Specific preadaptations of Rhodococcus equi cooperate with its Virulence-associated protein A during macrophage infection
Thomas Haubenthal and Philipp Hansen contributed equally to this work.
Abstract
Gram-positive Rhodococcus equi (Prescotella equi) is a lung pathogen of foals and immunocompromised humans. Intra-macrophage multiplication requires production of the bacterial Virulence-associated protein A (VapA) which is released into the phagosome lumen. VapA pH-neutralizes intracellular compartments allowing R. equi to multiply in an atypical macrophage phagolysosome. Here, we show that VapA does not support intra-macrophage growth of several other bacterial species demonstrating that only few bacteria have the specific preadaptations needed to profit from VapA. We show that the closest relative of R. equi, environmental Rhodococcus defluvii (Prescotella defluvii), does not multiply in macrophages at 37°C even when VapA is present because of its thermosensitivity but it does so once the infection temperature is lowered providing rare experimental evidence for ‘thermal restriction’. Using growth experiments with isolated macrophage lysosomes and modified infection schemes we provide evidence that R. equi resists the attack by phagolysosome contents at low pH for several hours. During this time, R. equi produces and secretes VapA which enables it to grow at the expense of lysosome constituents. We present arguments that, under natural infection conditions, R. equi is VapA-less during the initial encounter with the host. This has important implications for vaccine development.
Graphical Abstract
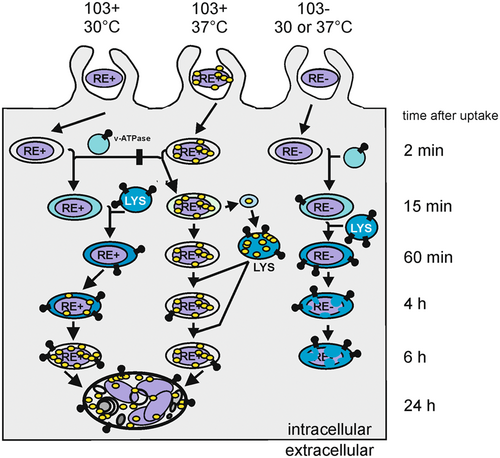
Rhodococcus equi bacteria entering macrophages can face different fates: Avirulent R. equi are delivered to a phagolysosome (bottom right) and killed. When virulent R. equi are cultivated at 30°C, they do not express the major virulence factor VapA (yellow), and they spend hours in an acidified phagolysosome (blue) before they have produced enough VapA to pH-neutralize the phagosome and grow. 37°C-grown virulent R. equi produce VapA to start with and find themselves immediately in a privileged compartment (bottom left).
1 INTRODUCTION
Rhodococcus equi (Prescotella equi; Sangal et al., 2022) is a Gram-positive actinomycete, a close relative of pathogenic mycobacteria, and both an environmental soil bacterium and a facultative intracellular pathogen. It can cause life-threatening pulmonary infections in young foals and in immunocompromised humans, particularly in AIDS patients (Albrecht, 1997; Prescott, 1991; von Bargen & Haas, 2009). The main host target cells of R. equi are lung macrophages (Johnson et al., 1983; Prescott, 1991) which phagocytose inhaled microorganisms into a phagosome where virulent R. equi multiply (Hondalus & Mosser, 1994; von Bargen et al., 2019; Willingham-Lane et al., 2016). Environmental non-equi rhodococci are rarely associated with human disease and it is particularly Rhodococcus erythropolis that has been isolated from patients with, e.g., septicemia (Baba et al., 2009; Park et al., 2011), osteomyelitis (Roy et al., 2009), pulmonary disease (Osoagbaka, 1989), endophtahalmitis (von Below et al., 1991) peritonitis (Brown & Hendler, 1989), or a skin infection (Vernazza et al., 1991). Also, very rare human cases associated with Rhodococcus fascians, Rhodococcus ruber, Rhodococcus gordoniae (Austin et al., 2016) or other rhodococci have been described.
It has been recognized early that a ~85 kbp virulence-associated plasmid (Takai et al., 1991; Tkachuk-Saad & Prescott, 1991) plays a key role during infection of foals. One plasmid-encoded factor which turned out to be the key for intracellular multiplication is the secreted Virulence-associated protein A (VapA) which remains attached to the bacterial surface during growth in broth culture but which is released into the phagosome lumen during infection (Giguère et al., 1999; Jain et al., 2003; von Bargen et al., 2019; Wright et al., 2018). VapA neutralizes the pH of the macrophage's endocytic/phagocytic continuum through its weak membrane permabilizing activities, allowing multiplication of R. equi within a membrane-bound vacuole that contains lysosome material (von Bargen et al., 2019). Confrontation with lysosome contents is considered a major bactericidal mechanism of macrophages (Szulc-Dąbrowska et al., 2020). Consequently, R. equi mutants lacking the vapA gene which do not neutralize phagosome pH do not multiply in mouse macrophages and they are avirulent in a chronic disease mouse model (Giguère et al., 1999; Jain et al., 2003). Next to the vapA-containing virulence plasmid (pVAPA), similar plasmids can be isolated from R. equi associated with other animals, such as a vapB-encoding plasmid (pVAPB) from pigs and a vapN-containing plasmid (pVAPN) from cattle. The basis of the apparent host tropism of defined R. equi strains is only partly understood (Willingham-Lane et al., 2016).
In pVAPA strains, expression of vapA is tightly regulated by the plasmid-encoded transcription factors VirR and VirS. They strongly stimulate vapA expression at environmental temperatures of 34°C or higher and increase it further in an acidic environment (Kakuda et al., 2014; Miranda-CasoLuengo et al., 2011; Ren & Prescott, 2003; Takai et al., 1992).
Several groups have recently reported the intriguing observation that endocytic uptake of purified recombinant VapA (rVapA) by macrophages promotes intracellular multiplication of avirulent derivatives of clinical R. equi, i.e., strains lacking either vapA or pVAPA altogether (Rofe et al., 2017; Sangkanjanavanich et al., 2017; von Bargen et al., 2019; Wright et al., 2018). VapA does not only lead to a reduced confrontation of phagosomal bacteria with an acidic environment but the raised pH also incapacitates several lysosomal hydrolases which otherwise kill many bacteria (Fu et al., 2020; Pires et al., 2016; Thorne et al., 1976; Turk et al., 1995). Hence, one could expect that extracellular addition and endocytosis of rVapA leads to a general inactivation of lysosomal defense mechanisms. In this case, many other bacteria might also grow in pH-neutralized macrophage phagosomes. To test this hypothesis, we analyzed whether rhodococci other than R. equi or some quite distantly related bacteria would multiply in macrophages provided that VapA was supplied in trans during an infection. We observed that this was not the case for nearly all tested bacteria. Only R. equi and its closest relative, R. defluvii (or Prescotella defluvii; Sangal et al., 2022), could make use of VapA under certain circumstances due to a number of preadaptations and the case of R. defluvii turned out to be a paradigm for how ‘thermal restriction’ limits bacterial growth in mammals.
2 RESULTS AND DISCUSSION
2.1 Selection of bacterial strains for infection experiments
Having observed that extracellularly added rVapA supports intracellular growth of R. equi in macrophages, we wondered whether this was also true for rhodococci other than R. equi. The corresponding intra-macrophage growth assays would have to be performed at 37°C—a temperature which is close to the typical core body temperature of a foal (ca. 37.5–38.5°C). The comparably high body temperature of homeothermic mammals apparently automatically results in an ‘unspecific’ natural immunity to many rhodococci and other microorganisms. Only very few studies have investigated this aspect, e.g., for environmental fungi (Casadevall, 2016). Therefore, we first screened a collection of strains representing different Rhodococcus species for their ability to grow at 37°C in BHI broth and observed that most isolates did not grow at all or they grew much slower than R. equi (which can grow at up to 42°C; Takai et al., 1992). For further analysis, we considered only strains that multiplied at 37°C with roughly similar kinetics as the virulent R. equi strain 103+ (Figure 1a–c; “+” denotes for clarity the presence of a virulence plasmid, “−” its lack). This included strains belonging to the species R. coprophilus, R. erythropolis, R. pyridinivorans and R. rhodochrous (Figure 1a).
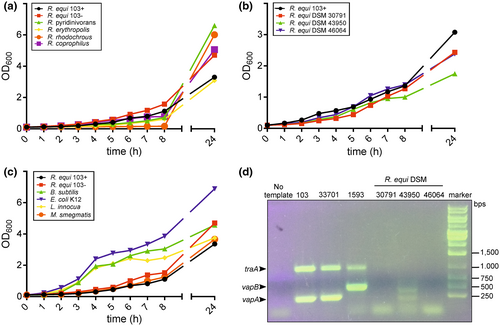
We further selected three environmental isolates of R. equi isolated from places that had likely not been in contact with horses recently, e.g., from a spacecraft-associated clean room (Figure 1b). These were analyzed for the presence of vapA and vapB genes and also for presence of the tra locus which is common to pVAPA, pVAPB and pVAPN (Bryan et al., 2018; Ocampo-Sosa et al., 2007). None of the three bacterial isolates contained such plasmid (Figure 1d). As representatives of non-Rhodococcus species we included avirulent strains of Escherichia coli, Bacillus subtilis, Listeria innocua and Mycobacterium smegmatis (Figure 1c). All these strains exhibited similar growth kinetics with R. equi strain 103+ actually growing slowest.
2.2 Only R. equi grows intracellularly when VapA is added at 37°C
Murine RAW 264.7 macrophage-like cells are frequently used host cells in R. equi virulence studies (Coulson et al., 2010; Rivolta et al., 2022; Vail et al., 2021; von Bargen et al., 2019). Here, RAW 264.7 cells were infected with the selected bacteria so that, on average, one to three bacteria were initially contained per macrophage. As recombinant VapA (rVapA) added to the macrophage growth media can well support intracellular multiplication of a vapA deletion mutant or even plasmidless R. equi 103−, we tested whether this also applied to the above bacterial strains. rVapA was added to the macrophage culture medium 5 h before and during infection. The numbers of robustly infected macrophages, defined as those that contained 10 or more bacteria (Fernandez-Mora et al., 2005; Hondalus & Mosser, 1994; Sangkanjanavanich et al., 2017; Willingham-Lane et al., 2016) were determined at 2 h of infection (just uptake measured) and at 24 h p.i. (multiplication has occurred). Without added rVapA, R. equi 103+ multiplied well whereas 103− did not (Figure 2a). With rVapA added, strain 103− multiplied to almost the same extent as strain 103+ as shown before (von Bargen et al., 2019). rVapA also supported multiplication of the avirulent environmental R. equi isolates (Figure 2a). None of the other tested rhodococci multiplied although R. erythropolis and R rhodochrous were sporadically associated with human disease. Also, neither of the apathogenic bacteria B. subtilis, Listeria innocua, E. coli, or M. smegmatis multiplied significantly in presence or absence of rVapA (Figure 2b). This indicated that blocking phagosome acidification and creating a ‘spacious phagosome’ did not suffice to make the macrophage phagosome a generally hospitable place in line with the observations by Wright et al. (2018) and Sangkanjanavanich et al. (2017) who observed no growth of E. coli in presence of rVapA.
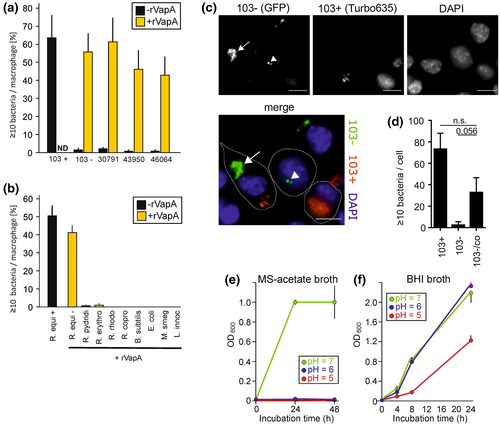
As rVapA added to macrophage culture media can make avirulent R. equi grow, one could expect that coinfection of macrophages with 103+ and with 103− could protect strain 103− from intracellular killing. VapA produced by 103+ could be transported from the 103+ phagosome by vesicular traffic throughout the macrophage (von Bargen et al., 2019) into a 103− phagosome and promote growth there. To test this hypothesis, we infected macrophages consecutively with strains 103+ and 103− to prevent their uptake into the same phagosomes. Strain 103+ was visualized by expression of a Turbo635 red fluorescent protein and strain 103− through expression of green fluorescent protein (GFP). Indeed, strain 103− multiplied significantly in RAW 264.7 macrophages when 103+ bacteria were present in the same cell proving the possibility of cross-protection (Figure 2c,d). Still, there are few reports about coinfection cases with R. equi and other pathogens (Portilho et al., 2019; Shimizu et al., 2010; Simsir et al., 2001) which may be attributable to the fact that only few bacteria can make use of VapA action (Figure 2b) and possibly also due to little diagnostic effort.
As stated above, pH-neutralization of phagosomes and lysosomes seems the major activity of VapA. It is, however, not known how raising the phagosome pH serves R. equi growth. Is it because neutral pH reduces macrophage defense against R. equi, e.g., through inactivation of lysosomal hydrolases or of antibacterial peptides? Or is it because the bacteria themselves do not well resist acidic pH or that they cannot take up nutrients at low pH? In soils, the natural habitat of R. equi, the bacteria survive better at neutral than at acidic pH (Hughes & Sulaiman, 1987). It has been reported that R. equi can resist strong acidification for short times (Benoit et al., 2000) but this does not necessarily translate into the ability for long-term growth in an acidic intracellular environment. Therefore, we analyzed growth of R. equi 103 at pH 5, 6 or 7 in either rich BHI broth or in minimal salt medium supplemented with acetate. We show that R. equi grew robustly in BHI broth at pH 6 and 7 but much less at pH 5 (Figure 2f) which is a typical phagolysosomal pH of macrophages (von Bargen et al., 2019). Yet in acetate-supplemented minimal salt medium which likely models the intra-phagosome environment far better than BHI broth (Kelly et al., 2002; Letek et al., 2010; Waddell & Butcher, 2007), no growth was noted at all at pH 5.0 or 6.0 (Figure 2e). This observation underlined the necessity for a near-neutral pH for R. equi multiplication when certain nutrients are used. This pH-dependency could in itself constitute a compelling reason why R. equi absolutely needs the pH-neutralizing activity of VapA for intracellular growth.
2.3 The environmental bacterium Rhodococcus defluvii has pathogenic potential
Unfortunately, we could not include R. defluvii (P. defluvii), R. equi's closest relative (Anastasi et al., 2016; Sangal et al., 2015), in our screen for intra-macrophage growth because R. defluvii grew extremely slowly at 37°C in rich BHI broth and in minimal salt medium with acetate (Figure 3a). R. defluvii was first isolated from a wastewater bioreactor (Kämpfer et al., 2014) and despite all similarities, forms a separate species from R. equi. This is well illustrated by the observation that R. equi genomes share average nucleotide identity indices of ~99% whereas the index between R. defluvii and R. equi is 83% (Anastasi et al., 2016). The fact that, at 37°C, R. equi 103 grows in BHI broth slower when it carries pVAPA but its growth rate at 30°C is pVAPA-independent (Figure 3a) has been described (Takai et al., 1994). No plasmids or vap genes have been described for R. defluvii. Based on the close relatedness of R. defluvii to R. equi and the ability of avirulent R. equi to multiply in macrophages in the presence of rVapA we reasoned that R. defluvii might be able to grow in macrophages if VapA were present. We set up an experiment in which the first hurdle to pathogenicity, i.e., temperature incompatibility, was bypassed by performing macrophage infections at 30°C whereas the second hurdle, lack of a virulence plasmid, was compensated by adding purified rVapA to infected macrophages.

These infection experiments at 30°C clearly showed that R. defluvii has pathogenic features similar to those of R. equi: R. defluvii without added rVapA did not multiply but did so when rVapA was present although less efficiently than R. equi 103− (Figure 3b,c). Also, vacuoles containing many R. defluvii were often seen at 24 h p.i. (Figure 3c). Similarly, strain 103− did not grow intracellularly in the absence of rVapA but multiplied in its presence (Figure 3b,c), as seen before (Figure 2a). These data show that the macrophages were fully competent to control the avirulent bacteria at the decreased temperature so that the multiplication in presence of rVapA clearly reflects some basic virulence potential. As expected, there was no growth of R. defluvii at 37°C with any experimental setup whereas the R. equi strains did grow at 37°C when VapA was produced by bacteria or added exogenously (Figure 3b,c).
Another, fortuitous and surprising result of this experiment was that strain 103+ multiplied in macrophages during infection at 30°C without added rVapA. Because vapA is not expressed at this temperature in broth culture (Byrne et al., 2001, 2007; Giguère et al., 1999; Kakuda et al., 2014) we had assumed that there would be no growth. We have recently reported that laboratory plasticware can strongly induce vapA expression at 30°C (Hansen et al., 2022) but a possible link to the situation here seemed far-fetched. To define whether intracellular VapA production at 30°C took place in this experiment we infected macrophages with strain 103+ for 24 h either at 30°C or 37°C, lysed the macrophages, produced postnuclear supernatants (lysed cell material without nuclei) and quantified the VapA contents of lysates by immunoblotting (Figure 3d). We observed that actually much less (but still some) VapA was produced intracellularly at 30°C compared to 37°C (Figure 3d). Note that, to our knowledge, these were the first macrophage infection experiments with R. equi cultivated at a temperature which does not induce VapA expression. Apparently, multiplication at 30°C did not require any or only little VapA production which was surprising given prior observations that a low expression level of vapA does not support intra-macrophage multiplication at 37°C (von Bargen et al., 2009). To further investigate this, we tested R. equi strains 103+, 103− and the vapA mutant for their colocalization with LysoTracker© at 24 h p.i. LysoTracker (LT) is a lysosomotropic, membrane-permeable and fluorescent compound that accumulates in compartments that have a pH of roughly ≤6.0 (von Bargen et al., 2009). The results clearly showed that 103+ -containing phagosomes were LT-negative (close to neutral pH) and 103− phagosomes positive (acidic) at 30°C, as they both also were during infection at 37°C (Figure 3e). This provides evidence that the quantities of VapA produced at 30°C suffice to pH-neutralize the phagosome at this temperature and that the defense system is fully capable to deliver strain 103− into acidified phagolysosomes. We propose that for pH-neutralization only the VapA on the bacterial surface and in its direct neighborhood is relevant. During infection at 37°C, large quantities of VapA are released by the bacteria and they travel throughout the macrophage using vesicular transport (von Bargen et al., 2019). At 37°C, this portion of VapA is likely bigger than the bacteria-associated one and causes the strong signal in the immunoblot in Figure 3d. One could argue that possibly other Vap proteins could functionally complement for VapA. But if this was true then phagosomes containing the vapA mutant would have to be negative for LT at 30°C which they are not (Figure 3e) and these mutant bacteria do also not multiply (Figure 3f, values <1.0 indicate killing). Therefore, the relatively low vapA expression at 30°C suffices for multiplication. Why much more VapA is produced and released by the bacteria at 37°C remains to be examined.
Our observations with varied infection temperatures and R. defluvii support the ‘thermal restriction’ hypothesis which states that the establishment of resource-intensive high body temperatures of most homeothermic animals was partly a consequence of the exposition to potentially damaging microorganisms. The temperature therefore established itself at a level which restricts the growth of as many potentially harmful microorganisms as possible. In fact, Casadeval's group showed that more than 4800 tested fungi grow in the lab at 30°C but with every centigrade above 30°C, 6% of the remaining isolates ceased to grow (Robert & Casadevall, 2009). Considering that the body temperatures of foals in the first weeks of their lives is ~38.5°C (Kang et al., 2022), a temperature at which R. equi still grows well (Takai et al., 1994), thermotolerance may be a key reason why R. equi became a pathogen and R. defluvii and similar bacteria did not. There is apparently very little precedence for this observation. The only related observaton of which we are aware is the infection of J774A.1 macrophages with Mycobacterium marinum which is, different from R. defluvii, already a (fish) pathogen. M. marinum has a much shorter generation time at 33°C (4.6 h) versus 37°C (14.3 h) in broth culture and is killed during macrophage infection at 37°C but multiplies intracellularly at 33°C (Ramakrishnan & Falkow, 1994). Similar observations have been made using a mouse foot-pad infection model where mice were kept at different temperatures to vary the foot-pad temperatures (Clark & Shepard, 1963).
It should be noted that a previously reported case of R. defluvii-caused pneumonia in an AIDS patient (Canetti et al., 2019) seems to contradict our data on R. defluvii thermosensitivity. However, this case did not likely involve R. defluvii but R. equi. The original identification was based on the sequence of a 16 S rDNA fragment. This sequence, upon reexamination, was identical not only to R. defluvii but also R. equi sequences (Diana Canetti, San Raffaele University, Milan, Italy, personal communication). Furthermore, our PCR analysis to distinguish between R. equi and R. defluvii based on their 16 S rDNA sequences (see Experimental Procedures) and analysis of growth patterns (data not shown) were consistent with the patient isolate being R. equi rather than R. defluvii. Taken together, R. defluvii is highly unlikely to be the cause of disease in mammals.
2.4 Differential growth of rhodococci in lysosome extracts
R. equi wildtype cells multiply well in purified macrophage lysosome material as their major source of nutrients at neutral pH (von Bargen et al., 2019). This fact was surprising because lysosome contents are generally considered bactericidal (Szulc-Dąbrowska et al., 2020). We have now tested whether rhodococci other than R. equi can also grow in lysosome material to see whether their possible inability to grow in macrophages could be ascribed to killing by lysosomes and/or an inability to use lysosome constituents as nutrients. Lysosomes were purified from macrophages and their identity and activity was confirmed by the observed strong enrichment of the hallmark marker protein of late endosomes and lysosomes, lysosome-associated membrane protein-1 (LAMP-1) in lysosome fractions (Figure 4a). A similarly high degree of enrichment was observed with lysosomal β-galactosidase, as determined through its enzymatic activity (Figure 4b). Furthermore, we confirmed the hydrolytic capacity of purified lysosomes by testing small quantities of lysosome material in a bovine serum albumin (BSA) digestion assay at acidic pH (Figure 4c). BSA is slowly degraded by trypsin from a 69 kDa full-length protein to a 51 kDa digestion intermediate (Markus et al., 1967) before it is degraded completely. Such intermediate was also observed here. The more lysosome extract was mixed with BSA, the more BSA fragment was generated and at 5 μg lysosome protein per assay, full-length BSA and intermediate were almost completely degraded (Figure 4c; compare 0 and 5 μg).
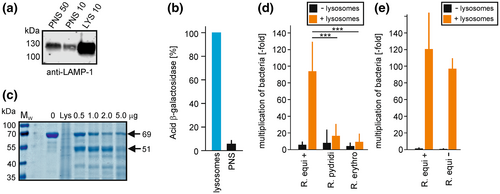
Such lysosomes were lysed by repeated freezing and thawing, incubated with bacteria at 37°C for 24 h (pH 7.2) and bacterial live counts (colony forming units) were determined on nutrient agar at 0 and 24 h p.i. R. equi 103+ grew well on lysosome material at neutral pH, as before (von Bargen et al., 2019) (Figure 4d) and so did avirulent strain 103− indicating that no plasmid genes were required for growth on purified lysosome material (Figure 4e). R. erythropolis and R. pyridinivorans were tested as suitable representatives of the other two Rhodococcus systematic lines (Anastasi et al., 2016). There was only little, statistically insignificant, increase in growth of R. erythropolis and R. pyridinivorans (Figure 4d) in presence of lysosomes at neutral pH. That their live cell numbers did not decrease indicated that both rhodococci were also entirely or largely resistant to lysosomes at neutral pH and that they likely did not profit as much as R. equi from lysosomes as a source of nutrition.
In summary, rhodococci that did not multiply in macrophages at neutral pH also lacked sturdy in vitro growth on lysosome materials possibly due to their reduced ability to use lysosome-derived nutrients such as lipid compounds.
2.5 Initially VapA-less R. equi multiply in macrophages and mice and withstand acidic phagolysosomes before they divide
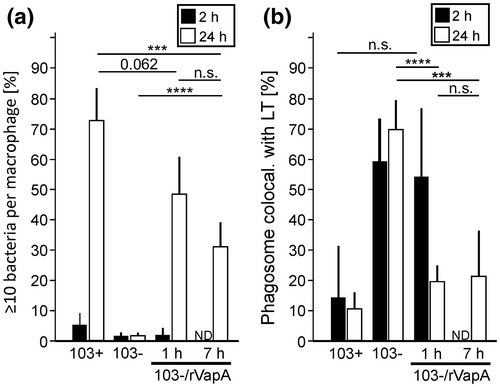
Virulent R. equi do not express VapA when grown at 30°C in brain heart infusion (BHI) broth but do so at 37°C (Byrne et al., 2007) (Figure 7a). We wondered whether R. equi multiplies intracellularly when it does not carry VapA on its surface initially but does produce it during infection. To this end, we quantified intra-macrophage multiplication of strain 103+ after growth in broth culture at 30°C versus 37°C by determining the percentages of robustly infected macrophages. Strikingly, virulent strain 103+ multiplied to the same extent within 24 h regardless of whether it was pre-grown at 30°C or at 37°C. The control strain 103− grown at either temperature did not multiply appreciably within 24 h, as expected (Figure 5a).

Our recent work has indicated that increasing the intraphagosomal pH by VapA action contributes dramatically to virulence of R. equi in macrophages. Therefore, 30°C broth-grown R. equi should be localized to strongly acidified phagosomes where they could be killed. To investigate this further, we analyzed the acidification of phagosomes containing virulent R. equi grown at 37°C using LT. We show that some phagosomes containing virulent R. equi grown at 37°C are acidified during the first minutes of infection but that, at 60 min p.i., only some 20% of phagosomes are positive for LT (Figure 5b). Conversely, at 60 min p.i., 70%–80% of phagosomes containing plasmid-less or vapA-deleted R. equi stained positive with LT (Figure 5b). These data underline the strong pH-neutralizing effect of VapA in early phagosome development.
We now changed the protocol and used for infection bacteria grown at 30°C. These bacteria were void of VapA (Figure 7a) and when they were ingested by macrophages, almost all phagosomes were positive for LT at 2 h p.i. However, at 4 h p.i. and more clearly at 5–6 h p.i., phagosomes containing 103+ were increasingly negative for LT whereas near all other phagosomes remained acidified (Figure 5c,d). To test whether an increasing VapA production by 103+ concomitantly reduced LT staining, we analyzed the kinetics of vapA expression during infection with virulent R. equi grown at 30°C. We observed that the bacteria reliably started to present VapA on their surface by 4–5 h p.i. (Figure 5e) which correlated very well with the loss of LT staining (Figure 5c,d).
Apparently, production of VapA by intracellular R. equi even hours after initiating infection still sufficed to promote intracellular growth. Consequently, R. equi must be quite resistant to acid phagolysosomes for several hours. To test this hypothesis, we used 30°C-grown avirulent R. equi for infection which were initially delivered to an acidic phagolysosome (Figure 5c). We added rVapA at 1 or 7 h after infection to the culture media of macrophages infected with these 30°C bacteria to mimic the delayed intracellular production of VapA which occurs when 30°C-grown virulent bacteria are used for infection. Would late substitution with rVapA still rescue the growth deficiency of VapA-nonproducers? In fact, rVapA added at 1 h or 7 h p.i. did support sturdy growth of avirulent 103−, though less pronounced than when it was added after 7 h (Figure 6a). Considering that we were using an avirulent strain not producing any virulence plasmid-encoded factors (compared to, e.g., a ΔvapA strain) and considering that endocytic uptake, lysosomal deposition of added rVapA and pH neutralization takes some time (von Bargen et al., 2019), this observation indicated that a substantial percentage of avirulent R. equi has remained in a multipliable state for some 10 h. Bacteria that multiplied in this experiment localized to a compartment negative for LT at 24 h p.i. (Figure 6b). As R. equi also resides in a compartment positive for the lysosomal marker protein LAMP-1 (lysosome-associated membrane protein-1) (Fernandez-Mora et al., 2005) this acidification experiment further underlined the resistance of virulent and avirulent R. equi to lysosome contents at acidic pH for several hours.
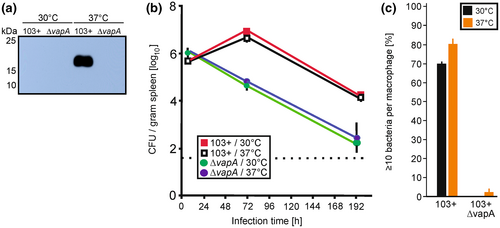
To test whether such lysosome resistance early in infection would also be observed during infection of a whole organism, we grew R. equi 103+ and the isogenic ΔvapA mutant at either 30°C or 37°C before using them in a well-established model of intravenous mouse infection (Pei et al., 2006; Sydor et al., 2013). Under these conditions, the inocula contain VapA only when grown at 37°C (Figure 7a). This mouse model is characterized by an approximately 10-fold increase in bacterial load in liver and spleen between days 1 and 2 and an almost complete elimination by day 8. Apparently, there was no difference in growth and elimination profiles in mice regardless of bacterial cultivation temperature (Figure 7b) just as there was no difference in bacterial multiplication in macrophages (Figure 7c).
3 FURTHER IMPLICATIONS
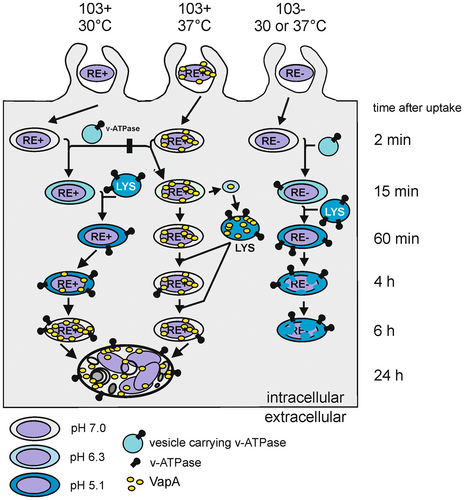
In summary, virulent R. equi, whether they were precultivated at 30°C or 37°C, multiplied to the same degree in macrophages and in mice although the bacteria were localized to acidified (30°C) versus pH-neutralized (37°C) phagosomes during the first hours of infection because of their differential expression of VapA (Figure 8). These conclusions have several important basic and applied implications:
3.1 Patterns of VapA expression during infection
VapA expression is strongly upregulated by temperatures around 37°C and secondarily by acidic pH. When virulent, 37°C-grown R. equi are phagocytosed by macrophages, their phagosomes acidify in the first 15 min to a weakly acidic pH of approximately 6.3, but at 2 h p.i. the phagosomes have returned to neutral pH (von Bargen et al., 2019). Why would vapA expression be co-regulated by acidification if the bacteria encounter only a brief and weak acidification? The apparnt puzzle would be resolved if an infection-relevant environment would be more acidic than pH 6.3–7.2 and the encounter was longer-lasting. We hypothesize that this environment is the phagosome of alveolar macrophages during the initial infection with R. equi from contaminated air. Unfortunately, no data are available on whether virulent R. equi from dust on affected farms (Muscatello, 2012; Muscatello et al., 2006) carry VapA on their surface yet it seems unlikely given soil dryness, temperatures and pH. In this case, virulent R. equi without VapA on their surfaces were inhaled by foals and phagocytosed by patrolling alveolar macrophages. According to our data (Figure 5c), phagosome pH would quickly drop and promote VapA production. Such infection scenario would explain why there was a selective evolutionary force to increase VapA production synergistically by pH 5.5 and at 37°C (Miranda-CasoLuengo et al., 2011; Rahman et al., 2005). After VapA-induced neutralization, strong VapA production would not be required anymore and downregulated due to the increased pH. To some degree, the data might also hint that soil-to-foal transmission of R. equi is a more relevant natural transmission pathway than foal-to-foal transmission (Muscatello et al., 2009). Coughed-up bacteria from a ~37°C lung environment would already express vapA and be quickly delivered to a pH-neutralized compartment (Figure 5b) and so, expression regulation by low pH would seem unnecessary.
3.2 Vaccination studies
Most vaccination studies to combat R. equi infection have used VapA-derived antigens such as vapA DNA, purified VapA, parts of VapA or VapA-enriched bacterial extracts (Giles et al., 2015). To challenge vaccinated animals, R. equi were in most if not all relevant studies cultivated at 37°C, so the bacteria had copious quantities of VapA on their surface and vaccination-induced antibodies in challenged animals had plenty of opportunity to bind to the bacteria and opsonize them before they were taken up by phagocytes. Opsonization on the other hand can support eradication of R. equi (Dawson et al., 2011; Sanz et al., 2014). As pointed out above, it is unlikely that virulent R. equi in dry soil or dust contain VapA. Therefore, would enhanced elimination after vaccination also be seen in a natural setting where the bacteria do initially not express VapA and the antibodies could not bind? To address this important point, future trials of promising (VapA-based) vaccines should incorporate challenge experiments with bacteria grown at 30°C or less in their protocol as they might better reflect the natural situation.
3.3 R. equi can persist for hours in an acidic phagolysosome
There are reports, particularly from in vivo experimental infections that pathogenic mycobacteria, such as Mycobacterium tuberculosis, can grow in acidified phagosomes or even phagolysosomes (Gomes et al., 1999; Gouzy et al., 2021; Levitte et al., 2016; Sundaramurthy et al., 2017) although there had been several earlier reports that provided convincing data that the bacteria inhabit a privileged compartment at pH 6.3 (Russell, 2001; Wong et al., 2011). R. equi, closely related to mycobacteria, may present a similar situation of inhabiting both types of compartments in different settings. Our data on resistance of R. equi to lysosome contents in vitro and the fact that even at pH 5.1, R. equi shows very little, but some, growth in lysosome contents in vitro (von Bargen et al., 2019) agree with the idea that the major reason why R. equi do not thrive in an acidic phagolysosome is their incapability to obtain or metabolize nutrients at this pH. VapA secretion removes this hurdle. This lifestyle discriminates R. equi from the two classical intracellular pathogens that grow in an (acidified) phagolysosome-like compartment, Coxiella burnetii (Pechstein et al., 2018) and Leishmania spp. (Saunders et al., 2021).
On a self-critical note, we had previously reported that R. equi arrests phagosome maturation at a stage before the phagolysosome (Fernandez-Mora et al., 2005). The deviation from our current view is likely due to the fact that much of our previous work looked at infection in the first two hours whereas in our recent studies we also address longer infection times. Furthermore, we had previously considered compartments only as phagolysosomal when they contained the classical lysosome markers throughout the vacuole. Now we understand for example why the paradigm lysosomal protease cathepsin B is absent from the R. equi vacuole which would erroneously indicate that it was not a phagolysosome: Cathepsin B is highly unstable at the neutral pH in this compartment and degraded (data not shown). We showed in our previous work (Fernandez-Mora et al., 2005) correctly that vacuoles containing virulent R. equi are non-acidified although LAMP-1 was present on the vacuoles at 2 and 24 h of infection similar as is the case with M. tuberculosis (Russell, 2001). We concluded correctly that these are not features of a functional phagolysosome. But taking together the recent data in von Bargen et al. (2019) and here we summarize that the R. equi-containing vacuole is an atypical, pH-neutral kind of phagolysosome. Such neutralized phagolysosome has also been proposed by Toyooka et al. (2005) although the causal link between pH-neutralization and bacterial multiplication was not tested or shown at the time.
3.4 Preadaptations and R. equi virulence
A hypothesis emerges that R. equi is a soil bacterium with many preadaptations (also called exaptations or co-opted features) which we define here as the possibility of a characteristic to adopt a new biological function without evolutionary modification (Ardila, 2016). Such preadaptations include the predilection for fatty acid-like compounds and short organic acid nutrients, particularly acetate and lactate (Hughes & Sulaiman, 1987; Letek et al., 2010; Vázquez-Boland & Meijer, 2019). Furthermore, the possession of a thick and partly hydrophobic cell wall (Sutcliffe, 1997) which likely supports the resistance of the bacteria to lysosome contents even at acidic pH as seen here. We speculate that ‘early’ virulent R. equi may have caused occasional disease in immunosuppressed animals just as it still does in immunocompromised persons but became a dedicated pathogen for foals once it had horizontally acquired the Vap protein-encoding pathogenicity island (Letek et al., 2008), the VapA secretion signal, and the ‘VapA boosters’ virR and virS. Once VapA increased phagosomal pH, the regulators for strong VapA production were likely positively selected with every round of infection, and eventually resulted in a pH-neutral phagosome which maximally fostered bacterial multiplication.
4 EXPERIMENTAL PROCEDURES
4.1 Strains, cell lines, plasmids, chemicals
Bacterial isolates used in this study are listed in Table 1. The bacteria were grown in brain heart infusion broth (BHI, Becton Dickinson) or in minimal salts medium as in Ashour and Hondalus (2003) with 100 mM sodium acetate as carbon source, for 16 h at 180 rpm and 30°C in a rotary shaker (if not indicated otherwise). MgSO4 and thiamine for media were sterile filtered. Tryptone Soy Agar (No. CP70.1) and Luria Broth (LB) medium (No. X964.1) were from Carl Roth (Karlsruhe, Germany). Medium pH was adjusted with HCl or KOH. RAW 264.7 (American Type Culture Collection; clone TIB-71) and J774E clone (Diment et al., 1987) macrophage-like murine cell lines were from Hubert Hilbi (University of Zürich, Switzerland) and Philip D. Stahl (Washington University, St. Louis, USA), respectively. These cells were cultivated in phenol red-free Dulbecco's Modified Eagle's Medium (DMEM; ThermoFisher Scientific, No. 31053028 or PAN-Biotech, No. P04-01161) with 2 mM GlutaMAX (ThermoFisher Scientific, cat. no. 35050061 or PAN-Biotech, No. P04-82100) and 10% calf serum (Sigma-Aldrich, No. F7524 or PAN-Biotech, No. P-30-3306) at 37°C in 5% CO2 atmosphere. Plasmids used were pCharge3 expressing Turbo-635 from Tanya Parish, Seattle (Carroll et al., 2010), USA, and pSC301 expressing green fluorescent protein (GFP) from Yossef Av-Gay, University of British Columbia, Canada (Cowley & Av-Gay, 2001) (agar media, 100 mg/L hygromycin, liquid media, 50 mg/L). R. equi containing these plasmids grow in BHI broth with the same kinetics as the non-recombinant strain. All standard chemicals were of p.a. quality. Immunoblotting was as in von Bargen et al. (2019), rat-anti-mouse-lysosome associated membrane protein-1 clone 1D4B (sc-19992) and anti-VapA mouse monoclonal were from Santa Cruz Biotechnology (sc-390576). Immunoblot development was with chemiluminscence (SuperSignal West Pico PLUS from ThermoFisher Scientific, No. 34579) and X-ray films. Ponceau staining was done with a brief additon of 0.1% Ponceau S in 5% acetic acid followed by decoloring in water.
| Name | Description | Source | Reference |
|---|---|---|---|
| Bacillus subtilis ΔspoIIGA | Non-sporulating mutant of strain AS3 | T. Schweder, University of Greifswald, Germany | Junne et al. (2011), McMinn et al. (2000) |
| Escherichia coli DH5α | K12-derivatized laboratory safety strain | ThermoFisher Scientific (order no. 18265017) | ThermoFisher Scientific |
| Listeria innocua serotype 6b | DSM 27575; ATCC 13932b | Department of Microbiology, Würzburg, Germany | DSMZa |
| Micrococcus luteus | DSM 20030; ATCC 4698b | DSMZa | DSMZa |
| Mycobacterium smegmatis mc2 155 | ATCC 700084b | G. Plum, University of Köln, Germany | McGuire et al. (2012) |
| R. coprophilus CUB1054T | Lake mud, Yorkshire, UK | A.C. Ward, University of Newcastle upon Tyne, UK; CUBb | McMinn et al. (2000) |
| R. defluvii Ca11(T) | DSM 45893; Isolate from bioreactor wastewater; type strain | S.P. Gläser, University of Giessen, Germany and DSMZ | Kämpfer et al. (2014) |
| R. equi 103+ | Isolated in 1979 from a pneumonic foal in Canada | J. Prescott, University of Guelph, Canada | de La Peña-Moctezuma and Prescott (1995) |
| R. equi 103+ ΔvapA | Strain 103+ with a targeted deletion in the vapA gene | This lab | von Bargen et al. (2009) |
| R. equi 103− | Obtained by curing strain 103+ from the virulence plasmid. | This lab | von Bargen et al. (2019) |
| R. equi DSM 30791 | Isolated from spacecraft-associated clean room class ISO 8, Herschel Space Observatory, French Guinea | DSMZa | DSMZa |
| R. equi DSM 43950 | Isolated from soil, New South Wales, Australia, ATCC 25694 | DSMZa | DSMZa |
| R. equi DSM 46064 | Isolated before 1979 from soil, country unknown; ATCC 25734b | DSMZa | DSMZa |
| R. equi PAM1593 | Isolated from an AIDS patient |
M. Gobernado, University Clinic La Fe, Valencia, Spain |
Ocampo-Sosa et al. (2007) |
| R. erythropolis DSM 43066 | Soil isolate, ATCC 4277 (type strain)b | DSMZa | DSMZa |
| R. pydridinivorans AK37 | Pyridine-degrading strain from crude-oil contaminated site in Hungary | J. Kukola, Szent-István University, Gödölló, Hungary | Kriszt et al. (2012) |
| R. rhodochrous DSM 43269 | Sampled before 1990, country of origin unknown | DSMZa | DSMZa |
- a Deutsche Sammlung Mikroorganismen und Zellkulturen (Braunschweig, Germany).
- b American Type Culture Collection (Manassas, VA, USA).
4.2 Bacterial growth experiments
For bacterial growth experiments in Figure 1, BHI broth or minimum salt medium with 100 mM sodium acetate as a carbon-source were incubated overnight at 250 rpm and 37°C. Precultures were diluted to a final OD600 of 0.05 (BHI) or of 0.1 (minimal media) and shaken at 250 rpm at 37°C for the indicated times. Aliquots were taken, the OD600 determined and plotted. Growth of R. equi at different pH values was determined from BHI and minimal salt/acetate cultures, adjusted to the indicated pH values taking either HCl or KOH. To this end, R. equi was precultivated for 16 h at 37°C and 250 rpm in the respective medium at pH 7.0, to obtain sufficient quantities. Bacteria were separated by centrifugation at 6200× g for 5 min, ODs600 were determined and the bacteria resuspended in the respective-pH medium at the desired final OD600. Cultures were then shaken in 50 ml flasks and OD600 was determined at the indicated times. Potential growth in lysosome-containing minimum salt medium was tested as in von Bargen et al. (2019). Briefly, lysosomes were prepared as in Becken et al. (2010) and 5 mg (final concentration) lysosome protein/ml minimal salt solution was mixed with PBS-washed bacteria in a final 8 μl culture volume. Purified lysosomes were lysed by three fast freeze (−80°C) and thaw (37°C) cycles. Incubation of the bacteria-lysosome mix was in microtubes in a tempered chamber with slow shaking for 24 h at 37°C. Dilution series were prepared in PBS at 0 and 24 h of infection, plated onto 0.5× Luria Broth (LB) medium (Carl Roth, X964.1), and colonies counted after 24 h at 37°C.
4.3 Polymerase chain reaction (PCR)
All R. equi strains were checked by multiplex colony PCR for the potential presence of virulence-associated plasmid. A small loopful of R. equi was taken from an overnight BHI agar culture, resuspended in 50 μl distilled water, and heated at 95°C for 10 min and centrifuged at 16,250× g in a reaction tube centrifuge. Of this, 1–2 μl were used as template. The primers were Traf-F1 and Tra-F2, pvapAF, VapAB and pVapABR as published (Ocampo-Sosa et al., 2007). The annealing temperature was 56°C, the elongation time 2 min. The following reactions were done as instructed in the Solis BioDyne (Tartu, Estonia) FIREPOL master mix RTL protocol.
To discriminate R. equi from R. defluvii (Ca11), we set up a new diagnostic PCR assay based on 16 S rDNA sequences (GU585589.1 for R. equi, NR_126278.1 for R. defluvii). Bacteria were grown on BHI agar plates at 30°C. For colony PCR, single bacterial colonies were suspended in 50 μl sterile aqua dest. and heated to 95°C for 5 min. Samples were centrifuged at 17,000× g for 5 min and the supernatant was used as template for analytical PCR. Oligonucleotide primers from Eurofins (Ebersberg, Germany) were used in all PCR reactions which were run with a constant reverse primer 16SrRNArev: 5′-GGAAGGAAACCCACACCTAGC-3′. In combination with the primer 16 S_rRNA_fw: 5′-GATAAGCCTGGGAAACTGGGTC-3′, R. equi yielded a 679 bp and R. defluvii a 675 bp product. In combination with the primer 16SrRNAequifw (5′-GATATGAGCTCCTGTCGCATG-3′), R. equi DNA generated a 646 bp fragment and R. defluvii DNA none, whereas in combination with the primer 16SrRNAdefluviifw (5′-CCTTGGACTGCATGGTTCTTGG-3′) a 642 bp fragment was generated with R. defluvii but none with R. equi DNA.
4.4 Macrophage infection experiments
J774E or RAW 264.7 macrophages were infected as in von Bargen et al. (2019) at a multiplicity of infection (MOI) of 3 except for R. defluvii (MOI = 3), B. subtilis (MOI = 5) and L. innocua (MOI = 10) to reach a similar level of infection. In brief, infections were for 30 min at 37°C, followed by a gentamicin pulse and incubation for a further 2 h or 24 h. DNA (bacteria and macrophages) was stained with 1 μM SYTO13 (ThermoFisher No. 57575) for 10 min at ambient temperature. Numbers of SYTO13-positive bacteria in infected macrophages were determined as in von Bargen et al. (2019) using an Axio Observer.Z1 (Zeiss) fluorescence microscope with a 100× or 63×/1.4 plan apochromate oil immersion objective. Pictures were taken using the manufacturer's Axio Vision 4.8.1. software. For quantification of multiplication, at least 50 infected macrophages were analyzed per experiment and per sample and the percentages of infected macrophages with 10 or more bacteria was plotted (Hondalus & Mosser, 1994; von Bargen et al., 2019). In the case of R. defluvii, live cell determination was not an option due to heavy clumping of intracellular bacteria. We determined the numbers of intracellular bacteria microscopically using SYTO13 for bacterial numbers up to 20. Beyond 20, numbers could not be reliably determined and in this case, the number was set as 21. In these experiments, all bacteria were grown at 30°C due to the thermosensitivity of R. defluvii. In rVapA supplementation studies, sterile-filtered recombinant VapA (rVapA) was generated, purified and used for macrophage feeding experiments as described in von Bargen et al. (2019). In the microscopic coinfection experiment, DNA was stained using 4,6-diamidino-2-phenylindole (DAPI; Sigma Aldrich No. D9542) using 0.2 μg/ml. LysoTracker staining was done as in von Bargen et al. (2019). For the experiments in Figure 3C, macrophage cultures on glass slides in 24-well plates were taken from a 37°C/5% CO2 incubator, the media discarded and fresh DMEM medium at ambient temperature was added. All R. equi in this experiment were pre-grown in BHI at 30°C. These bacteria were split between ‘30°C infected macrophages’ and ‘37°C infected macrophages’ which were further cultivated at 30°C/5% CO2 or 37°C/5% CO2. were then infected using bacteria that had been grown by 30°C and multiplication was quantified as before (von Bargen et al., 2019). Live cell determination assays as in Figure 3f were performed as in von Bargen et al. (2009) except that the bacteria were grown at 30°C and the infections were at 30°C and 37°C for 2 h (no multiplication yet) and 24 h (some multiplication has occurred) and the resulting live cell counts were divided through each other to determine the multiplication or killing factor.
Mouse infection experiments were done as previously (Sydor et al., 2013) according to the German Animal Protection Law and approved by the North-Rhine-Westphalia State Agency for Nature, Environment and Consumer Protection. Briefly, four mice (BALB/c mice from Charles River) per sample type were intravenously injected with 5 × 105 bacteria each as indicated, the mice sacrificed, the spleens removed and analyzed for live bacteria counts (colony forming units) at 2, 72 and 192 h using 0.5 × LB medium agar plates and incubation of plates at 37°C for 24 h before counting.
4.5 Quantifying VapA expression in macrophages
R. equi 103+ was grown in BHI broth at 30°C for 16 h and used to infect RAW 264.7 cells at an MOI of 20 for 30 min at 30°C or 37°C, as indicated. Cells were washed and further incubated for 23.5 h. Control samples remained uninfected. RAW 264.7 cell lysates were prepared in RIPA buffer (25 mM Tris/HCl (pH 8.0), 150 mM NaCl, 0.5% (w/v) dodium deoxycholate, 1% (v/v) nonidet P-40, 10% (v/v) glycerol, 2 mM EDTA, 1 μM leupeptin, 0.6 mM 1,10-phenanthroline, 2 μM pepstatin, 2 μM E-64, 1 mM pefabloc). Lysates were centrifuged at 4°C for 30 min at 21,000× g and supernatant equivalents of 50 μg protein were applied per lane. Lysates were blotted against anti-VapA (bottom) or against anti-LAMP-1 (top) as a loading control. As a VapA-detection control, R. equi 103+ was incubated in BHI medium for 24 h at 200 rpm and 30°C or 37°C and 0.05 OD600 units bacteria heated for 5 min at 95°C in sample buffer per lane.
4.6 BSA digestion assay
Lysosomes were prepared from J774E macrophages using a magnetic method as described (Becken et al., 2010), lysed by three fast freeze (−80°C) and thaw (37°C) cycles, and incubated with the indicated quantities of bovine serum albumin fraction V (BSA; Carl Roth No. 0163.4) in 75 mM citrate buffer (pH 4.5) for 30 min at 37°C and the digestion products were analyzed in SDS-10% polyacrylamide gels with colloidal Coomassie staining (Dyballa & Metzger, 2009). (Lys) describes a ‘postnuclear supernatant’, i.e. cell lysate after separation from unlysed cells and whole nuclei.
4.7 Protein analysis
Protein concentrations were determined using a Bradford assay (Bio-Rad, Munich, No. 5000001) with bovine serum albumin as a standard. Acid β-galactosidase was determined as in Lührmann and Haas (2000) using 6 μg protein from the postnuclear supernatant (PNS) or purified lysosome preparation (Becken et al., 2010). For lysosomes, the product was lysed by addition of 1/40 volume 20% triton X-100 and incubation at room temperature for 5 min. Ferrofluid (Ferrotec, Santa Clara, CA, USA; No. EMG508) which was used to purify lysosomes magnetically, was removed by centrifugation at 16,000× g for 5 min at 4°C and the protein concentration in the lysed material was determined from the supernatant. Gel electrophoresis, Western blotting and Coomassie staining were done as in Becken et al. (2010).
4.8 Statistics
p-values, were indicated, were calculated for selected pairs using a two-tailed heteroscedastic t-test. Either the absolute p-value is indicated or p indicated as * < 0.05; ** < 0.01; *** < 0.005; **** < 0.001.
AUTHOR CONTRIBUTIONS
Thomas Haubenthal: Conceptualization (supporting); Investigation (lead); Visualization (equal); Formal analysis (equal); Writing - review & editing (supporting). Philipp Hansen: Conceptualization (supporting); Investigation (lead); Visualization (equal); Formal analysis (equal); Writing - review & editing (supporting). Ina Krämer: Investigation (equal); Formal analysis (equal). Mélanie Gindt: Investigation (equal); Formal analysis (equal). Alexandra Jünger-Leif: Investigation (equal); Formal analysis (equal). Olaf Utermöhlen: Conceptualization (supporting); Investigation (equal); Formal analysis (equal). Writing - review & editing (supporting). Albert Haas: Conceptualization (lead); Funding acquisition (lead); Writing - first draft (lead); Writing - review & editing (lead); Visualization (equal); Formal analysis (equal); Supervision (lead); Resources (lead).
ACKNOWLEDGMENTS
We are grateful to Anna Kinzer (Bonn) and Ulrike Karow (Cologne) for expert technical assistance, to our colleagues for kindly sending us mammalian and bacterial cells and plasmids, and to the Deutsche Forschungsgemeinschaft (DFG) for financial support through grant HA 1929/14-1. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
Bacterial strains will be made available upon reasonable request. Mouse infection experiments were done according to the German Animal Protection Law and approved by the North-Rhine-Westphalia State Agency for Nature, Environment and Consumer Protection.
Open Research
DATA AVAILABILITY STATEMENT
Data will be provided upon reasonable request.