Type IV secretion systems: Advances in structure, function, and activation
Abstract
Bacterial type IV secretion systems (T4SSs) are a functionally diverse translocation superfamily. They consist mainly of two large subfamilies: (i) conjugation systems that mediate interbacterial DNA transfer and (ii) effector translocators that deliver effector macromolecules into prokaryotic or eukaryotic cells. A few other T4SSs export DNA or proteins to the milieu, or import exogenous DNA. The T4SSs are defined by 6 or 12 conserved “core” subunits that respectively elaborate “minimized” systems in Gram-positive or -negative bacteria. However, many “expanded” T4SSs are built from “core” subunits plus numerous others that are system-specific, which presumptively broadens functional capabilities. Recently, there has been exciting progress in defining T4SS assembly pathways and architectures using a combination of fluorescence and cryoelectron microscopy. This review will highlight advances in our knowledge of structure–function relationships for model Gram-negative bacterial T4SSs, including “minimized” systems resembling the Agrobacterium tumefaciens VirB/VirD4 T4SS and “expanded” systems represented by the Helicobacter pylori Cag, Legionella pneumophila Dot/Icm, and F plasmid-encoded Tra T4SSs. Detailed studies of these model systems are generating new insights, some at atomic resolution, to long-standing questions concerning mechanisms of substrate recruitment, T4SS channel architecture, conjugative pilus assembly, and machine adaptations contributing to T4SS functional versatility.
Graphical Abstract
Type IV secretion systems (T4SSs) are a functionally diverse superfamily of translocation nanomachines deployed by most species of bacteria. Collectively, T4SSs mediate the transfer of mobile genetic elements or protein toxins to other bacteria, or effector proteins or other macromolecules to eukaryotic cells to aid in infection. Recent structural and fluorescence approaches are generating exciting new insights into how T4SSs assemble in the cell envelope and recruit and translocate substrates to other cells.
1 INTRODUCTION
Many bacterial species deploy Type IV Secretion Systems (T4SSs) to deliver DNA, protein, or other macromolecules to bacterial or eukaryotic cell targets (Li et al., 2019; Waksman, 2019). The T4SSs are composed mainly of two subfamilies, the conjugation systems and effector translocators (Cascales and Christie, 2003). Conjugation systems are of considerable medical concern for their roles in dissemination of mobile genetic elements (MGEs), often encoding resistance to heavy metals or antibiotics (Cabezon et al., 2015; Huddleston, 2014; Koraimann, 2018). The effector translocators mainly deliver proteins to eukaryotic target cells, although recent studies have also documented the interkingdom transfer of DNA, peptidoglycan, or other macromolecules (Bleves et al., 2020; Cover et al., 2020; Grohmann et al., 2018). Many effector translocator systems are integral to the virulence of Gram-negative pathogens, and there is recent evidence for their deployment by Gram-positive pathogens (Grohmann et al., 2018; Jiang et al., 2016). Conjugation systems and effector translocators typically require direct cell-to-cell contact; however, a few T4SSs function independently of target cell interactions to import exogenous DNA or export DNA or proteins to the milieu (Alvarez-Martinez and Christie, 2009; Koch et al., 2020; Stingl et al., 2010).
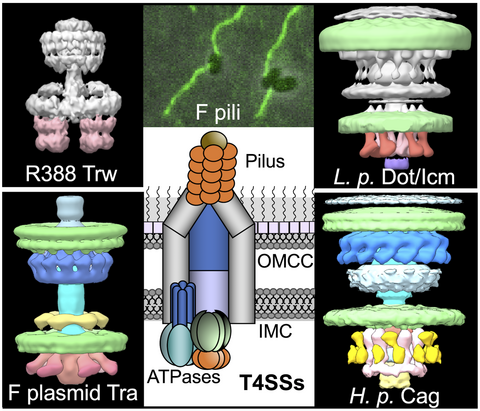
Recently, there has been exciting progress in defining the architectures and assembly pathways of the T4SSs. Structural studies of T4SSs began about 20 years ago with reports of X-ray structures of a few highly conserved T4SS subunits (Gomis-Ruth et al., 2001; Savvides et al., 2003; Terradot et al., 2005; Yeo et al., 2000). In the ensuing 10 years, larger (~1 megadalton; MDa) subassemblies from the R388 and pKM101 plasmid conjugation systems were solved by single-particle electron microscopy and crystallography (Chandran et al., 2009; Fronzes et al., 2009). In just the past few years, however, advances in electron microscopy have enabled visualization of larger subassemblies in isolation (Low et al., 2014; Rivera-Calzada et al., 2013) and of intact T4SSs in the native context of the bacterial cell envelope (Chetrit et al., 2018; Ghosal et al., 2017; Ghosal et al., 2019; Hu et al., 2019a; Hu et al., 2019b). These approaches, coupled with state-of-the-art fluorescence microscopy, have yielded new insights into T4SS assembly dynamics and spatial organization in intact cells (Chetrit et al., 2018; Ghosal et al., 2019; Jeong et al., 2017; Park et al., 2020). In this review, we will summarize the recent progress in structural definition of several model T4SSs functioning in Gram-negative bacteria, including the Xanthomonas spp. VirB/VirD4, Helicobacter pylori Cag, Legionella pneumophila Dot/Icm, and F plasmid-encoded Tra systems (Figure 1). At the outset, we acknowledge that a full understanding of the structural and functional diversity of T4SSs will require continued study of systems not covered in this review, such as those functioning in Gram-positive bacteria or obligate intracellular pathogens, machines that acquire substrates from the periplasm, and systems adapted for macromolecular import or export (Bhatty et al., 2013; Bleves et al., 2020; Grohmann et al., 2018).
1.1 T4SS substrates and translocation signals
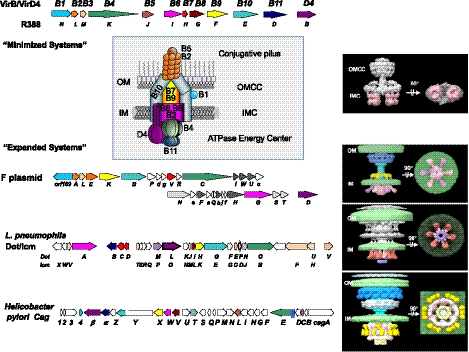
The T4SSs are minimally composed of a set of conserved subunits (Alvarez-Martinez and Christie, 2009; Grohmann et al., 2018). In Gram-negative bacteria, ~12 subunits are needed to build fully functional “minimized” systems (Bhatty et al., 2013). These are designated VirB1 through VirB11 and VirD4, according to a unifying nomenclature for the T4SS superfamily from the paradigmatic Agrobacterium tumefaciens VirB/VirD4 T4SS (Figure 1) (Alvarez-Martinez and Christie, 2009). Three ATPases (VirD4, VirB4, and VirB11) comprise the cytoplasmic energy center, which is situated at the base of the translocation channel. Of these ATPases, VirD4 serves as the receptor to which both DNA and protein substrates bind prior to entry into the translocation channel (Alvarez-Rodriguez et al., 2020b; Llosa and Alkorta, 2017). The channel itself consists of two large subassemblies, one spanning the inner membrane (IM) and the second situated in the periplasm and outer membrane (OM) (Christie et al., 2005; Low et al., 2014). The IM complex (IMC) consists minimally of VirB3, VirB6, VirB8, and the N-terminal region of VirB10 (Low et al., 2014). The VirB4 ATPase, which stably associates with the channel is also considered to be part of the IMC (Bleves et al., 2020; Low et al., 2014). The IMC connects via a stalk or cylinder to the outer membrane core complex (OMCC), which consists minimally of the lipoprotein VirB7, VirB9, and a C-terminal domain of VirB10 (Christie et al., 2005; Low et al., 2014).
The T4SS channel delivers substrates across the cell envelope, but it must first recruit these substrates to the cytoplasmic entrance. Recruitment of MGEs to cognate conjugation or “mating” channels is initiated by a set of processing factors termed DNA transfer and replication (Dtr) proteins, which bind the origin-of-transfer (oriT) sequence to form the relaxosome (Cabezon et al., 2015). The relaxosome, specifically the relaxase component, nicks the DNA strand destined for transfer (T-strand) and remains covalently bound to its 5′ end usually via a catalytic Tyr residue (Guzman-Herrador and Llosa, 2019). Recently, the interaction of the F plasmid-encoded TraI relaxase to its oriT target sequence was solved at atomic resolution (Ilangovan et al., 2017; Waksman, 2019). Notably, TraI harbors two translocation signals (TSs) similar in sequence to TSs carried by relaxases functioning in R388, R6112, and pKM101 plasmid transfer (Alperi et al., 2013; Ilangovan et al., 2017; Meyer, 2015; Redzej et al., 2013). These signals, designated TSA and TSB (Translocation Signals A and B), are not positioned at the N or C termini of F-encoded TraI or the related relaxases, as is characteristically the case for secreted proteins (Christie, 2019). Rather, the two TSs map to the C-proximal helicase domain, specifically, within the 2B/2B-like subdomains of an SF1A/B helicase structural fold (Ilangovan et al., 2017). These TSs likely contribute to binding of the relaxosome to cognate VirD4 receptors, also termed type IV coupling proteins (T4CPs) (Alvarez-Rodriguez et al., 2020b; Llosa and Alkorta, 2017), but details of these interactions are not yet available.
Contacts between other Dtr factors and cognate VirD4 subunits are currently better understood, in one case at atomic resolution. In the F system, the Dtr factor TraM binds the F plasmid oriT sequence and recruits the TraI relaxase to build the relaxosome (Lu et al., 2008; Wong et al., 2012). A crystal structure revealed that TraM is a member of the Ribbon–Helix–Helix (RHH) superfamily of DNA-binding proteins and that the RHH domain binds-specific sequences (sbm) within the oriT sequence (Peng et al., 2014; Wong et al., 2011). Upon binding, TraM induces a bend in oriT to allow access of the TraI relaxase to the nic site (Wong et al., 2011, 2012). TraM also has a C-terminal α-helical domain, which is involved inhomotetramerization, and it also specifically binds the C-terminal 13 residues of the VirD4-like receptor TraD (Lu et al., 2008). The TraM–TraD interaction thus forms a basis for recruitment of the cognate F plasmid to the F-encoded Tra (hereafter designated TraF) T4SS (Lu et al., 2008). Many conjugation systems of both Gram-negative and -positive species encode Dtr factors belonging to the RHH superfamily (Li and Christie, 2020; Rehman et al., 2019; Varsaki et al., 2009; Yoshida et al., 2008). Interestingly, however, deletion of the C-terminal helical domains of two such factors, TraK and PcfF, does not abolish transfer of pKM101 and pCF10, respectively, establishing that the RHH domain suffices for relaxosome assembly and at least a low level of substrate transfer (Li and Christie, 2020; Rehman et al., 2019). Taken together with other findings (see de la Cruz et al., 2010; Llosa and Alkorta, 2017), the current picture is that numerous relaxosome constituents, including the relaxase, Dtr factors, and even translocated DNA, must bind the VirD4 T4CP for specific and efficient conjugative DNA transfer.
As we await further details of relaxosome–VirD4 interactions, there has been important progress toward definition of TSs conferring recognition of protein substrates by effector translocator systems. Early on, two types of TSs were identified at the extreme C terminus, one composed of clusters of positively charged and a second of hydrophobic residues (Nagai et al., 2005; Vergunst et al., 2000, 2005). Many effectors carry such TSs, but in fact there is considerably variability among TSs recognized by different effector translocators, as illustrated by the following. First, in 2015, C. Farah and colleagues discovered a subfamily of T4SSs in Xanthomonas citri that promotes killing of neighboring bacteria through the translocation of protein toxins (Souza et al., 2015). All of these effector toxins carry C-proximal TSs consisting of a ~120 residue motif termed XVIPCD (Xanthomonas VirD4-interacting protein conserved domain) (Sgro et al., 2019). As implied by the name, effectors of the X. citri VirB/VirD4 T4SS were originally identified in screens for proteins that bind VirD4. As a result of bioinformatics screens, however, the list of candidate effectors bearing XVIPCD motifs exceeds several hundred; this list is dispersed among many species in the order Xanthomonadales and other proteobacteria (Sgro et al., 2019). The XVIPCD is characterized by a few conserved motifs in the N-terminal region and a Gln-rich C-terminal region, but the nature of the effector–VirD4 interaction is not yet specified.
Second, in Bartonella spp., effector proteins termed Beps (Bartonella effector proteins) are delivered through a VirB/VirD4 T4SS into eukaryotic cells during infection. Beps have a C-proximal TS termed the BID (Bep intracellular delivery) domain (Siamer and Dehio, 2015). BID domains are sequence-variable but adopt a conserved structural fold consisting of an extended four-helix bundle, which is intriguingly reminiscent of the C-terminal α-helical domains carried by TraM and other Dtr proteins discussed above (Stanger et al., 2017; Wagner et al., 2019). This structural fold is also implicated in VirD4 binding, yet, interestingly a given effector can have multiple BID domains distributed throughout the Bep. Furthermore, BID domains can confer effector function in the eukaryotic host cell by binding and altering protein functions (Siamer and Dehio, 2015; Stanger et al., 2017). The BID1 domain of BepA, for example, enhances proliferation of human endothelial cells by inhibiting apoptosis through binding of the catalytic subunit C2 of human adenylyl cyclase to potentiate cAMP production (Stanger et al., 2017). At least some BID domains thus have evolved dual roles as TSs for recognition by VirD4 receptors and as effectors through binding of eukaryotic cell target(s) to aid in infection.
Finally, early studies of the L. pneumophila Dot/Icm T4SS identified a number of translocated effectors that harbor C-terminal TSs composed of short polar and negatively charged amino acids (Burstein et al., 2009; Kubori et al., 2008; Nagai et al., 2005). A combination of bioinformatics and experimental approaches further identified at least 100 Dot/Icm effectors bearing clusters of glutamate residues (the E block motif) within 17 to 10 residues from the C terminus and one or more hydrophobic amino acids nearer the terminus (Huang et al., 2011). However, translocation of some effectors with the E block motif, as well as other effectors lacking this motif, can be modulated positively or negatively by adaptor proteins such as IcmS and IcmW (Burstein et al., 2016; Cambronne and Roy, 2007; Lifshitz et al., 2013). Collectively, these findings led to a proposal that Dot/Icm effectors engage with the T4SSDot/Icm in at least one of three ways, via the E block motif, through a combination of this motif and binding of adaptors, or by binding adaptors and possibly a TS other than the E block motif (Lifshitz et al., 2013). Exciting advances discussed further below have now described the structural bases underlying recruitment of potentially large numbers of effectors to the T4SSDot/Icm channel via specific contacts with one or more adaptor proteins.
1.2 VirD4 receptors and substrate docking
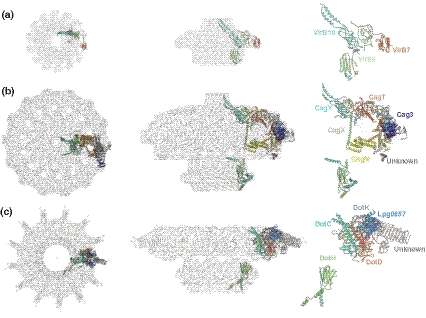
VirD4 receptors are associated with nearly all T4SSs; the few systems that lack VirD4 subunits appear to function exclusively in elaboration of antigenically variable pili or to export substrates recruited to the T4SS from the periplasm (Alvarez-Martinez and Christie, 2009; Llosa and Alkorta, 2017). R388-encoded TrwB is the structural archetype for the T4CP superfamily (Gomis-Ruth et al., 2001). On the basis of TrwB’s structure and other biochemical evidence, the VirD4 receptors are thought to assemble as homohexamers with an N-terminal transmembrane domain (NTD), a conserved cytosolic nucleotide-binding domain (NBD), and a sequence-variable all-alpha-domain (AAD) implicated in substrate binding (Gomis-Ruth and Coll, 2001; Llosa and Alkorta, 2017; Whitaker et al., 2015). Some T4CPs are considerably larger than TrwB (507 residues) due to the presence of sequence-variable extensions at one or both termini (Alvarez-Martinez and Christie, 2009; Whitaker et al., 2016). DotL associated with the L. pneumophila T4SSDot/Icm is presently the best-characterized representative of these larger receptors. DotL has a ~200-residue C-terminal domain (CTD) that binds the adaptor proteins IcmS and IcmW required for translocation of a subset of effectors through the T4SSDot/Icm (Bardill et al., 2005; Ninio et al., 2005; Sutherland et al., 2012; Vincent et al., 2012). In the last 3 years, significant advances were made in defining the roles of adaptors and DotL’s CTD to effector recruitment. First, two groups reported crystal structures of DotL’s CTD in complex with IcmS and IcmW and two other proteins, DotN and LvgA (Kwak et al., 2017; Xu et al., 2017). DotN, IcmW, IcmS, and LvgA bind successively from the N to C terminus of the CTD. In a pseudo-atomic model, the DotL holocomplex thus consists of a TrwB-like homohexamer joined to a larger bell-shaped CTD/adaptor complex, which was designated the substrate receptor or recognition module (Kwak et al., 2017). In the context of this model, most or all effector proteins are recruited to the DotL T4CP through contacts with distinct adaptor proteins.
Indeed, results of photocrosslinking assays confirmed effector contacts with an elongated hydrophobic surface of the IcmS/IcmW heterodimer (Xu et al., 2017). Furthermore, two groups have now reported crystal structures of DotL/adaptor–effector complexes (Kim et al., 2020; Meir et al., 2020). In one study, atomic structures were presented for the DotL/IcmS/IcmW/LvgA complex bound to the effectors VpdB, SetA, PieA, or SidH. Notably, a ~130 residue C-terminal fragment of VpdB is composed of 5 α-helices of which α1 forms specific and extensive contacts with a hydrophobic pocket of LvgA and α2– α5 form a four-helix bundle that weakly binds LvgA (Kim et al., 2020). C-terminal motifs of SetA, PieA, and SidH similarly bound the hydrophobic pocket of LvgA. An FxxxLxxxK motif was identified in α-helices of VpdB and SidH (but not SetA or PieA) that fitted into the hydrophobic pocket of LvgA. In a bioinformatics screen, 257 out of 2,930 proteins encoded by L. pneumophila strain Philadelphia-1 carry this motif, and of these 46 belong to the known effector repertoire of the T4SSDot/Icm (Kim et al., 2020). This FxxxLxxxK motif thus might constitute a previously unidentified TS for a large subset of Dot/Icm effectors, which mediates-specific contacts with LvgA.
In the second study, purification of the DotL/receptor complex yielded DotL bound to DotN, IcmS, IcmW, and LvgA, as well as to DotM and two previously unknown Dot proteins, DotY and DotZ (Meir et al., 2020). Modeling yielded an updated structure for the DotL homohexamer/receptor module and supplied new insights into the mechanism of recruitment of a second subset of effectors that are dependent on DotM and not IcmS, IcmW, or LvgA for translocation (Meir et al., 2018). These effectors carry the E block motif described above, and modeling of E block contacts with DotM revealed a structural basis for effector engagement with the DotL/receptor complex (Meir et al., 2020). Together, these recent findings have significantly advanced our understanding of how the T4SSDot/Icm channel recruits a large number of effectors bearing distinct TSs for translocation.
Upon binding of DNA or protein secretion substrates, how does the VirD4 receptor mediate translocation across the inner or cytoplasmic membrane? In fact, this remains one the most poorly understood aspects of type IV secretion. According to one model, VirD4 binds and directly shuttles substrates to the periplasm where they enter the VirB channel for passage to the cell exterior (Larrea et al., 2017; Meir et al., 2020). Alternatively, VirD4 recruits substrates, but then coordinates with the VirB4 and VirB11 ATPases to process and deliver them through a VirB channel that spans the entire cell envelope (Atmakuri et al., 2004; Cascales and Christie, 2004b). There is experimental support for both models, and discriminating between them is complicated by the fact that no studies have yet visualized an intact T4SS comprised of a VirD4 hexamer bound to the VirB channel. In fact, dimeric or unspecified forms VirD4 subunits were shown to bind cognate T4SSs (Hu et al., 2019b; Redzej et al., 2017), but it is not known whether these machines are competent for translocation. Indeed, there is considerable evidence that T4SSs undergo late-state assembly or structural transitions upon sensing of intracellular signals, such as substrate docking and ATP energy consumption, as well as extracellular signals such as target cell binding (see below and (Cascales et al., 2013; Cascales and Christie, 2004a; Lang et al., 2011; Li and Christie, 2020; Tato et al., 2007). Further definition of how such intra- or extracellular signals regulate channel assembly dynamics and substrate flow remain exciting areas for further exploration.
1.3 Structural and dynamic features of “minimized” systems
Recall that T4SSs in Gram-negative species consist of large OMCCs connected by stalks or cylinders to equally large IMCs. This current view of T4SS architecture arose from structures solved by G. Waksman and his colleagues of i) the OMCC associated with the pKM101-encoded Tra T4SS (T4SSpKM101) (Chandran et al., 2009; Fronzes et al., 2009; Rivera-Calzada et al., 2013) and ii) a nearly intact T4SS encoded by plasmid R388 (Figure 1) (Low et al., 2014). The OMCCs of both T4SSs adopt barrel-shaped structures of ~185 Å in width and height, and are composed of 14 copies each of the OM-associated VirB7 lipoprotein and VirB9 and the C-terminal region of VirB10. The barrel is composed of inner (I) and outer (O) layers, and an X-ray structure of the O-layer revealed an interior lining composed of VirB10 and an outer protective crown composed of VirB7 and VirB9 (Chandran et al., 2009). VirB10 has a two-helix bundle, originally termed the antennae projection (AP), and in the assembled OMCC the 14 APs form a helical channel that sits on top of the OMCC barrel and is presumed to span the OM. The I-layer consists of N-terminal domains of the 14 VirB9 subunits surrounding an elongated domain of VirB10, which extends to and across the IM (Jakubowski et al., 2009; Rivera-Calzada et al., 2013).
Remarkably, the IMC of the T4SSR388 nanomachine is highly asymmetric, with dimensions of 255 Å in width, 105 Å in thickness, and 134 Å in height. A prominent feature of the IMC is the presence of two side-by-side hexameric barrels of the VirB4 ATPase that extend from the IM into the cytoplasm. The IMC is composed of 12 copies of VirB3, VirB4, VirB5, VirB6, and VirB8, but 14 copies of the N-terminal region of VirB10; contacts between VirB10 and the rest of the IMC thus comprise the interface of a symmetry mismatch of possible functional importance. As noted above, an updated structure shows that two dimers of the VirD4 ATPase are integrated between the VirB4 hexamers (Redzej et al., 2017), although this likely corresponds to an incompletely assembled machine. The overall asymmetry of the T4SSR388 IMC is highly unusual among the known macromolecular translocation systems in bacteria (Christie, 2019), but at present is the only available structure for a purified IMC subassembly.
Recently, a structure of an OMCC from another “minimized” T4SS encoded by Xanthomonas citri T4SS (T4SSXan) was reported (Figure 2) (Sgro et al., 2018). Remarkably, the T4SSXan functions not in DNA transfer or delivery of effectors to eukaryotic cells but to translocate protein toxins to neighboring bacteria for niche establishment. Most VirB and VirD4 subunits of the T4SSXan closely resemble their counterparts in the A. tumefaciens or pKM101 systems. A prominent exception is the VirB7 lipoprotein, which is considerably larger (~14–20 kDa) than other VirB7 subunits (~4.5 kDa) due to the presence of a C-terminal N0 domain. Interestingly, N0 domains are prominent features of secretions associated with types II and III secretion systems, as well as the DotD lipoprotein associated with the T4SSDot/Icm (Nakano et al., 2010; Souza et al., 2011). In the X. citri OMCC, the N0 domains splay out from the central core of the OMCC, giving rise to an overall “flying saucer” shape, as opposed to the barrel shapes of the OMCCs from the R388, pKM101 and A. tumefaciens VirB/VirD4 systems (Figure 2) (Fronzes et al., 2009; Gordon et al., 2017; Low et al., 2014; Sgro et al., 2018). Other than the N0 extensions, the overall architecture of the OMCC from T4SSXan superimposes very well over that from the T4SSpKM101, and possesses characteristic features such as AP helical projections that likely form the OM channel. VirB10 also lines the interior and VirB9 forms the exterior of the O-layer, and VirB10 extends to and across the IM. Importantly, the entire Xanthomonas OMCC was solved at high resolution (3.28 Å), giving rise to the first atomic structure of the I-layer. Notably, this structure revealed the presence of linker regions between the domains of both VirB9 and VirB10, which allow for considerable flexibility and even the possibility of independent movement of the O- and I-layers.
The importance of an intrinsic flexibility in OMCCs is underscored by early findings that the A. tumefaciens VirB/VirD4 T4SS is activated by sensing and transduction of signals that accompany substrate binding and ATP energy consumption by the VirD4 and VirB11 ATPases. In turn, the distal portion of VirB10 undergoes a conformational change required for substrate passage (Banta et al., 2011; Cascales et al., 2013; Cascales and Christie, 2004a). Recently, the notion that OMCCs, and specifically the VirB10 components, undergo dynamic conformational changes has gained further support from extended molecular dynamics simulations of the C-terminal domains of native VirB10 from A. tumefaciens and a mutant variant (G272R), which is locked in the energy-activated state (Banta et al., 2011; Darbari et al., 2020). Most interestingly, the ATP-insensitive open state conferred by the G272R mutation exhibits a more rigid conformation compared to the WT complex, consistent with a reduced conformational flexibility that might impact channel gating in response to perception of intracellular signals (Darbari et al., 2020). Comparisons of OMCC architectures from quiescent versus energy-locked T4SSs will yield a better understanding of the structural consequences of signal activation. However, a full accounting of T4SSs in their open states remains a formidable challenge, as contacts between T4SSs and target cell receptors also have been proposed to stimulate late-stage channel assembly or gating as a prerequisite for substrate transfer (Kwok et al., 2007; Lu and Frost, 2005).
1.4 Architecture , assembly,and spatial organization of “expanded” T4SSs
Remarkable progress has been made in structural definition of three “expanded” T4SSs, the H. pylori Cag, L. pneumophila Dot/Icm, and F plasmid-encoded T4SSs by a combination of single-particle CryoEM, in situ CryoET, and fluorescence microscopy approaches (Figures 1 and 2).
1.4.1 The H. pylori Cag T4SS (T4SSCag)
The T4SSCag is deployed by H. pylori strains to attach and translocate substrates into human gastric epithelial cells during infection (Cover et al., 2020). These substrates include not only the CagA “oncoprotein,” but also chromosomal DNA (Varga et al., 2016), peptidoglycan (Viala et al., 2004) and D-glycero-b-D-manno-heptose 1,7 bisphosphate (HBP), which is an LPS metabolite (Gall et al., 2017; Stein et al., 2017; Zimmermann et al., 2017). Translocation of CagA induces various changes in eukaryotic target cells, including but not limited to cytoskeletal alterations and changes in cell polarity and adhesion, disruption of cell–cell junctions and increased cell motility and cell migration (Backert and Tegtmeyer, 2017; Hatakeyama, 2014). Independently of CagA translocation and, curiously, VirD4-like Cagβ, the T4SSCag stimulates interleukin-8 (IL-8) induction (Fischer et al., 2001), and also activates the Toll-like receptor 9 (TLR9) through DNA translocation (Cover et al., 2020; Lin et al., 2020; Varga et al., 2016). T4SSCag-mediated translocation of HBP was implicated in activation of the NF-kB pathway, and HBP was thus designated as a new pathogen-associated molecular pattern or PAMP (Zimmermann et al., 2017). In a recent update, however, H. pylori was shown to produce HBP at levels too low to account for NF-kB activation. Instead, a derivative of HBP, ADP-glycero-β-D-manno-heptose (ADP heptose), was identified as the key PAMP responsible for H. pylori-induced NF-kB activation in human epithelial cells (Pfannkuch et al., 2019).
The H. pylori T4SSCag is unique among the known effector translocator systems in its capacity to elaborate extracellular pilus structures when cocultivated with human epithelial cells. The T4SSCag thus resembles the Gram-negative bacterial conjugation machines and the A. tumefaciens VirB/VirD4 T4SS, which produce conjugative pili to promote attachment (Johnson et al., 2014; Rohde et al., 2003; Shaffer et al., 2011). Some similarities between the Cag and conjugative pili have been noted; for example, CagL and its homolog, VirB5, both localize on the respective pilus and are implicated in target cell binding (Aly and Baron, 2007; Kwok et al., 2007). Curiously, however, homologs or orthologs of VirB subunits required for production of conjugative pili are dispensable for Cag pili, such as CagC and CagY, which are orthologs of the VirB2 pilin and VirB10 subunits, respectively (Johnson et al., 2014; Shaffer et al., 2011; Skoog et al., 2018). H. pylori also has been reported to elaborate large sheathed tube structures whose relationship to Cag pili is not yet defined (Chang et al., 2018; Rohde et al., 2003; Tanaka et al., 2003). Finally, the CagA secretion substrate has been localized to the tips of Cag pili (Jimenez-Soto et al., 2009; Kwok et al., 2007). Among the conjugation systems, by contrast, there is no direct evidence for association of DNA or protein substrates within or attached to conjugative pili (Cascales and Christie, 2004b). Thus, at this time, the genetic requirements, nature of association with the T4SSCag, and functional roles of Cag pili and sheathed tubes are still under study.
The Cag OMCC was amenable to extraction from H. pylori for high-resolution analysis (Figure 2) (Frick-Cheng et al., 2016). Strikingly, purification of the Cag OMCC utilized an epitope tag to the chaperone CagF, which is thought to stably associate with the cytoplasmic portion (IMC) of the Cag machine. Yet, the purified complex lacks CagF and the IMC, and instead consists of the OMCC built from VirB10-like CagY, VirB9-like CagX, VirB7-like CagT, and two Cag-specific subunits, Cag3 and CagM. The OMCC is a ring-shaped structure with dimensions of ~400 Å in width and ~250 Å in height, and thus is considerably larger than OMCCs of minimized systems (Figures 1 and 2). Production of CagY, CagX, and CagM are required for detection of the OMCC, whereas OMCCs assembled in the absence of CagT and Cag3 are narrower, suggesting that CagT and Cag3 form the periphery (Frick-Cheng et al., 2016).
Recent reports have defined the 3D structure of the OMCC at a resolution as high as 3.4 Å (Figure 2) (Chung et al., 2019; Sheedlo et al., 2020). The OMCC has three distinct structural features, an outer membrane cap (designated as the OMC) consisting of an outer layer (O-layer) and inner layer (I-layer), a periplasmic ring (PR), and a stalk (Chang et al., 2018; Chung et al., 2019; Hu et al., 2019). Most strikingly, there is an apparent symmetry mismatch between the OMC (14-fold symmetry) and the PR (17-fold symmetry). The initial sub-4Å structure established that central portions of the OMC are composed of the three VirB orthologs, CagT, CagX, and CagY, but left unanswered the composition of the PR or locations of Cag3 and CagM (Chung et al., 2019). The latest ~3.4 Å structure reveals several remarkable features (Sheedlo et al., 2020). First, positions of all five components (CagX, CagY, CagM, CagT, and Cag3) within the OMCC and stoichiometries (1:1:2:2:5) were defined. The OMCC thus consists of 14 copies each of CagX and CagY, 28 of CagM, and CagT and 70 of Cag3, which explains its large size. The high-resolution structure also defines the nature of contacts among all five subunits within and between asymmetric units. Most importantly, the structure highlights the fact that both CagX and CagY form parts of the OMC and PR and, consequently, bridge the symmetry mismatch. Portions of CagX and CagY within the PR also resemble those comprising the I-layer of the X. citri, leading to the proposal that the T4SSCag PR and the I-layers of “minimized” systems are analogous structures (Sheedlo et al., 2020). If so, it is remarkable that the two highly conserved core subunits of T4SSs, VirB9, and VirB10, span two portions of OMCCs that in the “minimized” systems have equivalent 14-fold symmetries but in the T4SSCag have 14- and 17-fold symmetries.
Complementing these findings, the intact T4SSCag was recently visualized in the H. pylori cell envelope by in situ CryoET, albeit at a lower resolution (Figure 1) (Chang et al., 2018; Hu et al., 2019b). The T4SSCag machine is readily visualized as ring-shaped structures at several locations around the H. pylori cell. The outer portion corresponding to the OMCC resembles that solved by CryoEM in its 14-fold symmetrical ring-like features. Interestingly, the radial spokes of the OMCC, presumably corresponding to the 22-β-strand density of unknown composition in the CryoEM structure (Figure 2), exhibit a change in chirality. At the proximal face of the I-layer facing the IM, the spokes have an overall counterclockwise rotation, whereas near the OM they have a clockwise rotation. Furthermore, near the IM the spokes terminate in two distinct knobs, whereas near the OM they terminate in one knob. These findings might reflect a dynamic movement of the spokes, as well as the presence of additional subunits at the spoke termini that are dissociated during purification of the OMCC. As also shown by single-particle CryoEM, analyses of cag mutant machines confirmed that assembly of the OMCC in situ requires the Cag subunits CagX, CagY, and CagM, and also that CagT and Cag3 associate peripherally with the OMCC. Finally, at the OMCC–OM junction, the OM is pinched inward, indicating that the OMCC spans and causes local distortion of the inner and outer leaflets of the OM (Hu et al., 2019b).
In situ CryoET also enabled visualization of other portions of the T4SSCag not yet detected by single-particle CryoEM, including a central periplasmic cylinder and surrounding collar and a large IMC (Figure 1) (Hu et al., 2019b). The cylinder has a central channel through which CagA and other substrates are likely translocated. The IMC was visualized initially at a resolution sufficient to show the presence of four tiers of densities extending into the cytoplasm that were interpreted as side-by-side hexamers of the VirB4-like subunit CagE, reminiscent of the R388 structure (Figure 1) (Chang et al., 2018). However, a higher resolution structure clearly established that the IMC consists of three concentric rings of ~ 12, ~22, and ~ 36 nm in diameter, denoted as the I-, M-, and O-rings, respectively (Figure 1)(Hu et al., 2019b). Analyses of mutant machines lacking each one of the three ATPases, VirB4-like CagE, VirB11-like Cagα, and VirD4-like Cagβ, supplied evidence for specific contributions of each ATPase to the IMC architecture. First, CagE contributes to both the I- and M-rings by assembling as a hexamer of dimers that contribute to parts of the I- and M-rings. Second, VirB11-like Cagα is stably associated with the cytoplasmic face of the I-ring through direct contacts with CagE. Third, VirD4-like Cagβ is required for detection of densities contributing to the O-ring as well as cytoplasmic extensions of the I- and M-rings. It is unlikely that Cagβ accounts for all of the densities that are missing in a Δcagβ mutant, suggesting that the IMC is composed of additional subunits that are dependent on Cagβ for a stable association While further studies are needed to define contributions of Cagβ and unspecified subunits to the IMC, it is noteworthy that this is the first visualized ATPase energy center for any T4SS in which all three ATPases clearly make structural contributions. Overall, the results support an ordered assembly pathway for the T4SSCag, whereby the OMCC assembles first, and then, nucleates assembly of the integral membrane portion of the IMC. The ATP energy center assembles at the cytoplasmic base of the IMC through successive recruitment of CagE, Cagα, and Cagβ. Finally, one or more unspecified components are recruited through contacts with Cagβ and likely one or both of the other ATPases (Hu et al., 2019b).
1.4.2 The L. pneumophila Dot/Icm T4SS
L. pneumophila is an environmental parasite of amebae that coincidentally infects human hosts typically from inhalation of water droplets (Sherwood and Roy, 2016). The T4SSDot/Icm is used by L. pneumophila to colonize human alveolar macrophages, which triggers a severe pneumonia called Legionnaire’s disease (Sherwood and Roy, 2016). To evade killing by the host, L. pneumophila convert phagosomes into a protective compartment termed the Legionella-containing vacuole (LCV), whose formation requires the T4SSDot/Icm. As noted above, the T4SSDot/Icm translocates over 300 protein effectors during infection, many of which have been shown to target host cellular pathway controlling membrane transport processes (Isaac and Isberg, 2014; Sherwood and Roy, 2016). The T4SSDot/Icm also has retained a functional vestige of its ancestral conjugation machine in its capacity to transfer the mobilizable IncQ plasmid RSF1010 to recipient bacteria (Vogel et al., 1998).
The T4SSDot/Icm was first visualized as a ring-shaped complex on L. pneumophila cells by transmission electron microscopy (Kubori et al., 2014). The OMCC subcomplex was purified by ultracentrifugation and gel filtration (Kubori and Nagai, 2019). It is composed of five subunits, of which DotD, DotH, and DotG are the functional counterparts of VirB7, VirB9, and VirB10, and DotF and DotC are system-specific (Kubori and Nagai, 2019; Vincent et al., 2006). Interestingly, although deletion of VirB10 abolishes assembly of the OMCCs of “minimized” systems, deletion of DotG does not abolish ring formation by the other OMCC components. Rather, the central density is missing, supporting the notion that DotG forms part of the central channel (Kubori et al., 2014). Very recently, the structure of the OMCC was solved at a global resolution of ~4.6 Å without imposed symmetry (Durie et al., 2020). The OMCC approximates that of the Cag system in width (~400 Å) but is more compressed in height (~165 Å) (Figure 2). Also reminiscent of the Cag system, the OMCC is divisible into an outer ring-like OMC and central PR. The OMC has two prominent features, a central dome and a disk with 13 spokes that extend outward. The dome could not be resolved but its position on top of the disk and its size (~100 Å wide, ~50 Å in height) with a narrow opening of ~40 Å suggests it is equivalent to the α-helical channels built from VirB10-like APs. With symmetry imposed, the OMC and PR were solved at 3.5 Å and 3.7 Å resolution, respectively. The OMC and PR exhibit symmetry mismatch, but in this case the OMC has 13-fold symmetry and the PR has 18-fold symmetry. Positions of DotC, DotD, and DotH, as well as two subunits not previously identified, DotK and Lpg0657, were successfully mapped within the OMC and shown to exist in stoichiometries of 1:2:1:1:1, respectively. DotK and Lpg0657 have folds resembling peptidoglycan-binding domains, but no peptidoglycan was observed in the binding clefts of either protein. Despite the high resolution of this structure, several densities could not be definitively assigned, including a polypeptide sitting on top of the OMC that might link the OMC with the OM. Thirteen copies of a second, 22-strand β-helix extend outward to form the radial spokes (Figure 2). Initially, these were thought to correspond to a β-helix structure formed by a central repeat region of DotG, but a ΔdotG mutant machine retains these spokes as well as the OMC disk and instead lacks the dome and the PR. Thus, as shown for minimized systems and, more equivalently, the OMCC from the Cag system, VirB10-like DotG forms the central region of the OMCC as well as the dome presumptively attached to or spanning the OM. Another notable feature that recapitulates findings for the T4SSCag OMCC is that the VirB9-like DotH forms part of the OMC and PR, intriguingly bridging the symmetry mismatch between these two large subdomains (Kubori et al., 2014).
The Dot/Icm system is currently one of the most intensively studied T4SSs. Besides the high-resolution structure of the OMCC, recent studies with complementary live-cell imaging and in situ CryoET have identified a couple of important features. First, the channel preferentially assembles at the poles of L. pneumophila cells and, in fact, polar localization is essential for function of this T4SS during infection (Jeong et al., 2017; Ghosal et al., 2019). Cells in the exponential phase of growth have clusters of machines at the lateral mid-cell region, which can account for polar localization once cells divide and the septum becomes the new cell poles. However, even cells growing in stationary phase have lateral machines (Ghosal et al., 2019; Jeong et al., 2017; Park et al., 2020). The lateral machines might correspond to assembly intermediates, but it was recently shown that both polar and lateral machines consist of heterogeneous populations of assembly intermediates and presumptively intact machines (Park et al., 2020). Thus, the biological role of lateral machines in stationary phase cells, if any, remains to be determined. Second, analyses of mutant machines defined an assembly pathway for the T4SSDot/Icm, which resembles out-to-in assembly pathways proposed in early studies for the A. tumefaciens T4SSVirB/VirD4 and more recently for the H. pylori T4SSCag (Christie et al., 2005; Ghosal et al., 2019; Hu et al., 2019b; Park et al., 2020). A distinctive feature of the T4SSDot/Icm assembly pathway, however, is that two proteins, DotU and IcmF, are responsible for polar targeting of the T4SSDot/Icm (Ghosal et al., 20191). These proteins are not found associated with other T4SSs, but rather are homologs of two components (TssL, TssM) of type VI secretion systems (T6SSs). TssL and TssM do not promote polar targeting of T6SSs, yet, do function as nucleators of the T6SS in conjunction with TssJ (Durand et al., 2015). The DotU/IcmF complex is postulated to recruit the lipoprotein DotC, DotD, and DotH, which comprise the OMC (Durie et al., 2020; Ghosal et al., 2019). This ring-shaped complex then recruits the bitopic IM proteins DotG and DotF to build the PR and periplasmic densities including the central cylinder and surrounding collar. Once the OMCC/periplasmic substructures are formed, the IMC is assembled by recruitment of one or more unspecified IM subunits and finally the DotO and DotB ATPases (Chetrit et al., 2018; Ghosal et al., 2019; Park et al., 2020).
The architecture and dynamic activities of the IMC also have been further illuminated through in situ CryoET and fluorescence microscopy. In the first in situ CryoET structure, the OMCC presented as a “Wi-Fi” like structure of 400 Å in diameter consisting of the DotC, DotD, DotF, DotG, and DotH subunits (Ghosal et al., 2017). Initially, densities projecting into cytoplasm were interpreted as side-by-side hexamers of VirB4-like DotO, but higher resolution structures established that DotO adopts the same hexamer of dimers arrangement as visualized for VirB4-like CagE (Figure 1) (Chetrit et al., 2018; Hu et al., 2019b; Park et al., 2020). In end-on view, DotO presents as two concentric rings of approximately the same diameters as the I- and M-rings formed by CagE (Chetrit et al., 2018). A hexameric ring at the base of the I-ring was postulated to correspond to VirB11-like DotB, which was confirmed by density tracing of a GFP tag appended to DotB. The overall dimensions of DotB visualized in situ also fit well with the recently solved X-ray structure of the DotB homohexamer (Prevost and Waksman, 2018). Remarkably, DotB-GFP was shown to dynamically cycle off and on the Dot/Icm IMC by live-cell imaging. A mutant protein (E191K) that binds but does not hydrolyze ATP stably associates with the IMC, indicating that the ATP-bound state of DotB docks stably on the IMC (Chetrit et al., 2018). Very recently, a focused refinement of cytoplasmic complexes also supplied evidence for DotB-induced conformational changes in the IMC, which is interpreted as an opening in the channel in response to ATP hydrolysis by DotB (Park et al., 2020). Thus, ATP energy consumption by VirD4/VirB-like ATPases has now been shown to induce conformational changes in both the proximal (IMC) and distal (OMCC) portions of the T4SS channel (Cascales and Christie, 2004a; Park et al., 2020).
1.4.3 The F plasmid Tra T4SS
The F plasmid-encoded T4SSF was the first described bacterial conjugation system (Lederberg and Tatum, 1946). There has been a resurgence of interest in F plasmids largely due to their prominent in dissemination of genes encoding antibiotic and heavy metal resistance, virulence factors, and fitness traits among clinically important Enterobacterial species (Koraimann, 2018). The T4SSF also is an ideal model system for investigating fundamental mechanistic and structural properties of these nanomachines, because it is highly amenable to genetic manipulation and is presently the only T4SS known to produce pili that dynamically extend and retract (Clarke et al., 2008).
Recently, our views of how F pili assemble at the cell surface and how they mediate intercellular contacts have been completely reshaped through in situ CryoET studies of the F-encoded T4SS and structural analyses of F pili (Costa et al., 2016; Hu et al., 2019a; Zheng et al., 2020). Analyses of F-encoded structures in the E. coli cell envelope identified several distinct structures, including one interpreted as the translocation channel through which the F plasmid transfer intermediate passes to recipient cells (Figure 1). Like the other characterized T4SSs, this structure (named the F1-CH complex) is composed of OMCC and IMC subassemblies joined by a thin periplasmic density. The OMCC adopts a “flying-saucer” structure of 250 Å in width and 115 Å in height, which in top view has 13 knobbed-spokes joined to a central ring of 130 Å in diameter. Both in overall architecture and fold symmetry, the OMCC of the F1-CH more closely resembles that associated with the T4SSDot/Icm than those of the “minimized” systems or the T4SSCag (Figure 1). The OMCC surrounds a cylindrical density with a central channel that extends to and through the IMC. The cytoplasmic portion of the IMC closely resembles those of the Dot/Icm and Cag machines, insofar as VirB4-like TraC is configured as a hexamer of dimers surrounding the central channel. F plasmids lack VirB11 homologs and VirD4-like TraD was not visible, suggesting that TraD associates asymmetrically or transiently with the channel (Hu et al., 2019a).
Remarkably, three other F-encoded structures were bound at the cell surface by F pili (Hu et al., 2019a). One such structure, termed the F2-Channel/Pilus or F2-CH/P, resembles F1-CH complex but with an attached pilus. These structures are nonabundant (~5% of visualized F-encoded structures), and their most remarkable feature is that the F pilus sits on top of the structure and clearly does not extend across the OM. This observation, coupled with analyses of mutant machines, firmly establish that the mature F pilus assembles at the OM and not on an IM platform as previously envisioned. This distinguishes the F pilus from type IV pili (T4P; phylogenetically unrelated to conjugative pili) and the needle complexes of Type III secretion machines (T3SSs), which both nucleate on an IM platform and extend across the OM (see (Hu et al., 2019a). A second, a very abundant structure (~60% of visualized F-encoded structures) presents as a thin (~12 nm) stalk that spans the periplasm and a wider “mushroom cap” near the OM, to which the F pilus is attached at the outer surface of the OM. These F3-Stalk/Pilus or F3-ST/P structures lack a central channel or IMC densities corresponding to the VirB4-like TraC ATPase. Finally, a fourth structure, designated the F4-Outer membrane/Pilus or F4-OM/P complex, is again nonabundant (~5% of visualized F-encoded structures) and consists of the F pilus attached to a small density at the OM without any associated periplasmic density.
Taken together, these findings establish for the first time that F pili are docked onto alternative basal platforms around the cell surface (Hu et al., 2019a). In view of the genetic requirements for F pilus biogenesis, a working model posits that the F1-CH structure functions as a translocation channel when donor cells are in the “mating” mode, for example, in direct contact with target cells. However, in the absence of target cell contact, the F1-CH transition to a pilus assembly platform, giving rise to F2-CH/P structures. The F2-CH/P structure dynamically builds pili through a process involving extraction of TraA pilin subunits from an IM pool, assembly of a thin protofilament that extends through the central channel to the cell surface, and nucleation of the mature pilus as a helical fiber at the OM. Due to its highly dynamic nature, the F2-CH/P structure is difficult to capture in structural snapshots generated by CryoET, hence, its nonabundance. Once F pili polymerize, they can either retract through a reversal of the assembly process, or be deposited around the cell surface on alternative basal platforms conforming to the visualized F3 and F4 complexes. Because these complexes lack densities known to correspond to components that are essential for F pilus assembly/retraction dynamics, for example, TraC, the associated pili are presumed to be static structures. How these alternative platforms arise and what biological functions the associated F pili serve remain intriguing questions for future study (Hu et al., 2019a).
1.4.4 Structure–function studies of F pili
F pili are filaments that extend into the bacterial extracellular space to mediate attachment to target cells. Upon attachment, F pili retract to draw the two cells together, allowing for establishment of direct cell–cell contacts and, ultimately, an SDS- and shear-resistant “mating junction” (Arutyunov and Frost, 2013). Previous studies established that F pili are composed of thousands of TraA (pilin) molecules polymerized as a helical assembly (Paranchych and Frost, 1988; Wang et al., 2009). However, recent structures of two members of the F pilus family (encoded by the pED208 and pOX38 plasmids) solved by single-particle CryoEM revealed not only the expected electron density of the TraA pilin, but also a neighboring density attributed to a phospholipid from the PG (phosphatidylglycerol) family (Figure 3a) (Costa et al., 2016). Similarly, a recent CryoEM structure of an F pilus encoded by K. pneumoniae pKpQIL (Zheng et al., 2020) and a CryoET structure of the F pilus bound with MS2 phage, also shows the presence of both molecular species in the structure (Meng et al., 2019; Zheng et al., 2020). Thus, all F pilus structures solved to date confirm the peculiar arrangement of copious amounts of stoichiometric 1:1 pilin–phospholipid complexes as the building block.
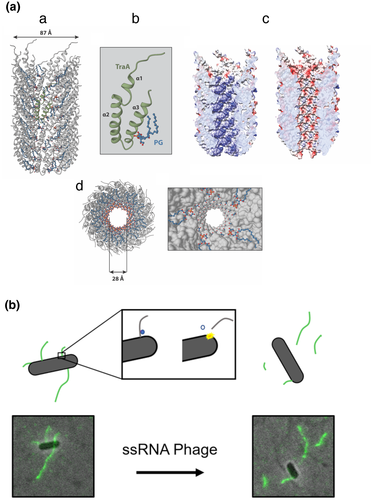
The pED208-encoded F pilus has been solved at the highest resolution (3.6 Å) among the available structures (Costa et al., 2016). The structure shows a filament with an internal lumen diameter of 28 Å and an external diameter of 87 Å (Figure 3a). The structure can be described as TraA-phosphatidylglycerol (PG) pentameric complexes stacked on top of each other related by an axial rise of 12.2 Å and with a rotation angle of 28-degrees or, alternatively, a five-start helical filament. Minor variations in terms of axial rise, rotation angle and start helix were observed in the CryoEM structures of F pili elaborated by the F plasmid and pKpQIL (Costa et al., 2016; Zheng et al., 2020). The high-resolution map obtained from pED208-encoded F pili yielded an atomic structure of TraA (Figure 3a). Each TraA subunit is composed of three α-helices (α1, α2, α3), where the loop between α2 and α3 projects to the lumen of the pilus and the N- and C-termini are exposed at the pilus surface where they are accessible for phage attachment (Zheng et al., 2020). The overall atomic structures of all individual TraA proteins are likely very similar given the very high degree of amino acid sequence identity between pilin subunits of different F systems.
In the vicinity of each TraA protein density, an unconnected electron density consistent with a phospholipid head group (pointing to the pilus lumen) and two acyl chains (embedded in the pilus wall) was resolved (Figure 3a) (Costa et al., 2016). Mass spectrometry experiments identified the two PG species present in the pilus as phosphatidylglycerol 32:1 and phosphatidylglycerol 34:1. The negatively charged head group from the PG phospholipids, reverses the electrostatic potential from the lumen surface from positive to negative. In addition to the electrostatic remodeling properties produced by the PG head group, the flexible acyl chains embedded in the pilus wall could likely contribute for the unique F pilus biomechanical properties such as bending, extension, and retraction (Clarke et al., 2008).
Our knowledge of F pilus structure and function has been further enriched by studies of F pili and their phages (Figure 3b). Many canonical ssRNA coliphages (e.g., MS2, R17, Qβ, etc.) utilize F pili to facilitate host recognition and delivery of viral RNA into the host cytoplasm during infection. Infection begins with phage adsorption to host pili. Recent CryoEM studies determined the molecular and structural details describing MS2 adsorption to the F pilus (Meng et al., 2019). Adsorption is mediated by the MS2 maturation protein (Mat), specifically, the β-region of Mat binds the N- and C- termini of four pilin subunits through a series of electrostatic interactions between residues at the Mat-pilin interface. This interaction can be abolished by eliminating charged residues at the interface (Manchak et al., 2002). Additionally, the orientation of F pilin monomers in assembled pili was revealed, where the N- and C- termini of each pilin are angled toward the cell envelope (Meng et al., 2019). This orientation may be favorable to promote pilus extension or retraction through the T4SS channel.
After adsorption, the ssRNA phage penetrates the cell envelope to deliver its genomic RNA into the cytoplasm. This penetration step is arguably the least understood aspect of the infection process. Since the F pili are naturally involved in conjugation, a process requiring intimate contact between donor and recipient cells brought together through pilus retraction, it seems intuitive that the pilus bound phage is brought to the cell surface through pilus retraction. As shown by use of dye-labeled phage R17 (Clarke et al., 2008), F pili undergo cycles of extension and retraction presumably as a means of surveying the area around the cells. Pilus retraction is typically completed within 5 min after initiation at a rate of 16 nm/sec, whereas extension occurs more rapidly at a rate of 40 nm/sec. Additionally, pili tend to supercoil when their distal ends contact a surface, suggesting that pili rotate during extension and retraction.
Direct evidence that F pili bound by phage are capable of retracting prompted further investigations of F pilus behavior in the presence of ssRNA phages. Recently, fluorescently labeled MS2 phage was used to detect and measure the length of F pili after ssRNA phage infection (Figure 3b) (Harb et al., 2020). These studies established that F pili detach from host cells during infection by ssRNA phages. The duration of pilus detachment coincided with the entry period of MS2, suggesting that pili detach during penetration of viral RNA. Additionally, the length of detached pili increased with higher multiplicities of infection, supporting the notion that pilus detachment, and likely viral RNA penetration, is facilitated through a process involving pilus retraction. In the context of the recent CryoET structure of the F2-CH/P complex (Hu et al., 2019a), it is envisioned that as the pilus-bound phage are drawn to the T4SS channel through pilus retraction, a torsional stress imposed at the channel–pilus junction causes breakage of the mature F pilus from the protofilament and release of F pili into the milieu (Figure 3). Interestingly, this process of phage-induced pilus release might represent a novel form of superinfection exclusion, insofar as it prevents subsequent binding of phages in the vicinity to cell-bound pilus receptors. These studies further established that MS2 phage retained the ability to bind F pili produced by mutant machines lacking the TraD T4CP, but the phage genomic RNA does not enter the cytoplasm of the ΔtraD mutant cells. TraD thus appears to serve as a gatekeeper of the F-encoded channel from both directions, in its capacity to regulate F plasmid transfer out of cells during conjugation and phage uptake during infection (Harb et al., 2020). Finally, the authors note that phage infection is also known to transiently inhibit cells from producing F pili, which represents another form of superinfection exclusion. Given the selective advantage of superinfection exclusion mechanisms, it is reasonable to propose that other phages that use pili as receptors might deploy similar strategies of pilus release and pilus assembly inhibition to prevent further infection.
The F pilus is the best-characterized member of the conjugative pili, but is unique among these pili in its demonstrated capacity to dynamically extend and retract. Other characterized conjugative pili naturally slough from cells, which has complicated efforts to study assembly dynamics. Indeed, such pili are thought to promote donor–target cell contacts through release and establishment of hydrophobic interactions that mediate nonspecific pilus clumping and cell aggregation. A common, and, perhaps, only function of conjugative pili thus might be to serve as attachment organelles for adherence of donors to surfaces and for biofilm development, rather than contributing directly to translocation of DNA or other substrates to target cells. At least two lines of evidence support this notion. First, “uncoupling” mutations have been isolated that block detectable pilus production without affecting substrate transfer (Jakubowski et al., 2005, 2009). Second, conjugation machines functioning in Gram-positive species do not elaborate pili, yet, can function highly efficiently in DNA transfer (Bhatty et al., 2013; Grohmann et al., 2017). Nevertheless, there is some evidence for transfer of the F plasmid from donor to recipient cells at a distance, which is proposed to be mediated by the extended F pilus (Babic et al., 2008). The latest structures of F pilus basal platforms, for example, the F2-CH complex, and the discovery of a phospholipid lining of the F pilus lumen, allow for this possibility at least as a secondary and low-efficiency mode of transfer. Clearly, further work is needed to “visualize” real-time transfer events through state-of-the-art fluorescence approaches or by capturing CryoET snapshots of donor-recipient pairs in the act of DNA transfer.
2 SUMMARY AND FUTURE DIRECTIONS
In this review, we have updated the reader on three remarkable advances in structural definition of model T4SSs obtained by single-particle CryoEM. First, the DotL/adaptor receptor module, solved in the absence and presence of docked effectors, offer exciting new insights into early stage substrate docking reactions generally as well as specific mechanisms for regulating the flow of hundreds of effectors through the T4SSDot/Icm. (Kim et al., 2020; Kwak et al., 2017; Meir et al., 2020; Xu et al., 2017). Second, structural resolution of F pili revealed TraA pilin–phospholipid as the fundamental building block, which raises intriguing new questions concerning the pilus biogenesis pathway, the possible functional significance of the PG-lined lumen, and the generality of these findings to other conjugative pili. Third, structural definition of the intrinsically stable OMCCs from the X. citri VirB/VirD4, H. pylori Cag and L. pneumophila Dot/Icm systems revealed that only the latter “expanded” systems display a striking symmetry mismatch between the outer and inner layers (Chung et al., 2019; Costa et al., 2016; Durie et al., 2020; Sgro et al., 2018; Sheedlo et al., 2020; Zheng et al., 2020). This symmetry mismatch is highly curious, especially in view of the fact that “minimized” systems are fully capable of translocating DNA or proteins to other bacteria as well as to eukaryotic cell targets, as best illustrated with the paradigmatic A. tumefaciens VirB/VirD4 T4SS. It should be noted that the “minimized” systems in fact exhibit symmetry mismatch, but between their OMCCs and IMCs. At this time, we can only speculate that symmetry mismatches between or within large subassemblies of T4SS nanomachines affords greater conformational flexibility. For example, symmetry mismatch between the IMC and OMCC may be necessary for conversion of intra- and extracellular signals into structural transitions associated with channel gating. Asymmetry within the OMCCs of the “expanded” Cag and Dot/Icm systems might afford more profound structural transitions necessary for recruitment of nonprotein substrates, spatiotemporal control in delivery of many hundreds of effectors, or elaboration of pili or large sheathed structures upon binding of eukaryotic cell receptors.
We also summarized exciting new insights gained through visualization of “expanded” T4SSs by in situ CryoET (Chetrit et al., 2018; Ghosal et al., 2017; Ghosal et al., 2019; Hu et al., 2019a #4672; Hu et al., 2019b; Jeong et al., 2017; Park et al., 2020). The studies have enabled comparisons of the OMCC structures solved in isolation and in the native environment of the cell envelope, and also defined for the first time structures of central periplasmic channels and IMCs from machines that have been refractory to purification. Live-cell imaging has further advanced our knowledge of T4SS machine assembly dynamics, as nicely illustrated by discoveries that the T4SSDot/Icm localizes and functions at cell poles, and the VirB11-like DotB ATPase associates dynamically and induces structural changes in the channel through rounds of ATP hydrolysis. With this new information in hand, it is clear that the T4SS field is well-poised for further basic studies aimed at deciphering the mechanistic details of substrate trafficking and exploring T4SS structural and biological diversity.
Continued structure–function studies of T4SSs also have important translational applications. One area of active investigation is in the design of small-molecule inhibitors of T4SSs with the broad goals of suppressing dissemination of antibiotic resistance or mitigating virulence of clinically important pathogens (reviewed in Alvarez-Rodriguez et al., 2020a; Boudaher and Shaffer, 2019). In some cases, inhibitors are being identified through high throughput screens for molecules blocking a T4SS-mediated process, for example, conjugation (Cabezon et al., 2017; Casu et al., 2017; Shaffer et al., 2016). In others, specific requirements for machine assembly or function are targeted for inhibition, such as VirB8 dimerization (Casu et al., 2016; Paschos et al., 2011; Smith, 2012 #3395), or catalytic activities of the VirD4, VirB4, or VirB11 ATPases (Arya et al., 2019; Garcia-Cazorla et al., 2018; Ripoll-Rozada et al., 2016). This latter approach, in particular, will benefit through structural definition of intersubunit contacts critical to T4SS integrity, as these are prime targets for small-molecule disruption of machine assembly. As we learn more of the infection processes of male-specific phages that parasitize T4SSs to gain entry into cells (Harb et al., 2020; Meng et al., 2019), novel strategies may arise for deployment of phages to kill cells harboring T4SSs or selectively inactivate these systems. Finally, there is developing interest in manipulation of T4SSs for therapeutic applications through delivery of toxic DNA or protein substrates to kill-specific target cells of interest. The T4SSs are excellent candidates as programed delivery systems as they are the only bacterial secretion system known to translocate DNA and proteins to both bacterial and human cell targets (Guzman-Herrador et al., 2017).
In summary, if the recent structural advances are any indication, the future of basic and applied studies of T4SSs shines brighter than ever.
ACKNOWLEDGMENTS
We thank the entire T4SS community for valuable contributions to this field, and we apologize for omissions of published work on the many other exciting aspects of type IV secretion not covered here due to page limitations. We thank members of the Zeng, Hu, and Christie labs for helpful comments and critiques. This work was supported by NSF Grant 1902392, NIH Grant R21 AI156846, and Texas A & M University X-Grant 290386 to L.Z, NIH Grant R21 AI 142378 to B.H. and P.J.C., McGovern Medical School start-up funds and Welch Foundation Grant AU-1953-20180324 to B.H., Wellcome Trust 215164/Z/18/Z grant to T.R.D.C., and NIH Grant R35 GM131892 to PJC. None of the authors declare a conflict of interest. Data sharing is not applicable to this article as no new data were created or analyzed in this study.