Landscape characteristics influence regional dispersal in a high-elevation specialist migratory bird, the water pipit Anthus spinoletta
Abstract
Species living in high mountain areas are currently threatened by climate change and human land use changes. High-elevation birds frequently inhabit island-like suitable patches around mountain peaks, and in such conditions the capability to exchange individuals among patches is crucial to maintain gene flow. However, we lack information regarding the dispersal ability of most of these species and the possible influence of landscape features on dispersal. In this study, we used population genomics and landscape resistance modelling to investigate dispersal in a high-elevation specialist migratory bird, the water pipit Anthus spinoletta. We aimed to assess the levels of gene flow in this species within a wide area of the European Alps, and to assess the effects of environmental characteristics on gene flow, by testing the isolation by distance (IBD) hypothesis against the isolation by resistance (IBR) hypothesis. We found clear support for IBR, indicating that water pipits preferentially disperse across suitable breeding habitat (i.e., high-elevation grassland). IBR was stronger in the part of the study area with less extended suitable habitat. Landscape resistance was slightly better described by habitat suitability models than landscape connectivity models. Despite the observed IBR, gene flow within the study area was high, probably also because of the still wide and relatively continuous breeding range. The forecasted reduction of range of this species may lead to stronger effects of IBR on gene flow. Other high-elevation specialist birds may show similar IBR patterns, but with possibly stronger effects on gene flow because of their more reduced and patchy habitats.
1 INTRODUCTION
High mountain ecosystems are currently subject to severe pressures including climate change, pollution, nitrogen emissions, and human land use changes such as increases in leisure activities and the intensification/abandonment of grazing in different areas (Catalan et al., 2017). In mountain areas, the strong variation of abiotic factors within short distances results in a wide variety of habitats and species occurring within relatively small areas and stratified across elevational gradients (Cadena et al., 2012). This often results in patchy distributions of species that are constrained to very specific elevational ranges (Cadena et al., 2012). Due to their specific adaptation to local conditions, species of high-mountain ecosystems are highly threatened by climate change and habitat loss/degradation due to human activities (e.g., Chamberlain, Pedrini, et al., 2016; De Gabriel Hernando et al., 2021; Goodenough & Hart, 2013). As a consequence of temperature increase, mountain bird populations are expected to change their distributions to track their thermal niches and/or to cope with habitat change (e.g., the uphill shift of wooded vegetation, Myers-Smith et al., 2011). While species adapted to warm conditions may gain new suitable areas at higher elevations (e.g., Ceresa et al., 2021; Scridel et al., 2017), high-elevation specialist species will, by contrast, lose suitable habitat with uphill distribution shifts, because ground area decreases with increasing elevation in mountain systems showing a pyramidal structure (Elsen & Tingley, 2015).
Up- or downhill elevational shifts in bird distributions have already been documented in different regions (e.g., Couet et al., 2022; DeLuca & King, 2017; Hallman et al., 2022; Maggini et al., 2011; Reif & Flousek, 2012), although in some studies this pattern was unclear or absent, probably because of the influence of factors other than climate change, such as human land use (Scridel et al., 2018). Changes in human land use may exacerbate the impact of climate change on mountain bird populations, for example by accelerating the upward treeline shift because of grazing abandonment (Gehrig-Fasel et al., 2007), or by increasing leisure activities at high elevations (Brambilla et al., 2016; Imperio et al., 2013). As a further threat to mountain birds, climate change may cause temporal mismatches between their reproduction phenology and food availability (Barras et al., 2019; Santisteban et al., 2012), potentially leading to lower breeding success. In the European Alps (hereafter Alps), strong range contractions have been forecasted for high-elevation specialists such as rock ptarmigan Lagopus muta, water pipit Anthus spinoletta, white-winged snowfinch Montifringilla nivalis and alpine accentor Prunella collaris (Brambilla et al., 2017, 2022). Most of these projected range reductions are based on the forecasted climatic scenarios for the next few decades, without accounting for potentially negative effects of land use changes or human-induced disturbance, and thus the actual population declines may be even more severe.
In a scenario of habitat reduction and fragmentation for high-elevation species, effective dispersal (i.e., dispersal followed by reproduction) of individuals among suitable habitat patches and the consequent gene flow will be crucial for population viability. Sufficient levels of effective dispersal increase the probability of recolonization of vacant habitat patches (or the colonization of new suitable habitats, which become available because of changing conditions), promote the maintenance of a wide distribution and a large population size, and reduce the probability of inbreeding and genetic drift (Frankham et al., 2010; Kvist et al., 2011). The dispersal ability of birds is generally high, but with strong interspecific differences that are apparently related to species' characteristics (such as migration strategy, breeding habitat, distribution range, population size) and to their phylogeny (Paradis et al., 1998; Sutherland et al., 2000). These relationships are complex and still poorly understood. Even in species with very high movement capability, such as long-distance migrants, dispersal rates can be restricted by strong philopatry (e.g., Ceresa et al., 2016; Hansson et al., 2002), which is probably due to high dispersal costs (Plissner & Gowaty, 1996; Waser et al., 1994).
Landscape characteristics also affect bird dispersal and, consequently, gene flow (Machtans et al., 1996; Newton, 1998). For species living in fragmented habitats, besides the distance among suitable patches, the characteristics of the landscape matrix separating the patches can also influence dispersal (e.g., Ceresa et al., 2015; Klinga et al., 2019). Unfortunately, current knowledge of the dispersal ability of high-elevation birds is extremely poor, with virtually no information for passerine birds. We also lack knowledge of the effects of the landscape matrix separating suitable habitat patches (i.e., wooded or farmed mountain slopes, valley bottoms) on dispersal and gene flow in these species. For high-elevation birds, which frequently inhabit island-like suitable patches around mountain peaks, population connectivity is crucial to survive under a changing climate, and the gap in knowledge regarding dispersal and gene flow is problematic for population viability analyses and conservation. More generally, the available information on the ecology of these species is still limited, possibly due to the logistic problems connected to data collection in mountain areas (Scridel et al., 2018).
Information about dispersal can be obtained from mark–resight data collected throughout several breeding seasons (e.g., Ceresa et al., 2016; Paradis et al., 1998, 2002). However, huge field efforts are needed for marking and monitoring birds during several years over an adequately large area, while small study areas invariably provide information only about short-distance dispersal (Paradis et al., 1998). Satellite telemetry is often used to investigate bird movements, but it is usually applied to just a few individuals due to high costs, and it is not feasible in small birds due to the excessive weight of the required equipment. As an excellent alternative to these approaches, genetic methods allow us to estimate differentiation among groups of individuals sampled at different locations, which indicates the level of gene flow among them. Based on genetic differentiation and geographical location of sampled individuals, it is also possible to look for dispersal limitations due to the geographical distance (isolation by distance, hereafter IBD). Genetic differentiation patterns can also be used to look for dispersal limitations due to landscape features (isolation by resistance, hereafter IBR) by relating genetic data to landscape resistance information, often represented by environmental layers, estimates of habitat suitability and/or landscape connectivity models that try to describe animal movements based on landscape characteristics (e.g., Klinga et al., 2019; Miller et al., 2018; Ruiz-Gonzalez et al., 2014).
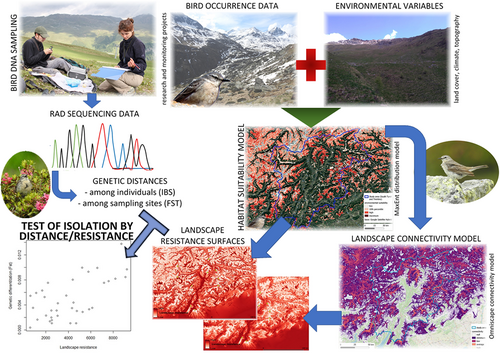
In this study, we combined population genomics and landscape resistance modelling to investigate population connectivity in a high-elevation specialist bird, the water pipit, across a wide area in the Alps. The water pipit is a small passerine bird (~18–20 g) that is strictly related to high-elevation grasslands and pastures for breeding and is distributed in mountain ranges of South and Central Europe, the Middle East and Central Asia, with the easternmost breeding areas in Northern and Central China (Tyler, 2019). This species is territorial during reproduction and usually performs elevational or short-distance migration movements, overwintering in lowland areas (Tyler, 2019). The water pipit is a high-priority species for conservation in the Alps, given the strong breeding range reduction and/or shift forecasted for future decades in the Alps (Brambilla et al., 2017, 2022) and the importance of the Alpine population at the European level, representing nearly 20% of the continental population (Brambilla et al., 2017). Despite the apparently stable population trend in Europe (BirdLife International, 2015; Lehikoinen et al., 2019), declines have been recently reported for Italy (Rete Rurale Nazionale & Lipu, 2021), Iberia (Lehikoinen et al., 2019) and some areas in Switzerland (Knaus et al., 2018). No information about dispersal ability is currently available for this species. In the next few decades, the importance of the Alps for the water pipit and for other high-alpine species will increase, as no other mountain chain in Europe offers the same amount of high-elevation habitats and hence of potential climatic refugia (Brambilla et al., 2022). Therefore, focusing on the water pipit as a model species, within a geographical area representative of broader contexts within the Alps, is relevant not only for this species, but also for a better understanding of the factors affecting isolation that could affect many high-elevation taxa threatened by the climate change.
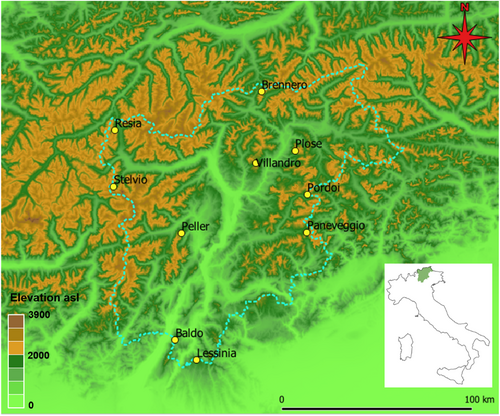
Our aims therefore were: (i) to assess the level of gene flow in the water pipit within an ~13,600-km2 mountainous area in the central–eastern Italian Alps (Trentino–South Tyrol region; Figure 1), using high-resolution population genomics data obtained through restriction site associated DNA sequencing (RAD sequencing; Davey & Blaxter, 2011); and (ii) to assess the effects of environmental characteristics on gene flow in the study species, considering land use, topographic and climatic factors. To this aim, we tested the IBD hypothesis against the IBR hypothesis, by comparing the genetic differentiation (among sampling sites as well as among individuals) with both geographical distances and landscape resistance surfaces obtained from habitat suitability and landscape connectivity models. The approach we followed is summarized in Figure 2. The landscape matrix separating the water pipit breeding areas is highly heterogeneous, and is dominated by woodlands, farmlands and valley floors anthropized to various extents. If water pipits avoid directional flights above large unsuitable areas during dispersal, we expected a clear effect of landscape characteristics on genetic differentiation patterns (i.e., IBR). In addition, we tested IBD versus IBR for the entire sample but also separately for two subareas that clearly differ in the availability of suitable habitat (details in Section 2), because the effects of landscape on dispersal can vary across different scales and habitat configurations (Amos et al., 2014; Segelbacher et al., 2010). Some species can indeed show dispersal restrictions locally, but without patterns detectable at large scales (Colson et al., 2013), or vice versa (Blair & Melnick, 2012). As an example, simulation models have shown that landscape effects on gene flow are often absent when habitat patches are aggregated and highly connected (Cushman, Shirk, et al., 2013). Our study area extends from the main Alpine divide in the north, where the suitable habitat for the water pipit is relatively continuous, to the lower relief areas at the southern margin of the Alps (Prealps), where high-elevation areas above the treeline are scarcer and more isolated (Figure 1). If water pipits avoid dispersing across unsuitable habitat, we expected to find a more pronounced effect of landscape resistance in the southern than in the northern subarea.
2 MATERIALS AND METHODS
2.1 Habitat suitability models
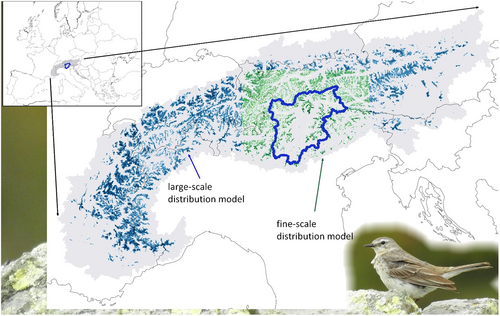
As a first step to obtain landscape resistance surfaces, we modelled habitat suitability for the water pipit by adopting a maximum entropy approach (maxent; Phillips et al., 2006). This approach is widely used for distribution modelling based on presence-only data collected through different protocols (Elith et al., 2011; Grimmett et al., 2020; Merow et al., 2013). We used the maxent approach as implemented in the sdmtool package (Vignali et al., 2020) in R 4.2.1. We used 1066 water pipit occurrence records collected between 2010 and 2020 in the Trentino–South Tyrol region during multiple studies and surveys carried out by the Museum of Nature South Tyrol, MUSE-Science Museum of Trento, Eurac Research, and associated researchers. We only used data collected during the water pipit breeding season (May 15–July 31). We then related bird occurrence data to environmental variables including land cover, topographic and climatic factors. While the bird distribution data and bird DNA sampling were limited to the Trentino–South Tyrol region, we modelled habitat suitability and landscape connectivity for a wider area (Figure 3), because dispersal can also occur by crossing neighbouring regions. This area covers a large portion of the water pipit breeding range in the Alps (25%–30%; Figure 3) and includes both inner-Alpine and peripheral massifs, and is therefore representative of the whole Alpine range of the species.
To model habitat suitability, we used the 2012 CORINE Land Cover (European Environment Agency, 2016) as the layer representing the land-use/land-cover. Climatic data were derived from the CHELSA version 2.1 database (Karger et al., 2017); in order to associate an accurate estimate of the average annual temperature to bird records, we calculated the average temperature for the study period (2010–2019; 2020 was not available) from monthly temperatures of all years with species records. Topographic variables were obtained in grass gis 7.8.4 (Neteler et al., 2012) from a Digital Elevation Model with a 25-m spatial resolution, made available by the EEA (European Environment Agency, 2016). We calculated slope and solar radiation, considering the daily total value (kWh m−2) and taking into account the potential shadowing effect due to surrounding mountains and reliefs. These variables were resampled at a 200-m resolution to include average values at a landscape scale, in order to better describe the site occupied by a breeding pair (and hence its home range) without giving too much emphasis to specific pixels. Variables used for modelling are summarized in Table 1.
| Environmental predictors | Permutation importance (±SD) | Source |
|---|---|---|
| Average slope | 16.9 ± 0.01 | Digital Elevation Model (25 m) |
| Solar radiation (June 21): average daily total value (kWh m−2) | 0.2 ± 0.00 | Digital Elevation Model (25 m) |
| Average mean annual temperature | 17.0 ± 0.01 | CHELSA database version 2.1 |
| Proportional cover of arable land | 2012 CORINE Land Cover | |
| Proportional cover of vineyards | 2012 CORINE Land Cover | |
| Proportional cover of fruit trees and berry plantations | 2012 CORINE Land Cover | |
| Proportional cover of olive orchards | 2012 CORINE Land Cover | |
| Proportional cover of pastures | 0.7 ± 0.00 | 2012 CORINE Land Cover |
| Proportional cover of annual crops associated with permanent crops | 2012 CORINE Land Cover | |
| Proportional cover of complex cultivation patterns | 2012 CORINE Land Cover | |
| Proportional cover of land principally occupied by agriculture, with significant areas of natural vegetation | 2012 CORINE Land Cover | |
| Proportional cover of broadleaved woodland | 0.3 ± 0.00 | 2012 CORINE Land Cover |
| Proportional cover of coniferous forest | 16.3 ± 0.01 | 2012 CORINE Land Cover |
| Proportional cover of mixed forest | 0.4 ± 0.00 | 2012 CORINE Land Cover |
| Proportional cover of natural grassland | 9.4 ± 0.00 | 2012 CORINE Land Cover |
| Proportional cover of moors and heathland | 3.0 ± 0.00 | 2012 CORINE Land Cover |
| Proportional cover of sclerophyllous vegetation | 2012 CORINE Land Cover | |
| Proportional cover of transitional woodland–shrub | 0.3 ± 0.00 | 2012 CORINE Land Cover |
| Proportional cover of beaches, dunes and sands | 2012 CORINE Land Cover | |
| Proportional cover of bare rocks | 9.9 ± 0.00 | 2012 CORINE Land Cover |
| Proportional cover of sparsely vegetated areas | 25.6 ± 0.01 | 2012 CORINE Land Cover |
| Proportional cover of burnt areas | 2012 CORINE Land Cover | |
| Proportional cover of glaciers and perpetual snow | 2012 CORINE Land Cover | |
| Proportional cover of inland marshes | 2012 CORINE Land Cover | |
| Proportional cover of peat bogs | 2012 CORINE Land Cover | |
| Proportional cover of water courses | 2012 CORINE Land Cover | |
| Proportional cover of water bodies | 2012 CORINE Land Cover | |
| Proportional cover of all urbanized areas | 2012 CORINE Land Cover | |
| Proportional cover of quarries and dumps | 2012 CORINE Land Cover | |
| Proportional cover of green urban areas and sport and leisure facilities | 2012 CORINE Land Cover | |
| Proportional cover of inland marshes | 2012 CORINE Land Cover | |
| Proportional cover of peat bogs | 2012 CORINE Land Cover | |
| Proportional cover of water courses | 2012 CORINE Land Cover | |
| Proportional cover of water bodies | 2012 CORINE Land Cover | |
| Proportional cover of all urbanized areas | Derived from 2012 CORINE Land Cover | |
| Proportional cover of quarries and dumps | Derived from 2012 CORINE Land Cover | |
| Proportional cover of green urban areas and sport and leisure facilities | Derived from 2012 CORINE Land Cover |
Environmental variables were calculated by means of focal features (or a moving window) within an area of 300 m radius around each occurrence and background location. Data about the true sampling effort were partial, and thus we limited the background to the areas that contained occurrence records, in order to properly describe the environmental conditions sampled by collectors (Brambilla, Scridel, et al., 2020). This was done by means of a 3-km buffer around all the occurrence records. Then, we randomly scattered 40,000 background points within such a buffer, in order to have background points in locations visited by observers, or very close to such sites. We adopted a two-step strategy to build robust, representative models. We first removed records too close to each other, by superimposing a 1-km grid on the study area and randomly selecting only one record per cell in the case of multiple observations. We then partitioned the remaining occurrence data into four spatially independent subsets, using the checkerboard 2 function of the package enmeval 2.0.3 (Muscarella et al., 2014), using 4 and 2 km as the size of cells. We used the data from three partitions to train the model, whereas those included in the fourth were used as independent data to test the model. The reduced data sets included 376 training records, while testing data sets included 130 records. maxent models were trained using only the linear and quadratic features, to reduce the risk of overfitting, following a training and tuning procedure based on Akaike's Information Criterion for small sample sizes (AICc) (Brambilla et al., 2022). We initially selected a value of the regularization multiplier. Then, we left out from the models all the variables with Lambda = 0 (no detectable effect on environmental suitability for a target species). With the remaining variables, we used AICc values to select the regularization multiplier value, the features to be included (linear and/or quadratic) and the number of iterations, and performed a variable selection, removing variables according to the respective value of permutation importance (Phillips et al., 2006), and keeping them out of the model if the resulting one was more parsimonious. The selected model included linear and quadratic features, 360 iterations and a regularization multiplier of 1.5.
To evaluate model accuracy, we computed the true skill statistic (TSS) and the area under the curve (AUC) of the receiver operating characteristics, over the training and test data sets. While the absolute value of such statistics is poorly informative (see, e.g., Lobo et al., 2008), their use over training and testing data sets allows an assessment of the models' robustness and generalizability. We also carefully checked whether the species–habitat relationships depicted by the models were consistent with current knowledge on species ecology (Guevara et al., 2018). Although necessarily qualitative, such an evaluation of the ecological meaning and reliability of species–habitat relationships highlighted by models is key to understand the “ecological realism” of models (Elith & Leathwick, 2009; Merow et al., 2014). We also evaluated the relative importance of each predictor included in the final model by means of the permutation importance, evaluated over 10 permutations (Table 1).
2.2 Landscape connectivity models and resistance surfaces
To describe landscape connectivity, we adopted a potential connectivity approach, which integrates distribution models and connectivity models based on the outcomes of the former (Rödder et al., 2016). Differently from Rödder et al. (2016), we did not include barriers, as our model species is extremely unlikely to be affected by the occurrence of “insurmountable barriers” (as defined in the cited study), due to its good flying ability. Given the lack of obligate dispersal directions for the target species within the study area, we selected a method based on circuit theory, allowing us to consider all potential connections among patches. Instead of the widely adopted, “original,” Circuitscape method (Hall et al., 2021; McRae et al., 2013), we selected the “omniscape” method. The omniscape algorithm can be regarded as a sort of “spatial generalization” of Circuitscape, as it extends the method implemented by the latter to continuous spatial data, and is explicitly recommended for habitat suitability maps (McRae et al., 2016). omniscape applies Circuitscape iteratively throughout the study landscape, by means of a moving window. Elaborations are based on a resistance raster, which can be derived from habitat suitability models, and a raster representing the source strength, which can also be derived from the same models and expresses the amount of current injected into a specific point (Landau et al., 2021). The omniscape algorithm evaluates the potential connectivity between all pairs of pixels in a given landscape that (i) act as sources, having a source strength higher than the threshold set by the user, and (ii) are closer than the radius set for the moving window procedure (Landau et al., 2021). We ran omniscape in the programming language julia 1.6 (Bezanson et al., 2017). Given the lack of information about dispersal distances for the water pipit, we set the radius for the moving window at an arbitrary value of 10 km, because this value allows us to include high-elevation areas potentially suitable for the target species of neighbouring mountain massifs, but do not encompass suitable sites located on the opposite sides of the major large valleys of the study area. Resistance was estimated as the inverse of habitat suitability (from maxent output); before the computation, all suitability values lower than 0.001 were set at that value, to avoid extremely large resistance values: the resulting raster has resistance values between ~1 for sites with the maximum suitability, and 1000 for sites with the lowest suitability. As source areas, we considered all those with a resistance value lower than the maximum resistance value at which the species was observed. Cumulative current fluxes were reclassified according to the cumulative current value recorded at all species locations.
To assess landscape effects on water pipit dispersal, we used two different landscape resistance surfaces, the first one calculated as the inverse of habitat suitability (from maxent output) and the second one as the inverse of landscape connectivity (from omniscape output). In both cases, we calculated values of accumulated landscape resistance among sampling sites as well as among single individuals by means of the Least Cost Path function in qgis 3.8 (QGIS.org, 2019). Relating multiple resistance surfaces to the observed genetic differentiation pattern is a widely adopted approach, but it is usually carried out by considering surfaces based on different landscape elements (e.g., Amos et al., 2014; Ruiz-Gonzalez et al., 2014), or on landscape changes through time (e.g., Miller et al., 2018). By contrast, we aimed to assess if landscape resistance was better represented simply by habitat “unsuitability” or by a conceptually more complex layer describing dispersal movements.
2.3 Sampling and DNA extraction
We obtained blood samples from 93 water pipits captured with mist-nets and spring traps during the 2021 breeding season at 10 sampling areas, spanning from the peripheral massifs of the Prealps in the south to the main Alpine watershed in the north (Figure 1). The sampling areas were located between 15 and 145 km from each other (mean distance ± SD: 78 ± 33 km). This distance range is large enough to study dispersal in passerine birds, as dispersal distances beyond 100 km have been found to be very rare in many different species (e.g., Paradis et al., 1998; Rushing et al., 2021; Winkler et al., 2005), and our range also largely overcomes such distance. Within each sampling area, we captured birds at several different sites up to a few kilometres from each other. We sampled 9–11 birds per area, although at the southernmost site (Lessinia) we only obtained three samples, because at this peripheral site the species is scarce. The sampling period (end of May to mid-July) allowed us to exclude the migration period of the water pipit (Spina & Volponi, 2008). In addition, all sampled individuals were sexually mature (in their 2nd year or older, aged following Demongin, 2016), showed well-developed incubation patches (females) or cloacal protuberances (males), and males showed territorial behaviour before and/or after the capture. This confirmed that we sampled reproductive individuals at their breeding sites. Blood samples (20 μL) were obtained by puncturing the brachial vein (Owen, 2011) and stored in ethanol. We extracted DNA using an E.Z.N.A. Tissue DNA Kit (Omega Bio-Tek) according to the manufacturer's protocol.
2.4 RAD sequencing and initial data filtering
Library preparation and sequencing were carried out at IGA Technology Services. The enzyme combination for double digest (dd)RAD library preparation was selected by analysing in silico closely related species (Motacillidae). Digested DNA fragments were ligated to barcoded adapters, pooled and purified and targeted DNA fractions were collected by setting the range to 510–650 bp. Fractions were then amplified, purified and sequenced with 150 cycles in paired-end mode on a NovaSeq 6000 instrument following the manufacturer's instructions (Illumina). Further details are provided in the Supporting Information.
Initial raw data analysis and single nucleotide polymorphism (SNP) calling were performed via the IGA Technology Services in-house bioinformatics pipeline. ddRAD reads were processed using stacks 2.53 (Catchen et al., 2013), using the following program utilities: process_radtags for raw Illumina reads demultiplexing, ustacks to assemble the short reads of each sample into exactly matching stacks, cstacks to create the catalogue of loci, sstacks and tsv2bam to match each sample against the catalogue, and gstacks to pull in paired-end reads (if available), assemble the paired-end contigs and merge with the single-end loci, align reads to the loci, and call SNPs. Finally, detected loci were filtered using the populations utility included in stacks 2.53 (Catchen et al., 2013), using option –R = 0.75 to retain only loci that are represented in at least 75% of the whole sample and with cutoff --max-obs-het = 0.8 in order to process a nucleotide site at a locus with an observed heterozygosity at maximum of 80%.
We used the program plink 1.90 beta (Chang et al., 2015) to filter the SNPs data set for linkage disequilibrium and missing data, setting minimum allele frequency at 0.01 and excluding individuals with >20% missing genotypes. This resulted in the exclusion of three individuals with excessive missing data, and therefore the final data set included 90 water pipits and 56,960 SNPs. We then used the program pgdspider 2.1.1.5 (Lischer & Excoffier, 2012) to convert the plink data set into the different formats required in downstream analyses.
2.5 Genetic distances and population structure
We first explored our data by calculating basic statistics such as expected and observed heterozygosity (HE and HO) in arlequin 3.5 (Excoffier & Lischer, 2010) and inbreeding coefficients (FIS) using plink. We then assessed genetic differentiation within our sample at both site and individual levels, by calculating pairwise FST values among sampling areas using arlequin and pairwise identity-by-state (IBS) distances among individuals in plink. Besides pairwise distances calculated to test IBD and IBR hypotheses (see the following section), we also investigated the overall genetic population structure, because population clustering may highlight the occurrence of especially important barriers to dispersal (e.g., Ceresa et al., 2018; Millions & Swanson, 2007). To this end, we adopted two different approaches, using both a discriminant analysis of principal components (DAPC) implemented in the package adegenet 2.1.5 (Jombart, 2008; Jombart & Ahmed, 2011) in program R 4.1.1 (R Core Team, 2021) and the Bayesian approach implemented in structure 2.3.4 (Falush et al., 2003; Pritchard et al., 2000). Using and comparing more statistical methods is indeed recommended especially in cases of weak population structure (Frosch et al., 2014; Kraus et al., 2016), which is a possible scenario within our study system. In structure, we estimated the most likely number of distinct genetic clusters (K) by fitting a model with population admixture and correlated allele frequencies (Falush et al., 2003), and we performed the analysis both with and without spatial information about sampling sites. For each value of K between 1 and 10, we carried out five independent runs with a burn-in period of 10,000 iterations and 10,000 Markov chain Monte Carlo replications. To better identify the actual number of genetic clusters, we used structure results to calculate the ΔK statistics (Evanno et al., 2005). DAPC was carried out through the find.clusters function of the R package adegenet 2.1.5, which identifies genetics clusters through the K-means algorithm (Jombart et al., 2010).
2.6 Testing isolation by distance and by resistance
To test our IBD and IBR hypotheses, we first explored the relationship between pairwise geographical distances/landscape resistances and values of both FST (site level) and IBS distances (individual level) by means of Mantel and partial Mantel tests (Amos et al., 2014; Cushman, Wasserman, et al., 2013). In addition, given the possible interpretation problems of Mantel test results in landscape genetics (Meirmans, 2015), we deepened our comparison of IBD and IBR hypotheses by using maximum likelihood population effects (MLPE) models (Clarke et al., 2002). These models allow us to account for the nonindependence of pairwise distances (Clarke et al., 2002) and have been found to outperform other regression-based approaches for model selection in landscape genetics (Shirk et al., 2018). Site-level analyses did not include birds from Lessinia, given the too small sample size (N = 3); however, individual-level analyses, besides using slightly more precise geographical and cost distances, allowed us to include these three individuals from the southernmost sampling area.
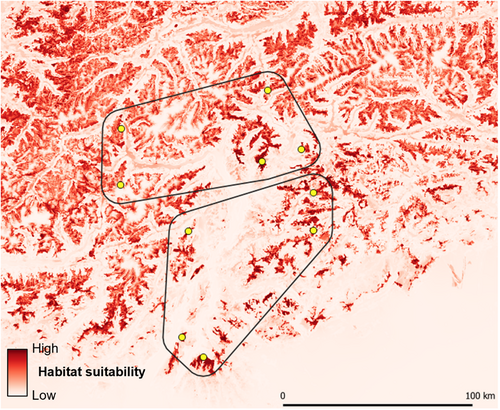
Mantel and partial Mantel tests were performed using the mantel function in the R package ecodist 2.0.7 (Goslee & Urban, 2007) with 10,000 permutations. When both IBD and IBR (hereafter, landscape resistance and IBR refer to 1/habitat suitability, see Section 3) were significantly supported, we used partial Mantel tests in a causal modelling framework to assess if the observed patterns of genetic differentiation were better explained by IBR or by IBD (Amos et al., 2014; Cushman, Wasserman, et al., 2013); that is, we calculated the relationship between landscape resistance and genetic distances after removing the effect of geographical distances, and vice versa. In case of nonsignificant Mantel tests and partial Mantel tests for both IBR and IBD, we concluded that genetic distances within our study area were not related to geographical distances or to the considered landscape resistance measures. We carried out these analyses for the entire sample but also separately for two subareas that clearly differ in the availability of suitable habitat. According to our habitat suitability model, areas potentially suitable for breeding covered ~29% and 16% of the northern and the southern subareas, respectively (Figure 4). The number of sampling sites for each subarea would have been very low, and therefore we carried out only the individual-level analysis. Given the repeated hypothesis testing (two subareas), we adjusted p-values by means of the Holm–Bonferroni correction for multiple testing (Holm, 1979).
MLPE models were fitted by using the MLPE.lmm function in the R package resistancega 4.2.4 (Peterman, 2018), with the default specification “scale = TRUE” to allow comparisons among the effects of the explanatory variables. We fitted a set of competing models using either geographical distances (IBD model) or cost distances (IBR model) as explanatory variables, and genetic distances as the response variable. We also fitted a null model without any distance or landscape resistance effect. We then ranked the models according to their AIC, and we considered models with ΔAIC <2 to be substantially supported (Burnham & Anderson, 2002). Furthermore, we calculated R2 for each model using the function r.squaredGLMM in the R package mumin 1.47.1 (Bartoń, 2019) and bootstrapped 95% confidence intervals of explanatory variables with the tidy function of the R package broom.mixed 0.2.9.4 (Bolker & Robinson, 2022). We considered a variable effect to be significant when 95% confidence intervals did not include 0. For MLPE models, we also carried out the analyses for the entire sample, and separately for the two subareas with different habitat availability. Differently from Mantel tests, in this more complex statistical approach we only considered the individual level, given the relatively low number of sampling sites.
3 RESULTS
3.1 Habitat suitability and landscape connectivity models
maxent modelling allowed us to obtain both an accurate and a robust description of breeding habitat suitability for water pipits in our study area (Figure 4). The validation procedure indeed showed no decline in model accuracy and discriminatory ability when tested on the independent data set (TSS train = 0.51; TSS test = 0.48; AUC train = 0.81; AUC test = 0.81). Land cover categories overall showed the highest relative permutation importance in describing habitat suitability (65.9%), followed by mean annual temperature (17.0%) and average slope (16.9%). The observed relationships between environmental factors and water pipit occurrence (details are provided in Figure S1) were largely consistent with previous information about the ecology of this species (e.g., Brambilla et al., 2017; Brambilla, Gustin, et al., 2020; Chamberlain, Brambilla, et al., 2016).
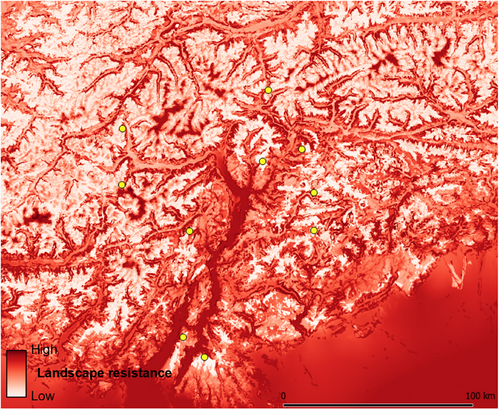
Cumulative current maps obtained from landscape connectivity models highlighted the occurrence of areas of low connectivity, especially in the southern part of the study area and along the main valley floors (Figure 5). The resistance surface obtained from habitat suitability indicated high resistance corresponding to the main valley floors and, to a lesser extent, also to the highest mountain ridges (Figure 6); the surface obtained as 1/connectivity was very similar (Figure S2).
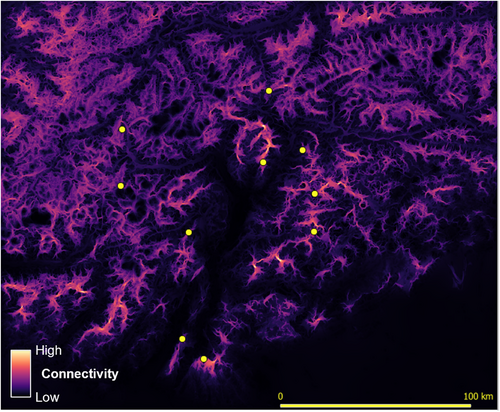
3.2 Genetic distances and population structure
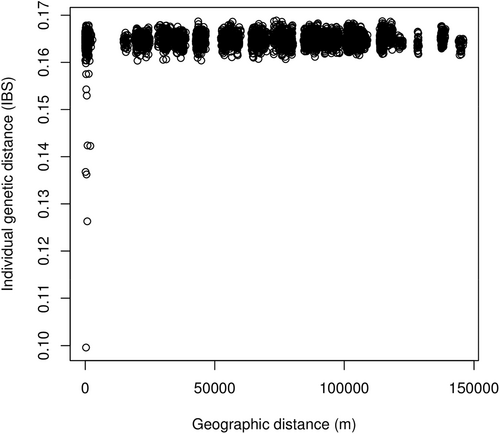
Pairwise genetic differentiation among sampling areas was always low, but in many cases statistically significant (Table 2). IBS individual genetic distances ranged between 0.100 and 0.169 (mean ± SD: 0.165 ± 0.002). We did not find evidence for population clustering within our sample, based on both structure and DAPC analyses. The K = 1 hypothesis indeed obtained the highest likelihood in structure analysis and the lowest Bayesian Information Criterion value in DAPC. Furthermore, values of ΔK statistics (which cannot be calculated for K = 1) did not show clear single peaks, indicating the lack of support for K > 1. Further details about population structure analysis are provided in Table S2, as well as basic statistics (HO, HE and FIS), which did not show clear differences among sampling sites (Table S1).
| Plose | Brennero | Villandro | Resia | Lessinia | Paneveggio | Pordoi | Stelvio | Baldo | |
|---|---|---|---|---|---|---|---|---|---|
| Brennero | 0.0075 | — | |||||||
| Villandro | 0.0063 | 0.0017 | — | ||||||
| Resia | 0.0055 | 0.0020 | 0.0014 | — | |||||
| Lessinia | 0.0084 | 0.0018 | 0.0049 | 0.0025 | — | ||||
| Paneveggio | 0.0059 | 0.0023 | 0.0011 | 0.0011 | 0.0025 | — | |||
| Pordoi | 0.0055 | 0.0016 | 0.0023 | 0.0011 | 0.0022 | 0.0004 | — | ||
| Stelvio | 0.0080 | 0.0043 | 0.0031 | 0.0035 | 0.0030 | 0.0029 | 0.0030 | — | |
| Baldo | 0.0138 | 0.0097 | 0.0085 | 0.0075 | 0.0088 | 0.0078 | 0.0082 | 0.0099 | — |
| Peller | 0.0093 | 0.0044 | 0.0035 | 0.0031 | 0.0040 | 0.0033 | 0.0035 | 0.0053 | 0.0100 |
- Note: Significant values (p < .05) are given in bold type.
3.3 Testing isolation by distance and by resistance
Despite the conceptual differences between the two landscape resistance surfaces, they turned out to be very similar (the two respective cost distance matrices were strongly correlated, r = .97), and the inverse of habitat suitability always performed slightly better in representing landscape resistance, being more strongly related to genetic distances. Therefore, all the reported results regarding IBR were obtained by using this surface. The results of the analyses using 1/connectivity are provided for comparison as Supporting Information, and the differences between the two surfaces are briefly commented on in Section 4. Considering genetic differentiation among sampling sites, the IBD hypothesis was not significantly supported by the Mantel test, while IBR was significantly supported, also after accounting for geographical distances (Table 3a). For individual genetic distances, both IBD and IBR were significantly supported, with IBR showing a slightly higher Mantel r (Table 3b). IBR was significantly supported also after accounting for geographical distances, while the inverse did not occur (Table 3b), clearly indicating a stronger support for IBR than for IBD. For both the site and individual level, Mantel correlograms indicated the occurrence of a threshold in the effect of landscape resistance, which disappeared for the highest accumulated resistance values (Figure 7).
| Area | Mantel test | Partial mantel test | ||||
|---|---|---|---|---|---|---|
| Model | r | p | Model | r | p | |
| (a) | ||||||
| All | IBD | .328 | .151 | IBD|IBR | −.348 | .234 |
| IBR | .630 | .024 | IBR|IBD | .637 | .035 | |
| (b) | ||||||
| All | IBD | .109 | <.001 | IBD|IBR | −.011 | .736 |
| IBR | .140 | <.001 | IBR|IBD | .088 | .036 | |
| North | IBD | .080 | .002 | IBD|IBR | −.015 | 1.000 |
| IBR | .088 | .006 | IBR|IBD | .045 | .511 | |
| South | IBD | .164 | <.001 | IBD|IBR | −.017 | 1.000 |
| IBR | .185 | <.001 | IBR|IBD | .089 | .066 | |
- Note: Significant values (p < .05) are given in bold type.
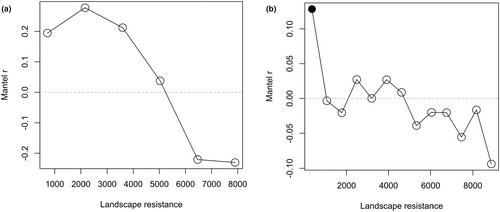
We obtained partly different results for the two subareas with different habitat availability (based only on individual genetic distances, see Section 2). In both areas, IBD and IBR were supported according to Mantel tests, with higher Mantel r for IBR (Table 3b). However, IBR was notably stronger in the southern than in the northern area (Mantel r = .185 and .090, respectively) and than in the entire sampling area (Mantel r = .140). After accounting for geographical distance and adjusting p-values for multiple testing, IBR was not significantly supported in either area, although the partial Mantel test was nearly significant for the southern area (Table 3b).
The MLPE model describing IBR performed clearly better than the IBD model, with a large difference in AIC values (ΔAIC = 41.04). The difference with the null model was even larger (ΔAIC = 115.04). Marginal R2 for the IBR model was low, but higher than for IBD and especially than for the null model (Table 4). Landscape resistance showed a positive and significant effect on individual genetic distances according to the bootstrapped confidence intervals (CI), which did not include zero (95% CI: 0.0004–0.0007).
| Area | Model | ΔAIC | R2 (marginal) | R2 (conditional) |
|---|---|---|---|---|
| All | IBR | 0.00 | .0386 | .1517 |
| IBD | 41.04 | .0204 | .1337 | |
| Null | 115.04 | .0000 | .1111 | |
| North | IBR | 0.00 | .0113 | .0861 |
| IBD | 4.91 | .0065 | .0794 | |
| Null | 10.62 | .0000 | .0730 | |
| South | IBR | 0.00 | .0477 | .1034 |
| IBD | 10.08 | .0349 | .0888 | |
| Null | 39.13 | .0000 | .0479 |
Considering the two subareas separately, the IBR model was the best supported in both cases, and again with a large difference in terms of AIC values compared to the IBD model and to the null model (Table 4). In the southern subarea, the effect of landscape resistance was stronger than in the northern one, showing a larger difference with the alternative models in terms of AIC values and marginal R2 (Table 4). Consistently, the effect of landscape resistance on individual genetic distances confirmed the stronger IBR in the south, although the effect was significant in both areas (95% CI: northern area: 0.0001–0.0004; southern area: 0.0004–0.0007).
4 DISCUSSION
The support we found for IBR indicates that landscape characteristics affect dispersal of water pipits, which apparently disperse more easily across the high-elevation grasslands and pastures suitable for breeding. Therefore, landscape resistance probably contributes to the observed (even if low) genetic differentiation, which indicates high but not unrestricted gene flow. Within the study area, low-elevation valley floors, wooded mountain slopes and, secondarily, the highest elevations (nival zone) lead to high resistance to dispersal according to our resistance surface (Figure 6). As a possible explanation, overflying unfamiliar landscapes during dispersal (probably solitary flights) may imply higher predation risks by avian predators; for example, in our study area the Eurasian sparrowhawk Accipiter nisus is very common across woodlands, and peregrine falcon Falco peregrinus breeding pairs are concentrated at low and middle elevations along the main valleys. By contrast, overflying suitable open habitat potentially provides more resting and foraging opportunities and allows a constant exploration of potential breeding sites. The partly different patterns found in the two subareas with different habitat availability are consistent with our initial expectations and confirm that observed effects of landscape on dispersal can vary according to habitat configuration and the considered spatial scale (Amos et al., 2014; Segelbacher et al., 2010). In the southern subarea, which includes more peripheral massifs, the unsuitable habitat matrix among breeding areas is more extended, potentially leading to higher dispersal costs and risks.
While IBR has been found in resident bird species/populations (e.g., Amos et al., 2014; Klinga et al., 2019; Unfried et al., 2013), it has been rarely reported for migratory birds such as the water pipit, possibly also because fewer studies have looked for IBR in migrant species, which are generally believed to be more mobile and less affected by habitat fragmentation during dispersal (e.g., Pruett et al., 2008; Unfried et al., 2013). More generally, birds are underrepresented in landscape genetic studies, possibly due to a preconceived assumption of lack of landscape effects on dispersal in this taxon (Kozakiewicz et al., 2018). García et al. (2021) found evidence of IBR in a long-distance migrant passerine bird, the bluethroat Luscinia svecica, when investigating more scattered populations over a wider area than in our study. The authors suggested that the known high philopatry of the investigated populations was probably involved in determining the observed restriction in gene flow (García et al., 2021). In fact, despite their high movement capability, dispersal of migratory birds can be restricted by high philopatry (e.g., Ceresa et al., 2016; Hansson et al., 2002), probably due to risks and costs connected to dispersal (e.g., increased mortality, physiological costs; Plissner & Gowaty, 1996; Waser et al., 1994). Such a scenario probably occurs also in our study system, as suggested by the occurrence of several more closely related individuals sampled at very short distances (up to a few kilometres, that is within the same study areas; Figure 8). This pattern is probably related to philopatry, because all sampled birds already had the opportunity to disperse and were reproductive individuals (see Section 2.3). In a harsh environment such as high mountains, detailed knowledge of local topography, foraging areas and predators is likely to be especially advantageous, increasing the costs of moving to a new, unknown grassland patch. In addition, the observed threshold in IBR (Figure 7) may indicate that the degree of resistance to dispersal is mainly determined by the landscape surrounding the sampling areas, and thus the characteristics of this matrix could influence the decision to disperse. This hypothesis is consistent with the detection of a clear IBR pattern also over relatively reduced extensions such as our two subareas (Table 4).
The resistance surface calculated as 1/habitat suitability explained genetic distances better than 1/connectivity according to MLPE models (Table S3), suggesting that it provided a better representation of landscape resistance to dispersal. It is noteworthy that a modelling framework explicitly designed to describe animal movements did not provide a better representation of landscape resistance than a description of habitat suitability. A possible explanation is that, in algorithms based on the circuit theory such as omniscape, animals move as random walkers (McRae et al., 2016), while according to several studies, bird dispersal cannot be adequately described as a random walk (e.g., Ceresa et al., 2016; Van Houtan et al., 2007, 2010). In fact, the distribution of bird dispersal distances often follows “fat-tailed” functions describing prolonged, directional movements, while random walk describes a slow diffusion through space and is probably more suited to describe routine movements within habitat patches (see Van Dyck & Baguette, 2005). In our case 1/habitat suitability was probably the best representation of landscape resistance because it adequately defined areas that are tracked or avoided by dispersing individuals, while the intent of modelling bird dispersal movements through random walk did not add useful information to the 1/connectivity surface. It is possible that further information currently unavailable (e.g., the radius for the moving window in omniscape) would improve models based on connectivity.
Despite the observed IBR, levels of gene flow in the study area were high. This indicates that water pipits can maintain an adequate exchange of individuals among breeding areas, including the most peripheral ones. This is not surprising, given the generally good dispersal ability of birds and the extent of the study area. The large amount of suitable breeding areas in the study area (and neighbouring regions; see Figure 3) implies relatively short distances among habitat patches, which probably concur in maintaining high levels of gene flow. Such a relatively wide and continuous breeding range within the study area probably also explains the quite low variance in genetic distances explained by landscape resistance (and even lower by geographical distances, in less supported models; Table 4). This interpretation is supported by the higher marginal R2 in the subarea with lower extent of suitable habitat (Table 4). However, low marginal R2 in MLPE models may also be partly due to the occurrence of a threshold in the effect of landscape resistance (Figure 7), that is a nonlinear effect, while MLPE are linear models.
While our results show high gene flow in the current conditions, a scenario of more restricted and patchy distribution in the near future is likely in the European Alps, given the high forecasted habitat loss due to climate change in the southern Alps (Brambilla et al., 2017) and the ongoing decline in some Alpine areas (e.g., Rete Rurale Nazionale & Lipu, 2021). In a context of scarcer and more fragmented suitable habitat, IBR and philopatry could have stronger effects on gene flow than we observed, especially in the most peripheral areas, such as the Prealps. In addition, recolonizations of isolated alpine grassland patches after local extinctions due, for example, to stochastic factors, could become more difficult, in the long term resulting in a more restricted breeding range than the potentially suitable one. Therefore, maintaining high habitat suitability through appropriate conservation measures would be important primarily for focal peripheral breeding areas, to buffer against climate change and maintain more continuous and interconnected peripheral subpopulations. Such measures include promoting extensive cattle grazing to maintain alpine pastures and avoiding shrub encroachment (Brambilla et al., 2018; Brambilla, Gustin, et al., 2020; Chamberlain et al., 2013), while at the same time avoiding intensive grazing, which leads to grassland and soil degradation (Garcia-Pausas et al., 2017) and can negatively affect open-habitat birds (Brambilla, Gustin, et al., 2020; Pavel, 2004). Limiting human disturbance due to outdoor activities and avoiding the building of new tourist infrastructures is also recommended, because human disturbance can affect bird settlement (Bötsch et al., 2017), cause avoidance of potentially suitable habitat (Coppes et al., 2017; Perona et al., 2019) and increase stress levels (Formenti et al., 2015); furthermore, ski-pistes negatively affect high-elevation bird communities (Caprio et al., 2011; Rolando et al., 2007). The same measures would also be beneficial across the entire Alpine range, in order to maintain the current population size also in the innermost Alpine areas. In these colder sectors, alpine grasslands are still apparently entirely within the water pipit temperature optimum (Ceresa et al., 2021) and the occurrence of the species is predicted to persist in the next few decades despite a changing climate (Brambilla et al., 2022). Avoiding habitat loss and degradation in these areas is therefore crucial to maintain the core water pipit populations in the long term. With average higher elevation and long-lasting snow cover, breeding areas in these Alpine sectors are especially vulnerable, because they will increasingly overlap with winter tourism infrastructures due to climate change (Brambilla et al., 2016). The aforementioned conservation measures would also be beneficial for other high-elevation species (e.g., rock ptarmigan, alpine accentor, white-winged snowfinch), for some declining open-habitat birds (e.g., skylark Alauda arvensis, rock partridge Alectoris graeca), and for raptors foraging mainly in open mountain areas, such as golden eagle Aquila chrysaetos and bearded vulture Gypaetus barbatus.
Given the area we considered and our aims, our study is obviously only focused on within-mountain chain dispersal, and we cannot provide information about gene flow among different mountain chains that could be obtained, for example, by continental-scale research. Future investigation at this scale could allow us to assess, for example, connectedness of small and isolated breeding areas of the lower mountain systems to large populations, such as the Alpine one. However, given that water pipits apparently prefer to avoid valley floors and woodlands during dispersal, even where the matrix of such unsuitable landscape is relatively reduced as it is in our study area, it is expected that very large lowlands or hilly areas separating different mountain systems could oppose a strong resistance to dispersal.
Although our results should be considered as species- and context-specific, detecting IBR in an area where the water pipit is relatively widely distributed suggests that other high-elevation specialist birds with more specific habitat requirements (e.g., white-winged snowfinch, alpine accentor, wallcreeper Tichodroma muraria) may show a similar pattern, but with possibly stronger effects on gene flow because of their more restricted and patchy distributions (see species distribution details in BirdLife International, 2022). Like the water pipit, all these species are vulnerable to climate change and their distribution area is predicted to decrease strongly in the next few decades across the Alps and other European mountain ranges (Brambilla et al., 2016, 2017; De Gabriel Hernando et al., 2021). Within our study area, the white-winged snowfinch is currently absent from peripheral massifs that are apparently suitable according to habitat suitability models (our unpublished data), as a possible consequence of restricted dispersal and decline of nearby subpopulations. For these reasons, our results indicate a possible current and, especially, future lack of connectivity in high-elevation specialist bird populations. Improving our knowledge about dispersal in these species is urgently needed to better understand the degree of vulnerability of high-elevation bird communities to current climate change, and habitat loss and degradation.
AUTHOR CONTRIBUTIONS
FC, MB and LK designed the study, FC, SV, MP and LT carried out fieldwork, FC performed DNA extraction, MB developed distribution and landscape connectivity models, FC analysed the genetic data supported by LK, PK led fieldwork and laboratory organization, PP provided fieldwork and laboratory support, and FC wrote the main manuscript text. All authors contributed to discussing the results and revising and editing the manuscript.
ACKNOWLEDGEMENTS
We are grateful to Matteo Anderle, Chiara Bettega and Jennifer Rossin for their help with fieldwork. The present study was financed by the fund “Research Südtirol/Alto Adige” of the Department of Innovation, Research and University of the Autonomous Province of Bolzano/Bozen, within the project “Population connectivity in high-elevation Alpine birds threatened by climate change,” CUP H32F20000020003. Fieldwork was carried out under permits of the relevant national and local authorities: “Prot. no. 2020/60029,” “Prot. no. 2020/61001,” “Prot. no. 2020/60041,” “Prot. no. 2020/60045” and “Prot. no. 2021/5190” of the Istituto Superiore per la Ricerca e Protezione Animale (ISPRA); “Decreto no. 1055/2021,” “Decreto no. 2415/2021” and “Decreto no. 2837/2021” of the Autonomous Province of Bolzano/Bozen; “Determinazione Prot. 87FAU/2021” and “Determinazione Prot. 88FAU/2021” of the Autonomous Province of Trento.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest.
BENEFIT-SHARING STATEMENT
A new scientific collaboration was developed specifically to carry out this study, with all collaborators included as coauthors or in the acknowledgements, depending on their level of input. The research addresses a priority concern, namely population connectivity in a high-priority species for conservation, and provides useful indications for conservation measures and priorities. In addition, benefits from this research accrue from the sharing of our data on a public database as described above.
Open Research
DATA AVAILABILITY STATEMENT
Data and script used in this paper, including raw sequence data, are publicly accessible through the institutional repository of the Università degli Studi di Milano, UNIMI dataverse (Ceresa et al., 2023a: https://doi.org/10.13130/RD_UNIMI/VTSVZQ; Ceresa et al., 2023b: https://doi.org/10.13130/RD_UNIMI/IFCPAX; Ceresa et al., 2023c: https://doi.org/10.13130/RD_UNIMI/6T6BCE).




