New insights into the disulfide stress response by the Bacillus subtilis Spx system at a single-cell level
Abstract
Spx is a global transcriptional regulator that orchestrates the Bacillus subtilis response to disulfide stress. The YjbH (SpxH) protein adapts Spx for ClpXP-mediated degradation, playing a critical role in the regulation of the cellular Spx levels. Upon stress, YjbH forms aggregates by a yet unknown mechanism, resulting in increased Spx levels due to reduced proteolysis. Here, we studied how individual cells use the Spx-YjbH system to respond to disulfide stress. We show, using fluorescent reporters, a correlation between the Spx levels and the amount of YjbH, as well as a transient growth inhibition upon disulfide stress. The in vivo dynamics and inheritance of YjbH aggregates are characterized by a bipolar distribution over time and appear to be entropy-driven by nucleoid exclusion. Moreover, we reveal that the population following disulfide stress is highly heterogenous in terms of aggregate load and that the aggregate load has strong implications for cellular fitness. We propose that the observed heterogeneity could be a mechanism to ensure population survival during stress. Finally, we find that the two YjbH domains (DsbA-like domain and winged-helix domain) contribute to its aggregation function, and show that the aggregation of the DsbA-like domain is conserved among other studied orthologs, whereas important differences are observed for the winged-helix domain.
Graphical Abstract
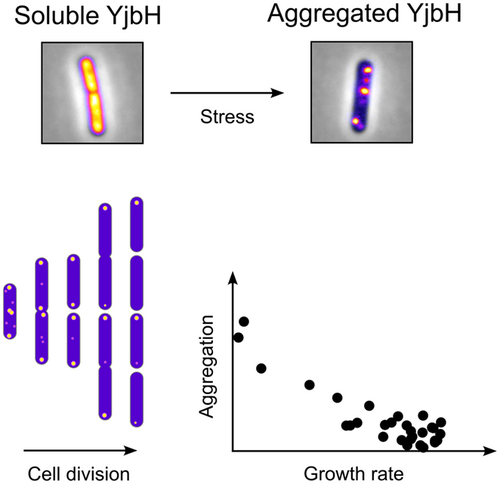
Upon stress, the adaptor protein YjbH forms aggregates. YjbH aggregation promotes increased levels of Spx, a key transcriptional regulator that orchestrates gene expression to mitigate stress. We investigated the localization and in vivo dynamics of YjbH aggregates, the impact of aggregates on cellular fitness, and the contributions of the two domains of YjbH to aggregation. Furthermore, we demonstrate that the aggregation properties of YjbH are conserved among members of the Bacillota (Firmicutes) phylum.
1 INTRODUCTION
To survive under adverse and fluctuating conditions, bacteria have evolved several mechanisms that sense and respond to stress. In the gram-positive bacterium Bacillus subtilis, the Spx protein (encoded by spxA) is a global transcriptional regulator controlling the expression of large number of genes in response to oxidative stress triggered by thiol-specific agents (Nakano et al., 2003; Rochat et al., 2012). Recent studies have shed light on the role of Spx in responding to other stressors, such as heat shock and cell wall-active compounds, in addition to its well-established role in the disulfide stress response (Rojas-Tapias & Helmann, 2018a; Runde et al., 2014; Schafer & Turgay, 2019). Thus, Spx is a versatile and important regulator that mediates the B. subtilis response to various stressors.
Spx modulates transcription of target genes by directly interacting with the C-terminal domain of the α-subunit of RNA polymerase (αCTD) (Lamour et al., 2009; Nakano et al., 2010; Newberry et al., 2005; Reyes & Zuber, 2008; Rochat et al., 2012; Shi et al., 2021; Zuber, 2004). Spx-induced genes include those involved in re-establishing the redox levels in the cytoplasm and repairing any caused damage, such as trxA (thioredoxin) and trxB (thioredoxin reductase) (Nakano et al., 2003; Rochat et al., 2012). Additionally, Spx is capable of repressing genes that are involved in normal cell growth, such as those encoding for ribosomal proteins and rRNA (Nakano et al., 2003; Rochat et al., 2012; Schafer et al., 2019). These findings highlight the importance of tightly controlling Spx cellular levels, as it regulates genes involved in both stress response and normal growth.
Indeed, the levels and activity of Spx are known to be controlled through many layers of regulation (reviewed in [Rojas-Tapias & Helmann, 2019b]). The transcriptional control of the spx gene involves the repressors YodB and PerR (Leelakriangsak et al., 2007). Furthermore, the Spx protein has a redox switch involving the cysteines of the C10-X-X-C13 sequence motif located in its N-terminal part. Under disulfide stress, these two cysteines are oxidized and form an intramolecular disulfide bond, which leads to the activation of Spx as a transcriptional regulator (Nakano et al., 2005). Spx appears to be subjected to arginine phosphorylation that may modulate the stability of Spx under certain conditions (Rojas-Tapias & Helmann, 2019a). One of the most important layers of Spx regulation, however, seems to be through its post-translational degradation by the ClpXP protease complex (Figure 1a). Under non-stress conditions, Spx is efficiently degraded by ClpXP, aided by the cytosolic adaptor protein YjbH (also referred to as SpxH) (Engman & von Wachenfeldt, 2015; Garg et al., 2009; Larsson et al., 2007; Nakano et al., 2003), comprised of an N-terminal DsbA-like domain connected by a proline-rich linker to a C-terminal winged-helix domain (Awad et al., 2019; Larsson et al., 2007). Typically, adaptor proteins interact with both the client and the protease, facilitating proteolysis. YjbH, however, does not seem to directly interact with ClpXP (Chan et al., 2012). Instead, YjbH reduces the conformational flexibility of Spx upon binding, such that its C-terminal region can be recognized by ClpXP, thus enhancing degradation (Awad et al., 2019; Chan et al., 2014; Larsson et al., 2007). Under some conditions, such as cell wall stress, the narrowly conserved anti-adaptor small protein YirB (also referred to as SpxO) binds to YjbH competitively inhibiting the binding of Spx (Kommineni et al., 2011; Rojas-Tapias & Helmann, 2018b). When the cell encounters stress leading to protein denaturation, YjbH aggregates, lowering its soluble levels and in turn decreasing proteolysis of Spx (Figure 1a) (Engman & von Wachenfeldt, 2015). Therefore, YjbH aggregation is understood as a key regulatory mechanism of the Spx stress response. YjbH forms aggregates not only under disulfide stress, but also by stress induced by other protein-denaturing conditions, such as ethanol or heat (Engman & von Wachenfeldt, 2015). Aggregates of YjbH were also observed when a non-native inclusion body-forming protein was produced in B. subtilis (Engman & von Wachenfeldt, 2015) suggesting that YjbH can interact with other aggregating proteins and form hetero-aggregates. However, and despite the major advances that have been made, the molecular mechanisms that trigger the aggregation of YjbH are not fully understood yet.
Spx and YjbH are widespread among the low-G + C-content gram-positive bacteria, including the human pathogens Staphylococcus aureus and Listeria monocytogenes. A role of YjbH in adapting Spx for ClpXP degradation has also been shown for S. aureus and L. monocytogenes (Engman et al., 2012; Pamp et al., 2006; Ruhland & Reniere, 2020). Spx orthologs show a high degree of sequence conservation in contrast to a sequence identity of about 30% among YjbH orthologs. However, the latter seems to share a similar function, judging from several complementation studies (Engman et al., 2012; Engman & von Wachenfeldt, 2015; Ruhland & Reniere, 2020). As an example, YjbH from Geobacillus kaustophilus, S. aureus and L. monocytogenes can complement a B. subtilis ΔyjbH strain (Engman et al., 2012; Engman & von Wachenfeldt, 2015). Notably, YjbH stress-regulated aggregation has also been observed in S. aureus (Panasenko et al., 2020).
Importantly, while there exists a large body of literature investigating the Spx-YjbH system at an averaged population level, there have been relatively few studies aimed at understanding how the system works at a single-cell level. Bulk analyses provide the average response of the population, which may result in cell-to-cell variations being masked by such averaging. As a clear example, cells with reduced growth rates (persisters) are present in exponentially growing populations of Escherichia coli, which means that a few cells exhibit a significantly lower growth rate than that of the population mean (Balaban et al., 2004). This phenotypic diversity can help the population to adapt to changing environments, as different phenotypes may be better suited to different conditions, such as, for example, persister cells that are less sensitive to certain antibiotics (Balaban et al., 2004; Sampaio et al., 2022). There are several examples of how cell-to-cell heterogeneity may provide advantages to bacterial populations in coping with stress (Alnahhas & Dunlop, 2023). The E. coli adaptive response, regulated by the Ada transcription factor, provides inducible protection against DNA alkylation damage. In the absence of stress, the distribution of Ada molecules between cells is heterogeneous, such that a majority of cells respond to the stress instantly but a few cells display a delayed onset of the adaptive response (Uphoff et al., 2016). When the population is exposed to DNA-damaging alkylating compounds, the latter subpopulation of cells is in a hypermutable state and may provide a pool of cells from which fitter individuals may arise (Uphoff et al., 2016). In E. coli, one of the principle global stress regulator RpoS is heterogeneously distributed and correlated with growth rate such that growth is reduced in cells with elevated RpoS activity (Patange et al., 2018).
Here, we aimed to get a more comprehensive understanding of the Spx-YjbH stress response by single-cell analyses and by dissecting the role of the two domains in aggregation of YjbH. Possessing information from both single and population levels should ultimately allow us to get an all-encompassing picture of the Spx-YjbH system. Our research at a single-cell level focused on different aspects of the Spx-YjbH system in response to disulfide stress: first, we demonstrate a negative correlation between Spx and YjbH levels, as well as reduced growth rate upon disulfide stress in individual cells; second, we put substantial focus in the YjbH aggregation property, suggesting that YjbH aggregates display a specific subcellular localization driven by the molecular crowding of the nucleoid and aggregate size. We show the in vivo dynamics of YjbH aggregates, and aggregate distribution pattern among daughter cells, which appears to follow a passive mechanism. Moreover, we report population heterogeneity in terms of YjbH aggregate carriage, and show a link between aggregation and cellular fitness of the progeny following disulfide stress. Finally, we report the contribution of the YjbH domains to its aggregation properties and identify conserved YjbH aggregation features by broadening the observations to other YjbH orthologs.
2 RESULTS
2.1 Decreased YjbH soluble levels correlate with increased Spx levels in single cells
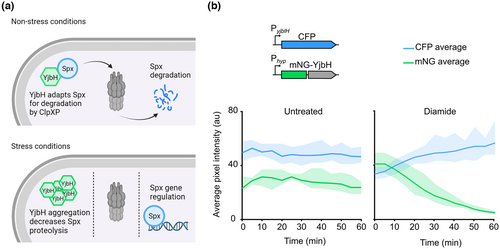
At the population level, a decrease in soluble YjbH levels and YjbH appearance in the insoluble fraction of a cell lysate correlates with higher Spx amounts in the soluble fraction (Engman & von Wachenfeldt, 2015). Here, we investigated whether this correlation would hold true at the level of single cells. For that, we created a dual-reporter strain (LUW780) to follow the Spx levels over time, as well as the YjbH levels and the presence of YjbH aggregates in individual cells. The reporter of Spx levels consisted of the gene encoding for the cyan fluorescent protein (CFP) expressed from the PyjbIH promoter, which is upregulated by Spx (Nakano et al., 2003). Note that we verified that the PyjbIH_CFP construct was a suitable reporter to indirectly estimate the cellular Spx levels (Figure S1). Also note that this reporter was used as a proxy for active Spx levels and that hereafter, the term “Spx levels” is used for simplicity. The second reporter consisted of the gene encoding for YjbH N-terminally fused to the bright fluorescent protein mNeonGreen (mNG-YjbH), expressed from the IPTG-inducible Phyperspank (Phyp) promoter. We also verified that mNG-YjbH served as a marker for YjbH localization by performing appropriate controls: mNG alone, or mNG fused to the B. subtilis truncated hemoglobin (trHb), which is known to be soluble, did not form foci upon addition of the disulfide stress inducing thiol-oxidizing agent diamide (Figure S2). Foci of mNG-YjbH (regarded as protein aggregates) could be seen already 15 min after diamide addition and disappeared after 60 min (Figure S2), as previously shown with a related fusion between superfolder GFP and YjbH (sfGFP-YjbH) (Engman & von Wachenfeldt, 2015). Next, and after induction of mNG-YjbH expression, we exposed the dual-reporter strain to diamide and observed the cells with time-lapse fluorescence microscopy. While the CFP intensity increased over time, indicative of Spx increase, the mNG-YjbH cytoplasmic intensity decreased (Figure 1b). A control experiment in which the cells were not exposed to diamide showed that the Spx levels did not increase, and the mNG-YjbH intensity did not decrease over time (Figure 1b). Taken together, our data show that an increase in Spx levels correlates with a decrease in soluble YjbH levels in individual cells.
2.2 Diamide exposure results in transient growth inhibition
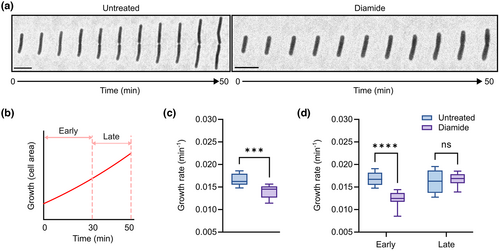
Next, we wondered whether single cells exposed to diamide—which, as shown above, have higher Spx levels—would display any growth defects. Previous research done at a population level has shown that a yjbH null mutant, in which the amounts of Spx are elevated, has a pleiotropic phenotype including reduced growth (Larsson et al., 2007). Moreover, Spx appears to repress genes related to normal growth and metabolism, as evidenced by transcriptomic analyses performed with a mutant version of Spx resistant to ClpXP proteolysis (SpxDD) (Nakano et al., 2003). We analyzed diamide-stressed single cells with time-lapse microscopy and observed a 15.5% decrease in growth rate following diamide exposure (0.014 ± 0.001 min−1), compared to the non-stressed cells (0.017 ± 0.001 min−1) (Figure 2a–c). When splitting the duration of the stress response into early stage (0 to 30 in after diamide exposure) and late stage (30 to 50 min after diamide exposure), we noted that with time (i.e., in the late stage of the stress response), cells seemed to adapt to the stress condition by increasing their growth rate to 0.017 ± 0.001 min−1 (similar to the growth rate of non-stressed cells: 0.016 ± 0.002 min−1; Figure 2d). Next, we investigated if a strain expressing mNG-YjbH (LUW536), which was to be used in later experiments, also displayed growth defects after diamide stress. Similar to the wild-type strain, the growth rate in the mNG-YjbH strain was lower upon stress (0.01 ± 0.002 min−1) when compared with the non-stressed cells (0.018 ± 0.001 min−1; Figure S3). As for the late stage of the stress response, the strain-bearing mNG-YjbH reporter did not resume its growth rate back to the levels of the non-stressed cells (Figure S3). This is presumably due to the fact that in this strain, YjbH is expressed from a non-native promoter, resulting in the absence of a feedback loop in which Spx induces the expression of YjbH. This feedback loop would allow rapid Spx degradation once the stress condition is gone, as YjbH would then be soluble instead of forming aggregates. It is also possible that the mNG-YjbH fusion protein is less efficient compared to the native YjbH at adapting Spx for degradation. Our observations at a single-cell level indicate that the diamide-activated Spx response leads to transient growth inhibition.
2.3 Large YjbH aggregates localize at nucleoid-free regions
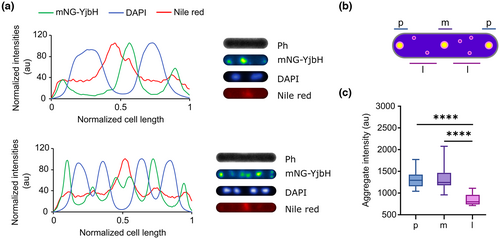
Next, we set a particular focus in the study of YjbH aggregates. We started by observing whether YjbH stress-induced aggregates displayed a specific subcellular localization. For that, we made use of the mNG-YjbH fusion protein (strain LUW536) and examined aggregate location in relation to the nucleoid regions by using the DNA fluorescent dye DAPI. In addition, we used the membrane stain Nile red to visualize cell borders. We imaged the cells after diamide exposure with fluorescence microscopy and extracted the intensity profiles of the mNG-YjbH, DAPI, and Nile red channels; we observed that the maximum fluorescence intensity of mNG-YjbH was on all occasions present at the polar regions of the cells or between the segregated nucleoids in pre-divisional cells (Figure 3a), suggesting that the large aggregates occupy the nucleoid-free regions in the cell. We also found additional aggregates of lower intensity, randomly distributed throughout the cell, which also seemed to occupy the nucleoid-free regions, although in rare occasions co-localized with the DAPI staining (Figures 3a and S4). We then measured and plotted the fluorescence intensity of mNG-YjbH foci (as a proxy of aggregate size) for different subcellular regions (polar, midcell, and randomly distributed). This confirmed that, while larger (more intense) aggregates seemed to have preference for polar and midcell positions, smaller (less intense) aggregates appeared randomly distributed in the cytoplasm (Figure 3b,c). These results indicate that the subcellular localization of YjbH aggregates depends on the aggregate size and on the accessible space within the cytoplasm.
2.4 Large YjbH aggregates are stabilized at polar positions over time
After observing the subcellular localization of the YjbH aggregates, we aimed to obtain information on the in vivo dynamics of YjbH aggregates during the diamide-stress response, and on how aggregates are distributed among the daughter cells after a cell division event. To study this, we performed time-lapse fluorescence imaging of the LUW536 strain expressing mNG-YjbH upon exposure to diamide (Figure 4a). First, we quantified the number of aggregates in different subcellular positions at different time intervals within a cell division event. Just after stress induction, larger aggregates were seen at the poles or midcell positions in the mother cells, and smaller aggregates appeared randomly distributed throughout the cytoplasm, as shown in Figure 4b,c. The aggregate pattern in daughter cells, however, was different from the one exhibited by their mother cell: in the daughter cells, most aggregates located at the poles, but not as much in other regions. This was primarily a consequence of cell division and stress adaptation: whereas the aggregates that were initially located at the old poles maintained their polar localization after cell division, aggregates formed at the midcell position (or forming septa) prior to cell division were inherited at the new poles of daughter cells, changing their relative cellular position (Figure 4c). We also observed that large YjbH aggregates appeared quite static over time, that is, they appeared fixed in their position (Figure 4d). By contrast, smaller aggregates (which were initially distributed throughout the cell; Figure 3c) diffused inside the cell (Figure 4d). Eventually, aggregates were either localized at bipolar or midcell regions presumably as a result of nucleoid occlusion, and/or cleared as a result of adaptation to stress. Our results suggest that unrestricted movement of small aggregates within the cell, together with stabilization of aggregates at nucleoid-free regions, drives the polar and midcell distribution of aggregates over time. Thus, it is likely that the observed YjbH aggregate distribution pattern is driven by a purely passive mechanism.
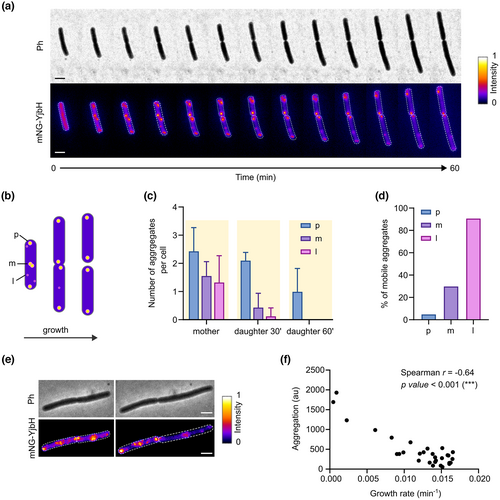
2.5 The YjbH aggregate load correlates with the cellular fitness
In our time-lapse data, we observed an unequal carriage of YjbH aggregates among the daughter cells. For instance, while some daughter cells were almost devoid of aggregates a few minutes after cell division (aggregates tended to be resolved presumably due to stress adaptation), others still contained some mNG-YjbH foci (Figure 4e). Here, we sought to investigate whether the YjbH aggregate load would be linked with differences in growth rate. We analyzed the time-lapse data and found a significant correlation between the presence of aggregates and cell growth rate: the more aggregation cells would bear, the lower their growth rate (Figure 4f). Thus, the presence of aggregates might have direct or indirect negative consequences for the progeny in terms of fitness. Since Spx represses genes involved in normal cell growth, it is also likely that the cells with higher aggregate load bear higher Spx levels and thus have a lower growth rate.
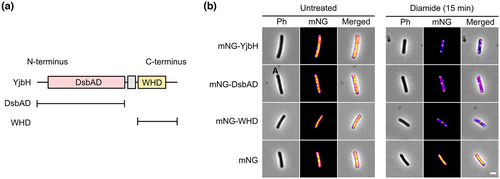
2.6 The two domains of B. subtilis YjbH contribute to its aggregation function
Little is known about the molecular determinants responsible for the propensity of YjbH to aggregate under stress conditions. Previous structural analysis of YjbH revealed that the protein is comprised of an N-terminal DsbAD-like thioredoxin domain (DsbAD) linked by an extended proline-rich sequence to a C-terminal winged-helix domain (WHD) (Figure 5a) (Awad et al., 2019). While the DsbAD contains the Spx-binding site (Al-Eryani et al., 2016), the function of the WHD remains unknown. It has been shown that the B. subtilis DsbAD localizes in the insoluble fraction of cell extracts, similar to the YjbH wild-type protein, suggesting that the WHD is not required for the aggregation to occur (Engman & von Wachenfeldt, 2015). Here, we aimed to investigate the contributions of the two domains to the stress-induced aggregation function of YjbH at a single-cell level. We N-terminally fused the two YjbH domains to mNG (mNG-DsbAD and mNG-WHD) and introduced them in single copy in the B. subtilis chromosome, under the Phyp promoter. Before the induction of disulfide stress, none of the fusion proteins was seen to aggregate and showed a fluorescent signal distributed homogenously throughout the cells' cytoplasm (Figure 5b). Distinct fluorescent mNG-DsbAD and mNG-WHD foci were observed upon diamide stress, similar to the aggregation behavior of full-length YjbH (Figure 5b). To test if the fusion proteins would functionally complement a B. subtilis yjbH null mutant when it comes to keeping low levels of Spx under non-stress conditions, we made use of a β-galactosidase reporter assay as a proxy for the functional levels of Spx (Awad et al., 2019). The fusion proteins were expressed to different levels by using increasing concentrations of the inducer (IPTG). mNG-YjbH lowered the levels of Spx in an IPTG-dependent manner (similar to the native YjbH) suggesting that the fusion protein is able to bind to B. subtilis Spx and enhance its proteolytic degradation (Figure S5). We observed that the mNG-DbsAD was capable of lowering the Spx levels to some extent, but less efficiently than the wild-type DsbAD domain (Figure S5). As expected, the Spx levels could not be kept low by the WHD fusion protein alone. These results show that both structural domains of B. subtilis YjbH display stress-induced aggregation properties in a similar manner.
2.7 YjbH orthologs and its DsbAD domains, but not WHD, display similar stress-induced aggregation
To identify conserved YjbH aggregation properties, we broadened our observations to YjbH orthologs from S. aureus (SaYjbH) and L. monocytogenes (LmYjbH), and assessed their aggregation properties in B. subtilis at a single-cell level. We aimed to investigate if the stress-sensing aggregation property of YjbH and its two domains is conserved between the different orthologs and if it is independent of host-specific factors. With a similar methodology as described above, we created B. subtilis strains containing the S. aureus and L. monocytogenes YjbH orthologs, as well as their two domains separately, fused to mNG, and assessed their aggregation upon diamide stress. Despite a low sequence similarity of about 30% (Figure S6), SaYjbH and LmYjbH were able to sense the stress in a non-native host by the same regulated aggregation mechanism as that shown for BsYjbH and displayed very similar aggregation profiles (Figures 6a,b and S7). Fluorescence microscopy also revealed distinct fluorescent foci for all DsbAD domains upon stress, similar to the aggregation behavior of the YjbH orthologs (Figure 6a,b). By using the β-galactosidase activity assay, we observed that all DbsAD orthologs were capable of lowering the B. subtilis Spx levels to some extent, but less efficiently than the intact YjbH proteins (Figures 6a,b and S5). This suggests that the stress-induced aggregation and the ClpXP adaptor function of the studied YjbH orthologs and their DsbAD domains are conserved and independent of host-specific factors.
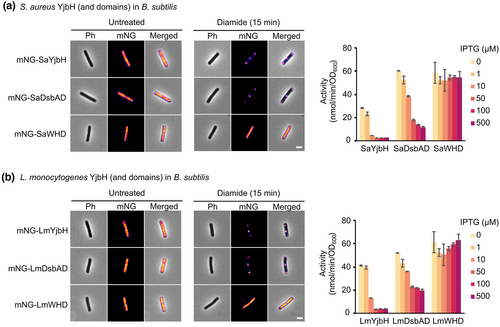
Surprisingly, no foci were seen for S. aureus and L. monocytogenes WHD (Figure 6a,b). The B. subtilis WHD has two cysteine residues (C236 and C297; Uniprot: O31606,) that may form intra- or inter-protein disulfide bonds after exposure to diamide and could therefore be involved in redox sensing and aggregation. No cysteine residues are present in the S. aureus and L. monocytogenes WHD. To test whether the different aggregation propensities observed for the WHD in the different orthologs could be due to the presence or absence of cysteines, we constructed a strain expressing an mNG-WHD variant in which the two cysteines (C236 and C297) in the WHD were replaced by serine residues. Aggregation of this fusion protein was observed under diamide stress (Figure S8), resembling the one observed for the YjbH and WHD fusion proteins. These findings suggest that the two cysteine residues of the WHD are not necessary for aggregation of the domain in response to diamide.
Finally, the fact that YjbH orthologs and their respective DsbAD exhibit similar aggregation patterns in B. subtilis without the need of host-specific factors suggests that the aggregation propensity might be inherent to the YjbH amino acid sequence. We predicted the aggregation propensity of the DsbAD as well as the WHD from the different orthologs in silico using the TANGO algorithm (Fernandez-Escamilla et al., 2004; Linding et al., 2004), which predicts aggregation propensity based on a protein amino acid sequence. The in silico predictions are in strong agreement with the in vivo observations (Figure S9) and are an indication that aggregation of YjbH and its respective domains is inherent to the amino acid sequence.
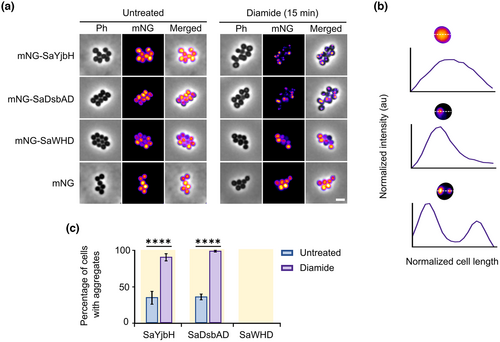
2.8 The S. aureus DsbAD, and not the WHD, aggregates upon stress in its native host
Our studies in B. subtilis suggest that the aggregation propensity of the different YjbH orthologs is inherent to the amino acid sequence and that the aggregation upon stress is independent of host-specific factors. We reasoned that if this is the case, studying the DsbAD and WHD of S. aureus YjbH (SaDsbAD and SaWHD) in their native host should show a similar aggregation pattern as that observed for these domains in B. subtilis, that is, aggregation for the SaDsbAD but not for SaWHD upon disulfide stress. Low-copy number plasmids (pSA_low) containing mNG-SaYjbH, mNG-SaDsbAD, and mNG-SaWHD under the IPTG-inducible Phyp promoter were introduced in S. aureus Newman, and the respective strains were subjected to diamide stress. Even though the cytoplasmic fluorescence intensity differed between cells, it was clear that mNG-SaWHD did not form any apparent foci, as we did not observe any sharp peaks of fluorescence intensity along the cells' diameter (Figure 7a,b). As for the mNG-SaYjbH and mNG-SaDsbAD fusion proteins, some foci (defined as sharp peaks of fluorescence intensity along the cell's diameter; Figure 7b) were present already under non-stress conditions (Figure 7a). However, the number of mNG-SaYjbH and mNG-SaWHD fluorescent aggregates increased from 35% to 90%, and from 36% to 99%, respectively, after exposing the cells to diamide (Figure 7c). This suggests that, similar to the intact SaYjbH protein, SaDsbAD is able to sense disulfide stress in S. aureus by an aggregating mechanism. These results show that the WHD is dispensable for the aggregation of YjbH in S. aureus, and further indicate that aggregation of the DsbAD is conserved among members of the Bacillota (Firmicutes) phylum. Also, the fact that the aggregation propensities for the S. aureus YjbH domains are similar in the non-native host B. subtilis confirms that the YjbH aggregation mechanism does not depend on host-specific factors.
3 DISCUSSION
Protein aggregation is usually associated with a loss of function, having deleterious effects such as reduced growth and survival in bacteria, or causing disorders in humans such as Parkinson's disease or Alzheimer's disease (Balchin et al., 2016). However, there are cases in which aggregation is a beneficial mechanism to regulate the activity, localization, or amounts of certain proteins (Schramm et al., 2020). This is the case of the bacterial adaptor protein YjbH, which aggregates upon disulfide stress—and other proteotoxic stress conditions such as heat and ethanol—and as a consequence, loses activity and can no longer adapt the Spx transcription factor for degradation by the protease complex ClpXP. YjbH aggregation, then, serves as a proteotoxic stress-sensing regulatory mechanism to rapidly elevate Spx levels, which in turn modulates gene expression to cope with the stress (Engman & von Wachenfeldt, 2015; Garg et al., 2009; Larsson et al., 2007). The molecular mechanism that induces YjbH aggregation, however, is still not fully understood.
To help shed light into this question, we show conserved and host-independent YjbH aggregation properties: despite a low sequence identity of around 30% (Figure S6), YjbH orthologs from B. subtilis, S. aureus, and L. monocytogenes display stress-induced aggregation in B. subtilis in a very similar manner (Figure 1b,d). We also show that the N-terminal DsbA-like domain (DsbAD) from the three orthologs aggregates similar to the full-length protein and is capable of adapting Spx for degradation to some extent. The fact that S. aureus and L. monocytogenes YjbH (and respective DsbAD) seem to adapt B. subtilis Spx for degradation indicates the existence of Spx-YjbH inter-species interaction. In relation to this, YjbH interaction with Spx has been shown to occur over a large surface area from the DsbAD that appears to be evolutionarily conserved (Awad et al., 2019). This interaction surface shows extensive charged electrostatic patches. This suggests that the mechanism by which YjbH recognizes and adapts Spx for degradation is also conserved and could explain the observed Spx-YjbH inter-species interactions.
These conserved properties of YjbH orthologs, which are highly divergent in sequence, show that many sequence combinations are compatible with the YjbH adaptor and stress-sensing functions. The aggregation mechanism is an important layer of the Spx-YjbH system regulation and has a key role in responding to stress. Thus, evolution has probably favored YjbH sequences which render a quite unstable and aggregation-prone protein, while conserving the residues that enable interaction with Spx.
Unexpectedly, while the B. subtilis WHD aggregated in a stress-dependent fashion on its own, the domains from S. aureus or L. monocytogenes did not (Figure 3b). Judging from models made by using the Alphafold machine-learning structure-prediction algorithm (Jumper et al., 2021; Varadi et al., 2022), the overall structure of the WHD orthologs is very similar (Figure S10) indicating that there are important residues present only in the B. subtilis WHD that make the domain aggregate upon stress. We can, however, rule out cysteine residues as required for aggregation of the B. subtilis WHD (Figure S8) which is in accordance with previous studies showing that the cysteine residues are not essential for aggregation of the full-length YjbH (Engman et al., 2012; Engman & von Wachenfeldt, 2015). More detailed characterization of the WHD orthologs could provide information about the aggregation mechanism.
The observation that the aggregation propensity of YjbH and its domains is independent of host-specific factors is also supported by our studies in S. aureus: the behavior of the two YjbH domains DsbAD and WHD from S. aureus in their native host resembled the one we observed for these domains in B. subtilis (Figure 4a).
Stress often leads to transient reduction of growth rate, such as our observed growth defects following diamide exposure (Figure 2). The reduced growth rate could be a direct consequence of the increased Spx levels, with Spx repressing genes for normal growth and metabolism; but growth defects could also be partly due to the presence of aggregates (YjbH and other aggregation-prone proteins). In some instances, the presence of aggregates has been associated with effects on growth rate (Lindner et al., 2008; Vedel et al., 2016; Winkler et al., 2010). In relation to that, we observed that the population of cells following disulfide stress differed in terms of aggregate load and that the presence of aggregates was negatively correlated with the growth rate (Figure 4f). Provided no further stress appears, the population would eventually be dominated by those faster-growing cells that experienced clearance of YjbH aggregates. On the other hand, the fraction of slow-growing cells containing YjbH aggregates could be more resistant to stress, conferring an advantage to the population in case of re-exposure to stress. Even though this remains to be tested in the Spx-YjbH system, studies in E. coli suggest that the presence of protein aggregates is linked to increased tolerance to subsequent stresses (Govers et al., 2018; Pu et al., 2019). Therefore, the observed heterogeneity might be a bacterial strategy to ensure the survival of the population.
We have also established a bipolar and midcell localization of mNG-YjbH aggregates, which has been reported previously for other proteins under proteotoxic stress conditions, both in E. coli (Coquel et al., 2013; Gupta et al., 2014; Neeli-Venkata et al., 2016; Oliveira et al., 2016; Winkler et al., 2010) and in B. subtilis (Hantke et al., 2019; Kirstein et al., 2008; Runde et al., 2014; Stannek et al., 2014). This suggests that upon stress, YjbH could co-aggregate with other misfolded proteins through, for instance, nonspecific hydrophobic interactions. A self-oligomerizing driven aggregation mechanism would favor unipolar localization.
The observed YjbH aggregate distribution pattern over time shows that large aggregates are stabilized at bipolar or midcell positions possibly by a passive entropy-driven nucleoid exclusion mechanism also observed for misfolded proteins in E. coli (Figures 3 and 4a–d) (Saberi & Emberly, 2010; Winkler et al., 2010). Smaller aggregates appeared randomly distributed in the cytoplasm as they presumably do not bear the same spatial constraints imposed by the nucleoid. Provided cells adapt to the trigger of stress and no more aggregates are formed over time, this observed pattern of aggregate distribution leads to asymmetry among the progeny: There will be faster-growing, aggregate-free daughter cells, and slower-growing daughter cells still containing aggregates at the old poles (until resolved). Such heterogeneity in the progeny could also be a mechanism to get rid of aggregates in the majority of the population, while ensuring survival of a small portion of the progeny in case of re-exposure to stress. Prior to the present study, little was known about protein aggregate inheritance, as well as the impact of aggregate carriage, in gram-positive bacteria. Most likely, a similar pattern of aggregate inheritance as seen for YjbH will also be observed for other aggregated proteins in future studies.
4 MATERIALS AND METHODS
4.1 Bacterial strains, plasmids, and growth conditions
The strains and the plasmids used in this work are listed in Tables 1 and S1, respectively. E. coli TOP10 or DH5α was used for plasmid amplification and isolation and was grown in lysogeny broth (LB) medium (10 g of tryptone, 5 g of yeast extract, 5 g of NaCl per liter of broth) or on LB agar (LA) plates. All B. subtilis strains were grown on tryptose blood agar base (TBAB), or in nutrient sporulation medium with phosphate (NSMP) supplemented with 0.5% glucose (NSMPG) (Winstedt et al., 1998). S. aureus strains were grown in tryptic soy broth (TSB) or on tryptic soy agar (TSA). All bacterial strains were grown at 37°C. For liquid cultures, baffled flasks were used and shaking was done at 200 rpm. The antibiotic concentrations used for growth media were the following: ampicillin (100 μg mL−1), chloramphenicol (5 μg mL−1 for B. subtilis and 20 μg mL−1 for S. aureus), erythromycin (0.5 μg mL−1), kanamycin (5 μg mL−1), lincomycin (12.5 μg mL−1), and tetracycline (20 μg mL−1). Isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to the growth media when induction of the Phyp promoter was required, to a final concentration of 500 μM for B. subtilis and 50 μM for S. aureus, unless otherwise stated. To induce disulfide stress, cells were exposed to diazenedicarboxylic acid bis(N,N-dimethylamide) (diamide) at mid-exponential growth phase to a final concentration of 0.5 mM for B. subtilis and 5 mM for S. aureus, unless otherwise stated.
| Strain | Relevant characteristicsa | Sourceb |
|---|---|---|
| 1A1 | trpC2 | BGSC |
| LUW393 | ΔyjbH::tet, TcR | Awad et al. (2019) |
| LUW533 | amyE::Phyp_mNG, CmR | This work |
| LUW534 | amyE::Phyp_mNG-BsYjbH, CmR | This work |
| LUW536 | amyE::Phyp_mNG-BsYjbH, ΔyjbH::tet, CmR TcR | This work |
| LUW588 | thrC::PyjbIH_lacZ, EmR | Awad et al. (2019) |
| LUW608 | amyE::Phyp_BsYjbH, thrC::PyjbIH_lacZ, ΔyjbH::tet, CmR EmR TcR | This work |
| LUW747 | amyE::Phyp_mNG-BsWHD(-Cys), thrC::PyjbIH_lacZ, CmR EmR | This work |
| LUW767 | amyE::Phyp_mNG-BstrHb, CmR | This work |
| LUW773 | bglS::PyjbIH_CFP, KmR | This work |
| LUW780 | bglS::PyjbIH_CFP, amyE::Phyp_mNG-YjbH, KmR CmR | This work |
| LUW851 | amyE::Phyp_mNG-LmWHD, thrC::PyjbIH_lacZ, CmR EmR | This work |
| LUW852 | amyE::Phyp_mNG-SaYjbH, thrC::PyjbIH_lacZ, CmR EmR | This work |
| LUW853 | amyE::Phyp_mNG-BsYjbH, thrC::PyjbIH_lacZ, CmR EmR | This work |
| LUW854 | amyE::Phyp_mNG-BsDsbAD, thrC::PyjbIH_lacZ, CmR EmR | This work |
| LUW855 | amyE::Phyp_mNG-SaDsbAD, thrC::PyjbIH_lacZ, CmR EmR | This work |
| LUW856 | amyE::Phyp_mNG-LmDsbAD, thrC::PyjbIH_lacZ, CmR EmR | This work |
| LUW857 | amyE::Phyp_mNG-BsWHD, thrC::PyjbIH_lacZ, CmR EmR | This work |
| LUW859 | amyE::Phyp_mNG-SaWHD, thrC::PyjbIH_lacZ, CmR EmR | This work |
| LUW860 | amyE::Phyp_mNG-LmYjbH, thrC::PyjbIH_lacZ, CmR EmR | This work |
| LUW861 | amyE::Phyp_mNG-LmWHD, thrC::PyjbIH_lacZ, ΔyjbH::tet, CmR EmR TcR | This work |
| LUW862 | amyE::Phyp_mNG-SaYjbH, thrC::PyjbIH_lacZ, ΔyjbH::tet, CmR EmR TcR | This work |
| LUW863 | amyE::Phyp_mNG-BsYjbH, thrC::PyjbIH_lacZ, ΔyjbH::tet, CmR EmR TcR | This work |
| LUW864 | amyE::Phyp_mNG-BsDsbAD, thrC::PyjbIH_lacZ, ΔyjbH::tet, CmR EmR TcR | This work |
| LUW865 | amyE::Phyp_mNG-SaDsbAD, thrC::PyjbIH_lacZ, ΔyjbH::tet, CmR EmR TcR | This work |
| LUW866 | amyE::Phyp_mNG-LmDsbAD, thrC::PyjbIH_lacZ, ΔyjbH::tet, CmR EmR TcR | This work |
| LUW867 | amyE::Phyp_mNG-BsWHD, thrC::PyjbIH_lacZ, ΔyjbH::tet, CmR EmR TcR | This work |
| LUW869 | amyE::Phyp_mNG-SaWHD, thrC::PyjbIH_lacZ, ΔyjbH::tet,CmR EmR TcR | This work |
| LUW870 | amyE::Phyp_mNG-LmYjbH, thrC::PyjbIH_lacZ, ΔyjbH::tet, CmR EmR TcR | This work |
| LUW1047 | amyE::Phyp_BsDsbAD, thrC::PyjbIH_lacZ, ΔyjbH::tet, CmR EmR TcR | This work |
| LUW1104 | amyE::Phyp_BsWHD, thrC::PyjbIH_lacZ, ΔyjbH::tet, CmR EmR TcR | This work |
- a All B. subtilis LUW strains are derived from B. subtilis 1A1. Therefore, they all contain the trpC2 auxotrophic marker. CmR, EmR, KmR, and TcR are the abbreviations for chloramphenicol, erythromycin, kanamycin, and tetracycline resistance, respectively.
- b BGSC Bacillus Genetic Stock Center, Department of Biochemistry, Ohio State University, Columbus. http://www.bgsc.org/.
4.2 DNA manipulations and transformations
E. coli cloning and transformation, as well as other molecular biology techniques, were performed as described by (Russell & Sambrook, 2001). Extraction of B. subtilis chromosomal DNA and B. subtilis transformation was performed according to (Hoch, 1991). The oligonucleotides used in this study are shown in Table S2.
4.3 Construction of plasmids
The plasmids constructed in this study (Table S1) were cloned using E. coli TOP10 or DH5α. For B. subtilis, plasmid fragments of pCW101-derived plasmids were integrated in the amyE locus of the chromosome by double-crossover homologous recombination. Plasmid pCW101 and derived plasmids only replicate in E. coli but not in B. subtilis; plasmid pSA_low and derived plasmids replicate in both S. aureus and E. coli. Details on how the different plasmids were constructed can be found in the Supplementary material.
4.4 Construction of B. subtilis strains expressing genes under control of the Phyp promoter
To create B. subtilis strains expressing genes of interest from the Phyp promoter, cells were transformed with pCW101 plasmids that contained the genes encoding the protein of interest under the IPTG-inducible Phyp promoter, and transformants were selected for resistance to chloramphenicol. To verify the successful integration of all desired fragments into the amyE locus, the transformants were grown on plates containing 1% (w/v) starch followed by exposure to 1:1 iodine: water solution. A lack of halo formation confirmed the correct integration. Deletion of the wild-type yjbH gene was done by transforming the strains with chromosomal DNA from strain LUW393, selecting for tetracycline resistance.
4.5 Construction of B. subtilis strains expressing the CFP-encoding gene under control of the PyjbIH promoter
To create B. subtilis strains expressing the gene encoding for the cyan fluorescent protein (CFP) under control of the PyjbIH promoter, cells were transformed with plasmid pCFPbglS_PyjbIH, selecting for kanamycin resistance. The successful integration of the promoter regions fused to the CFP encoding gene into the bglS locus was checked by the lack of halo formation surrounding bacteria streaked on plates with 1% (w/v) lichenan (Sigma-Aldrich®), and exposed to Congo red solution.
4.6 Construction of B. subtilis strains expressing the lacZ gene under control of the PyjbIH promoter
To introduce the lacZ gene under the PyjbIH promoter in the thrC locus, chromosomal DNA from strain LUW588 was used to transform the desired B. subtilis strains, selecting for erythromycin and lincomycin resistance. If needed, deletion of the wild-type yjbH gene was done by transforming the strains with chromosomal DNA from strain LUW393, selecting for tetracycline resistance.
4.7 Construction of S. aureus strains
To create S. aureus Newman strains expressing mNG, mNG-SaYjbH, mNG-SaDsbAD, and mNG-SaWHD fusion proteins, pSA_low plasmids containing the genes encoding for these proteins were introduced by electroporation in the respective strains, selecting for chloramphenicol resistance.
4.8 β-Galactosidase activity measurements
The bacteria were grown in 2 mL LB medium with the indicated concentrations of IPTG in 15 mL glass test tubes. Triplicates were prepared for each sample. After a 16 h incubation at 37°C with shaking (200 rpm), 1 mL of each sample was centrifuged for 5 min at 20,000g and at 4°C. After centrifugation, pellets were resuspended in 500 μL Z- buffer (Miller, 1972) containing 50 mM mercaptoethanol, and 5 μL of 10 mg mL−1 lysozyme and 1 mg mL−1 DNase were added. Samples were incubated at 37°C for 30 min and then centrifuged for 30 min at 20,000g and at 4°C. Pellets were resupended in 600 μL Z-buffer with mercapthoethanol, and incubated at 28°C for 10 min. β-galactosidase activity was then measured using 2-nitrophenyl-β-d-galactopyranoside as a substrate, as previously described (Atkinson et al., 1990).
4.9 Fluorescence microscopy
For fluorescence microscopy experiments, cells were grown in 25 mL NSMPG cultures at 37°C with 200 rpm of shaking. If induction from the Phyp promoter was needed, IPTG at a final concentration of 0.5 mM was added at OD at 600 nm of 0.2. When necessary, stress was induced with diamide at mid-exponential growth phase (OD at 600 nm of 0.6).
To image cells (with exception of time-lapse microscopy), 1 mL of samples was taken from the liquid cultures, and they were centrifuged at 16,000g for 2 min. After discarding the supernatant, the pellet was resuspended in 200 μL of phosphate-buffered saline solution (PBS). Samples were kept on ice until visualized under the microscope. Cells were placed on microscope slides coated with a layer of agarose (1% agarose in PBS), and protected with a cover slip.
To perform time-lapse microscopy with strain LUW780 (Phyp_mNG-YjbH and PyjbIH_CFP), cells were exposed to 0.25 mM diamide. Two minutes after diamide exposure, cells were placed on microscopy slides coated with a 1% agarose pad (10% NSMPG in PBS). Cells were imaged at 5 min of frequency, at 37°C.
Microscopy was done with a Zeiss Axio Imager.Z2 upright light microscope equipped with a Hamamatsu ORCA- Flash4.0 V2 sCMOS camera or, in the case of time-lapse microscopy, with a Zeiss Axio Observer.Z1 inverted light microscope equipped with a Hamamatsu ORCA-Flash4.0LT sCMOS camera. Zeiss ZEN 2 (blue edition) software was used to acquire images, which were saved in TIFF format, and analyzed and processed with Fiji (Schindelin et al., 2012). Fluorescence signal intensities were obtained with MicrobeJ (v 5.13n beta) (Ducret et al., 2016).
4.10 Membrane and DNA staining
To visualize membranes and DNA, samples from mid-exponential growth cultures (OD at 600 nm of 0.6) were taken. 4,6-diamino-2-phenylindole (DAPI) (Sigma-Aldrich) was first added to a final concentration of 0.5 μg mL−1. After 5 min, Nile red (Sigma-Aldrich) was added to a final concentration of 10 μg mL−1 and samples were incubated for 5 min at room temperature. Cells were stressed with diamide (Sigma-Aldrich) after staining.
4.11 Growth rate analyses
To perform growth rate analyses, cells were placed on microscope slides coated with a 1% agarose pad (10% NSMPG in PBS, 1% agarose) with or without diamide. Cells were imaged with time-lapse microscopy. Cell area or cell length values (extracted with Fiji [Schindelin et al., 2012]) were used to calculate the growth rates.
AUTHOR CONTRIBUTIONS
Judith Matavacas and Claes von Wachenfeldt conceived the study and designed experiments; Judith Matavacas, Deepak Anand, and Claes von Wachenfeldt performed experiments; Judith Matavacas, Deepak Anand, and Claes von Wachenfeldt analysed data; and Judith Matavacas and Claes von Wachenfeldt wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
ACKNOWLEDGMENTS
This work was funded by grant 2019-05578_3 from the Swedish Research Council awarded to C.v.W. D.A. was supported by a fellowship from the Carl Trygger Foundation for Scientific Research.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.