). Most individuals were sighted annually. The age of each dolphin was obtained from birth year records. If we could not observe the individual continuously (for example, a calf weaned with no natural marks), we did not include the individual in our analysis. Kogi et al. (2004) defined adults as having a large girth with spots on the ventral area. Adults are also darker in color and usually have many scars with swelling in the genital area. Females often have a calf with them. We confirmed the first calf for each female using past sighting records. We defined older adults (35+ years in 2019) as those identified as adults in 1994, this category contained 13 dolphins (Kogi et al., 2004). On average, female Mikura dolphins initially deliver their first calf at 10.3 years of age, with the range from 7–12 years (Kogi, 2013). Our identification of a mother–calf pair was based on the study by Kogi et al. (2004). The study defined an adult female as a mother with a particular calf in more than 50% of all sightings. The sex of each individual was determined by observing the genital and mammary slits (Smolker et al., 1992) and/or by observing an erection in males.
Images from video recordings of dolphins were only included in the analyses if the dolphins were within 3 m of the camera with an in-focus image and body condition could be determined. Additional video footage was obtained by a skin-diver using a digital camcorder (DCR-TRV7, HDR-CX430, or HDR-SR12; Sony, Japan & Hero 7 Black; Gopro, San Mateo, CA) in a waterproof housing (DIV, Japan; MPK-TRV7, Sony, Japan; or Recsea, Japan). Natural marks (e.g., fin notches and body scars) were used to identify individual dolphins.
2.3 Speckle classification
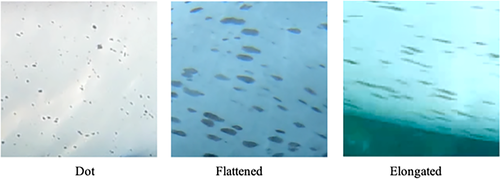
The speckles were classified into four density types, similar to those used by Krzyszczyk and Mann (2012) (Figure 1). Due to the limited detailed descriptions by Krzyszczyk and Mann (2012), we provide descriptions of these four categories as follows: None: There are no speckles. Few: There are some speckles on part of the skin surface, Moderate: speckles appear extensively on the skin surface, and Heavy: speckles appear extensively and coalesce on the skin surface. In this final group, the original skin coloration may be obscured by the speckles. The spot shapes were classified into the following three types (Figure 2): Dot: most spots in the area had only slight variations in their long and short diameters; Flattened: most spots in the area appeared to be between Dot and Elongated, and Elongated: most spots in the area had a long diameter that was over three times longer than the shortest diameter.
2.4 Body parts
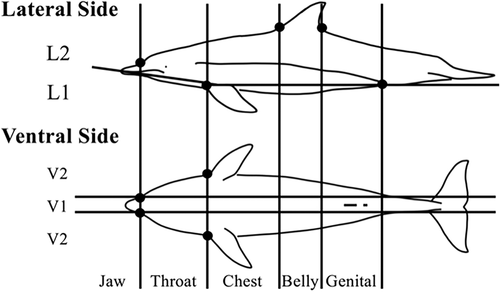
The body surface area was divided along two body axes: the cranial–caudal and dorsal–ventral (Figure 3). Body surface area was first classified using five body parts that formed part of the cranial–caudal axis and the border point between each area. The border point between each area was determined by the base of the rostrum, flipper, and dorsal fin. The body parts were defined as the jaw, throat, chest, belly, and genital. Additionally, four body parts on the dorsal–ventral axis were defined. First, the body part was divided into lateral and ventral views. In the ventral view, the area around the midline (V1) and both ends of the ventral view (V2) were defined. The lateral view was divided into two parts (L1 and L2) using the line from the anus to the base of the front edge of the flipper and the rostrum line. The upper limit of the L2 area was defined by the boundary contour between the darker gray dorsal surface and the light ventral surface. The jaw was divided into two parts, lateral and ventral. Eighteen body parts were identified in total. We ensured that the observable body part was as large as possible. V2 and L1 partially overlapped in several samples, but did not fully overlap.
2.5 Sample sizes
The analyses focused on 102 identified dolphins of known ages and 13 older adults. There were 52 female, 38 male, and 12 unknown sex neonates and juveniles between the ages of 0 and 25 years, and 13 older adult females. The sample sizes for each body part and speckle details are provided in Tables 1 and 2, respectively. A speckle rating of each body part was collected from an individual once a year and treated as independent data still-image data were used as much as possible to avoid misclassification of the speckle rating (Min: 1 video clip, Max: 10 video clips per individual) within the year. Any ambiguous data, such as obscure images of the speckles, were excluded from the study. Only one experimenter (the first author) analyzed the speckle data to exclude interobserver errors. Age at the first appearance of speckles was determined using annual continuous observation data of newborn individuals until the first speckle onset. A total of one male and four females were included in the age at the first appearance of speckles analysis.
| Body part | None | Few | Moderate | Heavy | |
|---|---|---|---|---|---|
| Jaw | V | 98 | 4 | 0 | 0 |
| L | 194 | 7 | 1 | 0 | |
| Throat | V1 | 74 | 15 | 8 | 0 |
| V2 | 86 | 15 | 9 | 0 | |
| L1 | 177 | 11 | 5 | 0 | |
| L2 | 193 | 6 | 3 | 0 | |
| Chest | V1 | 57 | 14 | 11 | 0 |
| V2 | 53 | 12 | 44 | 1 | |
| L1 | 125 | 26 | 51 | 2 | |
| L2 | 183 | 26 | 21 | 0 | |
| Belly | V1 | 52 | 7 | 21 | 0 |
| V2 | 44 | 20 | 54 | 2 | |
| L1 | 86 | 31 | 77 | 4 | |
| L2 | 132 | 37 | 44 | 0 | |
| Genital | V2 | 39 | 28 | 57 | 2 |
| L1 | 71 | 36 | 97 | 4 | |
| L2 | 98 | 20 | 78 | 5 | |
| Body part | None | Dot | Flattened | Elongated | |
|---|---|---|---|---|---|
| Jaw | V | 98 | 4 | 0 | 0 |
| L | 195 | 7 | 0 | 0 | |
| Throat | V1 | 74 | 12 | 9 | 2 |
| V2 | 86 | 13 | 11 | 0 | |
| L1 | 177 | 10 | 6 | 0 | |
| L2 | 193 | 7 | 2 | 0 | |
| Chest | V1 | 57 | 9 | 12 | 4 |
| V2 | 53 | 16 | 27 | 10 | |
| L1 | 126 | 39 | 28 | 11 | |
| L2 | 185 | 37 | 10 | 0 | |
| Belly | V1 | 51 | 5 | 13 | 8 |
| V2 | 42 | 24 | 33 | 18 | |
| L1 | 87 | 40 | 50 | 19 | |
| L2 | 134 | 35 | 40 | 6 | |
| Genital | V2 | 39 | 34 | 33 | 18 |
| L1 | 71 | 44 | 68 | 22 | |
| L2 | 96 | 22 | 60 | 19 | |
2.6 Descriptions of age-related changes
We analyzed age-related changes of increasing density and flattened speckles in individuals in the population. All individuals were grouped into three age groups (0–2, 3–5, and 6–8 years) to compensate for varying sample sizes. Each age group contained at least five individuals, with a maximum of 55 individuals. The analyses enabled us to clarify the area where speckles first appear, and the age at which speckles initially appear. We also examined the correlation between age at first parturition with the initial speckle appearance age using Spearman's rank sum correlation test. We then examined the direction of the speckle increase with the growth stage classification. The age of speckle onset in an area was determined when over 50% of the individuals in an age group had speckles on each body part. We clarified the order of speckle appearance in each area. We also examined individual variations in speckle expansion.
2.7 Speckle phase analyses
The analysis involved 201 samples. Our aim was to test whether age groups can be estimated from speckle appearance. We initially investigated the youngest age group in which over 50% of the individuals had speckles on each body part. Utilizing our background knowledge of the age groups, speckle phases were defined by the group of body parts where the spots appeared in individuals of a similar age. We numbered the phases from I to V. Throat, chest, and belly of V1 were not used in this analysis because of the small sample size.
2.8 Evaluating sex differences
We examined sex differences within each speckle phase and in genital patch shape. The genital patch is a speckle-free area around the genital slit. Genital patches were classified into two types. Oval patch was defined as a large round patch covering the slits, and line-like patch was defined as a patch covering a narrow area around the genital slit.
3 RESULTS
3.1 Description of age-related changes
All dolphins had no speckles in the year they were born (n = 14). Continuous observation data showed the speckles first appeared in the genital-V2 area at a mean age of 6.5 ± 0.53 years (females at 6.5 years, n = 8; males at 7 years, n = 1; range of 6–7 years). The noncontinuous observation data showed that the youngest speckled individuals were 6 years old (n = 4) in females and 7 years old (n = 1) in males. The data also showed that the oldest nonspeckled individuals were 12 years old in females (n = 1) and 13 years old in males (n = 1). The age of speckle onset did not significantly correlate with weaning age (Spearman's rank correlation analysis, r = 0.14, p = .83, n = 9) or with first parturition age (Spearman's rank correlation analysis, r = −0.11, p = .83, n = 8). Fourteen out of 15 females had confirmed speckling onset before their first parturition. Only one female did not have speckles before the first parturition.
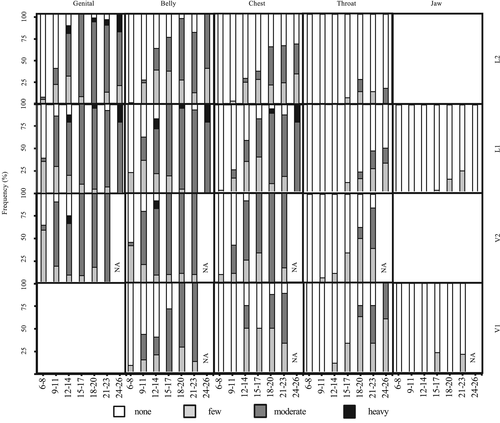
Figures 4 and 5 provide the proportion of individuals with each speckle density and shape as it changed with age. The younger age groups tended to possess a lower density (such as none and few) with smaller and more circular shapes (such as dots). In the older age groups, the speckles were larger and flatter, with flattened and elongated spots. There were differences in the speckle onset among the body parts (Figures 4 and 5). In all age groups, most dolphins were classified as having no speckles on jaw-V, jaw-L, and throat-L2. In all individuals from 0 to 26 years old, if the body parts possessed speckles, they were classified as either few or dot. However, several older adults (n = 6) had moderate or heavy density speckling and flattened or elongated speckles in the jaw-V and jaw-L parts. Furthermore, two older females had speckles on the dorsal side of their flippers.
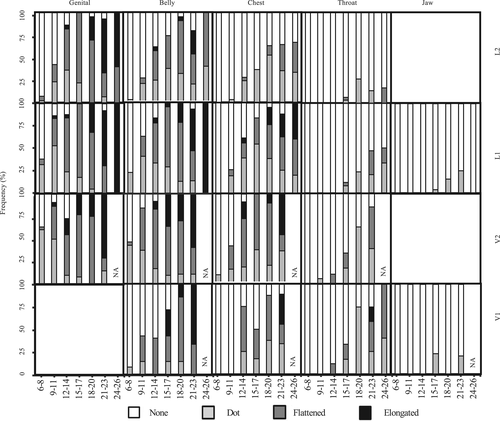
3.2 Direction of speckle increase and growth stage classification
3.2.1 Direction of speckle increase
The onset of speckling and the expansion directions were examined for each body part (Figures 4 and 5). Speckling first appeared in the genital-V2 area and finally appeared in the jaw-L and throat-L2 areas. In any age division, <50% of individuals showed speckles in the Jaw-L, V, and Throat-L1, L2. Speckles tended to increase from the genital to the throat area along the caudal-cranial axis. Conversely, along the dorsal-ventral axis, speckling onset in the ventral area was earlier than the lateral area (except in the V1 area). Speckles in the V1 area emerged later than in the V2 and belly-L1 areas.
3.2.2 Speckle phase analyses
Speckle phase classification was created to describe the development of the speckle pattern with the increasing number of speckles. The age distributions of both sexes in each phase were recorded (Figure 6) along with the interquartile age range in each speckle phase.
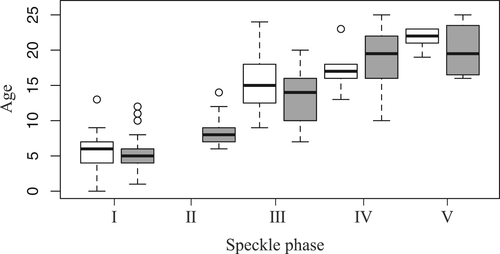
Phase I. Dolphins with no speckles, including calves, were classified as phase I. This phase mainly contained animals up to ~7 years old (range 0–13, mean 5.6, median 6.0, n = 21) for males and ~ 6 years old (range 1–12, mean 5.6, median 5.0, n = 34) for females.
Phase II. This phase was defined as speckles appearing on the genital-V2, L1, belly-V2, and belly-L1 areas. Ventral speckling usually started between 7 and 9 years of age (range 6–14 years, mean 5.6, median 8.0, n = 39) for females and increased throughout phase II. The limited sample size did not allow us to analyze males in Phase II.
Phase III. Dolphins were classified as Phase III when speckles appeared in the genital-L2, belly-L2, chest-V2, and L1 areas. The dolphins were usually classified in this phase when males were 12.8–17.5 years of age (range 9–24, mean 15.5, median 15.0, n = 16) and females were 10–16 years of age (range 7–20, mean 13.2, median 14.0, n = 41).
Phase IV. Dolphins were classified as Phase IV when they had speckles on the chest-L2 and throat-V2 areas. The dolphins were usually classified as being in this phase when males were 16–18 years old (range 13–23, mean 17.0, median 17.0, n = 10) and females were 16.3 years of age (range 10–25, mean 18.9, median 19.5, n = 22).
Phase V. Dolphins were classified as Phase V when speckles appeared on the throat-L1, L2, and jaw-L, V areas. The dolphins were usually classified in this phase when males were 21–23 years of age (range 19–23, mean 21.6, median 22.0, n = 6) and females were 16.8–23.2 years of age (range 16–25, mean 20.0, median 19.5, n = 12).
We found no statistically significant differences between the sexes in each phase (Figure 6, Wilcoxon rank sum test, p > .05). The speckling of each individual always increased and never decreased.
3.3 Evaluating sex differences
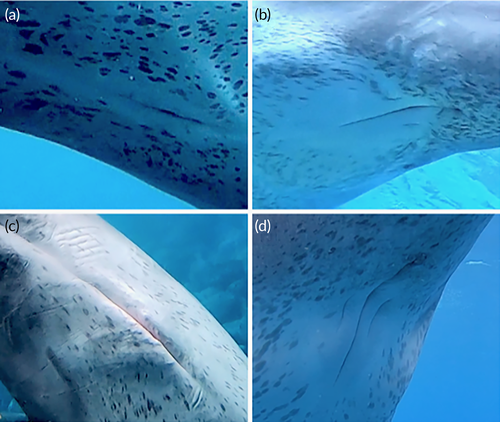
Genital areas of 10 adults and subadults were observed. All males had a line-like patch (n = 6), and all females had an oval patch (n = 4). There were statistically significant differences between the sexes in the shape of these patches (Fisher's exact test, p < .01; Figure 7).
4 DISCUSSION
4.1 Speckling as an indicator of age
Speckles first appeared at the mean age of 6.5 years around the genital area. The speckles increased in two directions. The directions were from the genital area to the jaw and the ventral to the lateral side. Speckles increased in density and flattened in shape after their initial onset in each area. Less than 50% of the relatively old individuals (21–23 and 24–26 age groups) had speckles on the ventral and lateral sides of the jaw and the lateral side of the throat area. Six of the 13 older adults (estimated age > 35 years) had moderate or heavy speckle densities in these areas. In addition, one of the older adults showed anomalous fused speckling, similar to a travertine pattern in the lateral chest area. Older adults were estimated to be over 35 years of age. The maximum life span of the Indo-Pacific bottlenose dolphin is 40–50 years (Connor et al., 2019; Wang, 2018). Accordingly, the speckles continued to increase with age after 26 years and may increase throughout their life span. On the other hand, the speckle phase analysis showed that speckles over several body parts tended to merge within the same age division in all five phases. The age range of each phase was 2–7 years. Most individuals showed the same speckle-merging tendencies. Previous studies have described the relationship between speckle phase and age groups (e.g., Atlantic spotted dolphin: Herzing, 1997; Pantropical spotted dolphin: Kasuya et al., 1974; Perrin et al., 1976). We described the relationships in Indo-Pacific bottlenose dolphins using similar methodology to previous studies. The results suggest that the dolphin speckles can be used for age estimation because the number of speckles continues to increase with age, and the changes in the speckling area with age are generally consistent amongst individuals.
4.2 Speckles as an indicator of weaning and sexual maturity
In the Atlantic spotted dolphin, speckles begin appearing around weaning (Herzing, 1997). However, speckle onset did not significantly correlate with weaning age in this study or in a previous study by Krzyszczyk and Mann (2012). Speckling may not be related to weaning in the Indo-Pacific bottlenose dolphin.
The age of speckle onset correlated with the age of sexual maturity in a study on bottlenose dolphins (Tursiops aduncus) using specimens off the coast of Australia (Ross & Cockroft, 1990). Krzyszczyk and Mann (2012) reported that speckle onset in the genital area correlated with age at first parturition. In this study, 13 out of 14 females had speckles before the first parturition year. Therefore, no speckles might be an indicator of female immaturity in the population we are investigating.
Females initially give birth at 10.3 years of age in the Mikura population (Kogi, 2013). Most females classified as phase III were over 10 years of age. Therefore, females classified as phase III can be considered mature individuals. Funasaka et al. (2013) reported that a 14-year-old male and a 10-year-old male of the Mikura population had reached sexual maturity by texture observation of testis. At other study sites, the timing of sexual maturity for male Indo-Pacific bottlenose dolphins was described at 10–15 years of age (Cockcroft & Ross, 1990; Kemper et al., 2014). In this study, most males classified into Phase III were 12.8–17.5 years of age. Therefore, male dolphins with phase III speckles can be considered sexually mature. All individuals above Phase III can also be considered mature dolphins.
4.3 Sexual dimorphism
This study is the first to report that there was a patch-like, nonspeckling area around the genital slit, and the shapes of the patches differed with sex. We did not confirm this patch when a dolphin had no speckles in the Genital-V2 area. We observed this patch more easily as this area became more speckled (moderate or heavy speckling). Sexually dimorphic coloration of the genital region has been reported in several delphinid species, such as Cephalorhynchus commersonii (Goodall et al., 1988), C. hectori (Slooten & Dawson, 1988), Phocoenoides dalli (Morejohn et al., 1973), and Lissodelphis borealis (Leatherwood & Walker, 1979). The results of the current study demonstrates that the size of the nonspeckled area was larger in female Indo-Pacific bottlenose dolphins than in males. Therefore, speckles may be used to distinguish between the sexes or to emphasize the genital area, and this could be used to confirm the sex of wild individuals. However, we could not find sexual dimorphism in the speckle patterns on other body parts.
4.4 Geographical variation
The speckles on the bottlenose dolphins in Shark Bay began appearing in the genital area and expanded to the throat area with age (Krzyszczyk & Mann, 2012). Furthermore, speckles appeared on the ventral side earlier than on the lateral side in the genital and belly areas (Krzyszczyk & Mann, 2012). In the current study, speckles first appeared in the genital region and expanded to the throat. Mikura dolphins also showed ventral speckle onset earlier than laterally. The same pattern as the Shark Bay bottlenose dolphins was evident. Krzyszczyk and Mann (2012) reported that the axillae displayed speckles faster than the chest area. In this study, the chest V2 and L1 areas corresponded to the axillary area in the previous study by Krzyszczyk and Mann (2012). In this region, Mikura dolphins showed a similar speckling expansion to the bottlenose dolphins in Shark Bay (Figure 4). The speckles appeared later in the V1 area of the ventral view in this study. This result may not suggest geographical variation because the observation area of this study was more intensive than the previous study.
The speckles on the Shark Bay bottlenose dolphins first appeared at a mean age of 10.2 ± 0.35 years (Krzyszczyk & Mann, 2012). However, speckles first appeared much earlier (at 6.5 years) in this study. Speckles in the genital, belly, and chest areas appeared earlier in the Mikura population than the Shark Bay population. However, speckles appeared earlier in the throat area of the Shark Bay population (Krzyszczyk & Mann, 2012) than the Mikura population (Table 3).
| Shark Baya | Mikura Island | |||
|---|---|---|---|---|
| Body part | Body view | Mean ± SE (year) | Body part | Over 50% speckled (age division) |
| Genital | Ventral | 10.2 ± 0.35 | Genital V2 | 6–8 |
| Belly | Ventral | 11.2 ± 0.52 | Belly V2 | 9–11 |
| Genital | Lateral | 11.5 ± 0.57 | Genital L1 | 9–11 |
| Belly | Lateral | 13.4 ± 0.46 | Belly L1 | 9–11 |
| Axillae | ? | 12.3 ± 0.79 | Chest V2 | 12–14 |
| Chest | ? | 14.9 ± 0.75 | Chest V1 | 12–14 |
| Throat | ? | 15.7 ± 1.21 | Throat V2 | 18–20 |
- a Shark Bay data cited from Krzyszczyk and Mann (2012). They did not distinguish between the jaw and throat areas.
Females initially gave birth at 12–15 years old, and calves were typically weaned at 2.7–8.0 years old in Shark Bay (Mann et al., 2000). At Mikura, the mean age of the initial birth by a female was 10.3 years (range = 7–13 years) and the dolphins were weaned at 3.5 years (range = 3–6 years) (Connor et al., 2019; Kogi, 2013). Indo-Pacific bottlenose dolphins in South Australia reached physical maturity when males were 208–238 cm long and females were 195–233 cm (Kemper et al., 2014, 2019). Morisaka et al. (2016) examined 13 mature dolphins of both sexes using a 3D camera and estimated a general length of 249.6 ± 10.7 cm. The dolphins around Mikura Island tended to mature relatively earlier at a longer body length than the Shark Bay bottlenose dolphins. Kita et al. (2017) reported that Mikura dolphins were a genetically closed population. The differences in speckle onset among populations are thought to be a biological reflection of geographical variation.
4.5 Conclusion
This study demonstrates that speckles can be used to estimate the ages of Indo-Pacific bottlenose dolphins. The estimated age requires statistical analysis, and the accuracy should be further examined. Clarifying the mechanisms underlying speckle pigmentation may elucidate the relationship between age and speckle conditions. A physiological approach with longitudinal observations of spot changes is needed to clarify the mechanisms controlling speckling ontogeny.
Our results suggest that speckles may function as age signals because speckles continue to increase throughout dolphin lives. Dolphins might use speckles to determine the age of other individuals. Our results indicate sexual dimorphism in the genital patches. In addition, the genital patch became clearer with age. These findings strongly support the hypothesis that speckles function as a signal of sexual maturity. Cognitive studies are required to examine whether dolphins can recognize the speckles of other individuals.
ACKNOWLEDGMENTS
We thank the Mikura Island Tourist Information Centre for their financial and logistical support. We also thank the people of Mikura Island for their kind support during our stay on the island. Many people supported us and helped with data collection on Mikura Island, such as boat captains, tour guides, members of the individual identification survey, Mikura-jima Bandouiruka Kenkyukai, and the MIDO team. We would like to thank Dr. Tadamichi Morisaka, Mie University, Japan, for his constructive comments. This study was supported by the Cooperation Research Program of the Wildlife Research Center of Kyoto University. This research was partially supported by JSPS KAKENHI (Grant Number 20 K12584), which was awarded to M. Sakai. We would like to thank Editage (https://www.editage.com) for English language editing.
AUTHOR CONTRIBUTIONS
Genfu Yagi: Conceptualization; formal analysis; funding acquisition; investigation; methodology; project administration; writing-original draft. Mai Sakai: Funding acquisition; investigation; methodology; project administration. Kazunobu Kogi: Data curation; investigation; resources.




