Baculoviruses hijack the visual perception of their caterpillar hosts to induce climbing behaviour thus promoting virus dispersal
Handling Editor: Camille Bonneaud
Abstract
Baculoviruses can induce climbing behaviour in their caterpillar hosts to ensure they die at elevated positions to enhance virus transmission, providing an excellent model to study parasitic manipulation of host behaviour. Here, we demonstrate that climbing behaviour occurred mostly during daylight hours, and that the height at death of Helicoverpa armigera single nucleopolyhedrovirus (HearNPV)-infected larvae increases with the height of the light source. Phototaxic and electroretinogram (ERG) responses were enhanced after HearNPV-infection in host larvae, and ablation of stemmata in infected larvae prevented both phototaxis and climbing behaviour. Through transcriptome and quantitative PCR, we confirmed that two opsin genes (a blue light-sensitive gene, HaBL; and a long wave-sensitive gene, HaLW) as well as the TRPL (transient receptor potential-like channel protein) gene, all integral to the host's visual perception pathway, were significantly upregulated after HearNPV infection. Knockout of HaBL, HaLW, or TRPL genes using the CRISPR/Cas9 system resulted in significantly reduced ERG responses, phototaxis, and climbing behaviour in HearNPV-infected larvae. These results reveal that HearNPV alters the expression of specific genes to hijack host visual perception at fundamental levels—photoreception and phototransduction—in order to induce climbing behaviour in host larvae.
1 INTRODUCTION
One feature of long-term coevolutionary relationships between parasites and their hosts, whether involving microbes, fungi, helminths or arthropods, is the ability of parasites to manipulate host behaviour (Poulin & Maure, 2015). Not all changes in host behaviour following parasitism represent manipulations on the part of the parasite, and many are simply pathological side-effects of parasitism (Poulin, 1995). However, any alterations in host behaviour that happen to benefit parasite fitness, typically by aiding its reproduction or dispersal, have the potential to evolve into adaptations that will be maintained by selection (Adamo, 2013; Poulin, 1994; Poulin & Maure, 2015). For example, when the Ophiocordyceps fungus infects an ant in the forest, the ant eventually leaves the nest, scales up a plant, and then dies with its mandible embedded in a leaf approximately 25 cm above the soil surface, where temperature and humidity are suitable for fungal sporulation and spore dispersal (Andersen et al., 2009). When the acanthocephalan Corynosoma constrictum infects the freshwater amphipod Hyalella azteca, an intermediate host, it induces higher responsiveness to red light and lower responsiveness to green light, causing increased wandering behaviour of the amphipods in the water column which enhances parasitoid transmission via increased predation risk (Benesh et al., 2005). The parasitoid wasp Ampulex compressa “zombifies” its cockroach host by injecting a calibrated amount of venom into its cerebral ganglia, allowing the wasp to guide the paralysed cockroach to its nest, where it remains a living food source for the wasp's larva (Gal & Libersat, 2008). Although parasite manipulation of host behaviour is a topic that has received considerable attention (Heil, 2016), the physiological mechanisms underpinning these manipulations remain poorly understood in most cases (Hoover, 2019).
Baculovirus-mediated changes in caterpillar behaviour are among the classic cases of host behavioural manipulation (Gasque et al., 2019). The Baculoviridae comprise a large group of double-stranded DNA viruses, including nucleopolyhedroviruses (NPVs) and granuloviruses, that infect more than 800 species of insects, primarily lepidopteran larvae (Herniou & Jehle, 2007). It is thought that baculoviruses have been coevolving with their insect hosts for 200–300 million years (Thézé et al., 2011). Following ingestion and subsequent infection, baculoviruses typically induce hyperactivity and climbing behaviour in their host caterpillars, sometimes referred to as “tree-top disease” (van Houte et al., 2014). Goulson (1997) speculated that behavioural changes in NPV-infected M. brassicae larvae most probably benefit the virus, while climbing behaviour favoured horizontal transmission of Spodoptera exigua MNPVs (SeMNPVs) via intraspecific necrophagy in S. exigua (Rebolledo et al., 2015). Moreover, cadavers at exposed positions can be visited by parasitoids, predators and scavengers that may actively disperse the virus over much greater distances (Entwistle et al., 1993; Olofsson, 1989; Raymond et al., 2005; Vasconcelos, 1996).
Climbing behaviour has been observed in larvae of many lepidopteran species, including Mamestra brassicae, Bombyx mori, Lymantria dispar, Lymantria monacha, Spodoptera exigua, Trichoplusia ni, and Helicoverpa armigera infected with NPVs (Gasque et al., 2019; Goulson, 1997; Han et al., 2015; Hoover et al., 2011; van Houte et al., 2012; Kamita et al., 2005; Katsuma et al., 2012). Although caterpillar climbing behaviour was first described more than 100 years ago, its physiological basis has not been studied in detail (Gasque et al., 2019). The viral egt gene encodes the ecdysteroid UDP-glucosyl transferase (EGT) that inactivates 20-hydroxyecdysone (20E) and has been implicated in the induction of climbing behaviour. Expression of the egt gene of L. dispar multiple nucleopolyhedrovirus (LdMNPV) is required to induce climbing behaviour in L. dispar larvae (Hoover et al., 2011), although the behaviour is not affected by egt gene expression in S. exigua or T. ni larvae infected by Autographa californica MNPV (AcMNPV; Ros et al., 2015) or in Helicoverpa armigera larvae infected by H. armigera NPV (HearNPV; Georgievska et al., 2010). Therefore, the role of the viral egt gene in mediating host climbing behaviour does not appear to be conserved across all baculovirus-host relationships. Previously, we demonstrated that juvenile hormone and 20E were linked to climbing behaviour in HearNPV-infected H. armigera larvae via their roles in mediating viral replication (Zhang et al., 2018).
Light is a factor guiding many important insect behaviours. For example, many insects depend on visual cues to find and select host plants, to choose mates, and to orient long-distance migrations (Jiggins et al., 2001; Sauman et al., 2005). Light is also an important factor mediating baculovirus-induced climbing behaviour in caterpillars (Bhattarai et al., 2018; Gasque et al., 2019; Han et al., 2018; van Houte et al., 2014). Exposure to light from above, during a specific period post-infection, is required to elicit climbing behaviour in SeMNPV-infected S. exigua larvae (Han et al., 2018). However, the mechanisms underlying the light-mediated behavioural changes induced by baculoviruses have not been explored. Insects perceive light through photoreceptors that are located mainly in the primary compound eye or the stemmata, light sensing organs in larvae that do not produce clearly focused images (Fain et al., 2010; Gilbert, 1994). The compound eyes of adult insects have three types of photoreceptors containing opsins, membrane-bound proteins sensitive to long wavelength, blue, and ultraviolet (UV) light (Kitamoto et al., 1998, 2000). Opsins change physical conformation when light signals are received by a photoreceptor cell (von Lintig et al., 2010). Light rays impinging on these receptors trigger Ca2+-permeable light-sensitive transient receptor potentials (TRPs) and transient receptor potential-like (TRPL) channels are opened, allowing extracellular calcium ions to flow into the cell. This triggers a wave of depolarization down the cell membrane for the length of the axon, converting the light signal to an electrical impluse (French et al., 2015; Hardie & Minke, 1992; Hardie & Raghu, 2001; Katz & Minke, 2018; Macias-Muñoz et al., 2019; Niemeyer et al., 1996).
Helicoverpa armigera (Lepidoptera: Noctuidae) is a polyphagous cosmopolitan pest of many crops, especially cotton, corn, and pepper (Gonçalves et al., 2019). Earlier studies have identified three opsin genes in adult H. armigera: the blue light-sensitive opsin gene HaBL, the long wave-sensitive opsin gene HaLW, and the UV-sensitive opsin gene HaUV (Yan et al., 2014), but the expression of these genes has not been studied in larvae. Based on our existing knowledge, we hypothesized that baculoviruses might manipulate the visual signalling pathway of their hosts by altering the expression of these genes, thus inducing positive phototaxis and climbing behaviour in infected caterpillars. Therefore, we used assays of phototaxis and electroretinogram (ERG) recordings to test whether virus-induced climbing is caused by an enhanced phototaxic response. Then, we ablated the stemmata in HearNPV-infected larvae to verify the role of the stemmata in mediating this phototaxis. Next, using transcriptomics and qPCR, we identified differentially expressed genes (DEGs) in the host's light transduction signalling pathway following HearNPV infection. Finally, we used CRISPR/Cas9 gene editing to confirm the roles of specific DEGs implicated in mediating climbing behaviour.
2 MATERIALS AND METHODS
2.1 Insect rearing and baculovirus infection
Larvae of H. armigera were reared on an artificial diet comprised of maize powder (300 g), soybean powder (100 g), yeast (100 g), vitamin B complex (1.5 g), and agar (25 g), all combined in 1.4 L of deionized water. Rearing was conducted under standardized conditions of 26 ± 1°C, 70 ± 10% RH, and a 14:10 (L:D) photoperiod. Neonate larvae were kept in glass test tubes (85 mm × 22 mm diameter) plugged with cotton, with 3–5 larvae per tube. When they reached the third-instar, larvae were held one per tube to prevent cannibalism until they pupated (after completing the fifth instar). Pupae were removed from the tubes within 3–4 days of pupation and placed in a plastic frame cage (40 × 20 × 20 cm) covered with gauze. Emerging adult moths were fed a 10% honey solution on cotton balls, refreshed daily, the gauze serving as an oviposition substrate.
The baculovirus used was the H. armigera single nucleopolyhedrovirus strain HearNPV-G4, which was originally isolated from H. armigera cadavers in Hubei province in 1981 (Chen et al., 2001). The virus was obtained as purified and freeze-dried powder (5 × 1011 OBs/g) from Henan Jiyuan Baiyun Industry Co., Ltd. In all HearNPV-infection experiments, newly molted fourth instar larvae were divided into mock- and HearNPV-infected groups and starved for 3–5 h. Then, each larva was fed for 12 h on a block of artificial diet (8 × 4 × 2 mm) contaminated with either 5 μl of a HearNPV suspension (2 × 108 OBs/ml; infected group) or 5 μl of sterile water (mock group). After this exposure, larvae were moved to uncontaminated diet blocks in tubes (as above), with one larva per tube, and time-stamped 0 h post-infection (hpi). We defined 0–24 h as 0 day post infection (dpi), 24–48 h as 1dpi, 48–72 h as 2 dpi, 72–96 h as 3dpi, etc. Both healthy and infected larvae entered the fifth instar after 48 hpi under conditions of 26 ± 1°C, 70 ± 10% relative humidity. In a preliminary experiment, mortality in the infected group ranged from 90% to 100%, with < 5% mortality in the mock group. These methods are henceforth referred to as the “standard infection procedure”.
2.2 Behaviour assays
2.2.1 The climbing apparatus
The climbing apparatus was comprised of glass tubes (300 mm height × 50 mm diameter) as in a previous study (Zhang et al., 2018). Each tube contained a vertically suspended strip of wire mesh (300 × 20 mm) as a climbing substrate (Figure S1A). A piece of opaque black fabric was used to shade the bottom of the tube and a transparent plastic sheet covered the top. An LED light source (c. 500 lux) was placed 20 cm above the tubes in a climate-controlled growth chamber as described above for standard growth conditions. Preliminary experiments indicated that varying the intensity of LED light (100, 500, or 1500 lux) did not affect climbing behaviour or height at death, so 500 lux was selected. The larvae of mock or infected group (n ≥ 20 larvae per treatment) were each placed on a piece of diet (20 × 10 × 10 mm) at the bottom of a climbing tube, provided in case some individuals had not yet entered the climbing phase, when feeding ceases. All larvae were observed to record mortality and climbing heights daily until all larvae had either died or pupated. Larvae in the HearNPV-infected group that either pupated or died from causes other than viral infection were excluded from analyses. Larvae in the mock group that died were excluded from analyses.
2.2.2 Observations of climbing behaviour
The climbing tubes described above were used to monitor larvae (24 larvae per treatment) in real time using an infrared camera to record climbing behaviour in the tubes on day and night until all larvae died or pupated. Each video recorder was connected to six cameras and one display, with each camera mounted in a cubical box (50 × 50 × 50 cm) to capture larval behaviour in eight tubes simultaneously (Figure S1B). The height of each larva was recorded hourly so that the average height of larvae in each group could be depicted graphically on a per-hour basis. Linear trend lines (y = mx + b) of hourly average height changes during day or dark can be constructed in excel. The slope values (m) of trend lines were used to assess the extent of larval climbing during light and dark periods under a 14:10 (L:D) photoperiod; the higher the value of m, the higher the height climbed during that interval. The data of m values (mean ± SE) during light and night were analysed by independent t test to analysis the difference between light and dark.
2.2.3 Climbing behaviour in response to light
To test the climbing response to light along the vertical plane, the climbing tubes (as described above) were used with white light (500 lux) positioned 5 cm from the glass tubes at either the top, middle, or bottom of the tubes. In each treatment, nonilluminated portions of the tubes (2/3 of the length) were covered with opaque black fabric to prevent the passage of light. One larva was placed in each tube after mock- or HearNPV-infection (40 larvae per treatment) using the standard infection procedure described above and the height at which each larva died was recorded.
In order to verify climbing response to light source on living plants in the vertical direction, we performed another laboratory assay on potted cotton plants that included two treatments, one with the light source at the top of the plant, and the other with the light source at the bottom of the plant (n = 3 independent plants per treatment); 30 infected larvae were distributed throughout each plant. The plants were about c. 45 cm tall (at budding stage, about 60 days post-germination) and each was contained in a plastic frame screen cage (50 × 50 × 50 cm). After c. eight days, when all larvae had died due to infection, the location of their death was categorized as either on the upper or lower half of the plant. The numbers of larvae dying on upper and lower parts of the plant was recorded. The percentage (mean ± SE) of larvae that died on the upper and lower half of the plant in different treatments were counted and a graph made. The data of the larval numbers were used to analysis the differences between upper and lower death distribution in treatments with different light source locations.
2.2.4 Assay of horizontal phototaxis
To directly observe the phototaxic responses of larvae in horizontal orientations over a short period (about 15 min), a hexagonal light box was constructed (15 cm height × 20 cm a side), with a 5 cm diameter hole cut in each side to allow the passage of light (Figure S1C,D). A smaller black hexagonal box (15 cm ht × 11 cm a side), was placed centrally within it, with a rectangular aperture cut centrally in the base of each side to permit the passage of larvae toward a selected light source. The exterior corners of the inner box were then connected to the interior corners of the larger box using opaque black cardboard (90 cm × 150 mm) to create six outer chambers, each illuminated separately, to which larvae placed in the central chamber could respond selectively (Figure S1D). One face of the apparatus was illuminated with white LED light (9 W, c. 500 lux) and the other five sides were left in darkness (Figure S1C). The spectrum of the LED light ranged from 400 to 700 nm (Yang et al., 2020). Experimental larvae (n = 3 replicates of 30 larvae each) were held in darkness for 30 min prior to testing and then placed in the central arena of the apparatus. After 15 min of light exposure, the number of larvae that had crawled into each compartment of the outer chamber was counted. Phototaxis was assayed using larvae 1, 2, 3, and 4 days post-infection (dpi) and responses were tallied as n/N × 100%, where n = number of larvae responding to the light source and N = total number of larvae. The data of response larval numbers in different treatments were used for statistical analysis, followed by other horizontal phototaxis experiments.
2.3 The role of the stemmata in phototaxis and climbing behaviour
To verify the role of larval stemmata in mediating phototaxis and climbing behaviour, we ablated the stemmata of mock and infected larvae at 2 dpi using a pair of No. 55 dissecting forceps (WPI, Dumont #55, 14099) under a dissecting microscope. The stemmata on both sides of the head were completely ablated o produce blind larvae (Figure 2c,d), a sham operation of both mock and infected larvae was performed by making a similar wound on the head near the stemmata (Figure 2a,b). Operations were performed carefully to minimize wound size to avoid excessive loss of haemolymph. At 2 days post-operation, mock and infected larvae were assayed for phototaxic behaviour as Section 2.2.4 and climbing behaviour as Section 2.2.1. The phototaxis response larval numbers and the final climbing heights reached by mock and infected larvae were recorded for groups of blind, sham, and sham under continuous darkness.
2.4 Electroretinogram assays
To directly compare retinal responses to light between mock and HearNPV-infected larvae, ERG recordings were performed as described previously by Wang et al. (2008). Using No. 55 dissecting forceps (WPI, Dumont no. 55, 14099), a small hole was made in the shell of the larval stemmata, avoiding damage to the stemmata, and a small hole was also made in the center of the head capsule 1.5 mm away from the stemmata. One glass microelectrode filled with Ringer's solution was inserted into each of these two holes. A Newport light projector (model 765) was used for stimulation. ERG signals were amplified with a Warner electrometer IE-210 and recorded with a MacLab/4s analogue-to-digital converter and the clampelx 10.2 program (Warner Instruments). Light pulses (intensity = c. 3000 lux) of 2 s on and 7 s off were used to test the light sensitivity of larvae. ERG recordings were performed on mock- and infected larvae at 1, 2, 3, 4 dpi (n = 5 larvae per treatment).
2.5 Transcriptome analysis
The Illumina high-throughput sequencing platform was used to identify DEGs in head tissue of mock- and infected larvae. Infected larvae were produced using the standard infection procedure and head tissue samples were dissected at 54 and 78 h post infection. Total RNA was extracted with TRIzol reagent (TaKaRa) with three replications (10 heads per replicate). This produced twelve libraries for RNA-seq that were paired-end sequenced on the Hiseq 4000 platform. Clean data were obtained by removing reads containing adapter sequences, poly-N sequences, and low-quality reads. All downstream analyses were based on clean data of high quality which were mapped to a reference genomic sequence of H. armigera (Genbank Accession: GCA_002156985.1) using the software tophat2 (Trapnell et al., 2012). A transcriptome of mapped reads was assembled using the software stringtie (Pertea et al., 2015). The HearNPV sequence derived reads were filtered before assembly to ensure precise evaluation. Gene expression levels were estimated by RSEM (Li & Dewey, 2011) and differential expression analysis was performed using the software degseq (Anders & Huber, 2010). The screening criteria for a significantly different expression were padj < 0.05 and a >2.0-fold change in gene read counts. KEGG pathway enrichment analysis was performed using kobas software (Xie et al., 2011). A total of 10 DEGs were randomly selected and verified using Quantitative PCR (qPCR) primers (Table S4) to validate the results of transcriptomic DEGs.
2.6 Quantitative PCR analysis
Quantitative PCR was used to assay levels of gene expression in healthy and infected H. armigera larvae across different tissue types and different developmental stages. Total RNA of infected and uninfected larval samples was extracted using the RNAiso Plus kit (TaKaRa). First-strand cDNA was synthesized from 1 μg of total RNA using the PrimeScript RT reagent kit with gDNA Eraser (Takara). qPCR was performed using SYBR Green Supermix (TaKaRa). The qPCR program was 95°C for 1 min, followed by 40 cycles of 95°C for 5 s, 60°C for 30 s. The ribosomal protein L32 (RPL32) gene was used as a reference, as it has been identified as a stable house-keeping gene in previous HearNPV studies (Zhang et al., 2015). The relative expression of each gene was calibrated in each of three biological replicates using the 2−ΔΔCt method (Livak & Schmittgen, 2001) and the primers listed in Table S4. The RNA samples of different tissues and developmental stages were extracted to characterize spatiotemporal gene expression. Samples of stemmata, brain, ventral nerve cord, midgut, Malpighian tubules, cuticle, salivary glands, fat body, testes, tracheas and haemocyte, were all dissected from healthy fifth instar larvae using fine tweezers under a microscope. The developmental stages samples included 1, 2, and 3 day-old eggs (n = 50 per sample); 1 and 2 day-old (post molt) first, second, third and fourth instar larvae (n = 5 per sample); the molting stage of first, second, third and fourth instar larvae; 1, 2, 3, 4, and 5 day-old fifth instar larvae (n = 5 per sample), and 1 and 2 day-old pupae (n = 5 per sample). All samples were ground in liquid nitrogen prior to extraction of RNA. To compare gene expression levels between mock and infected larvae by qPCR, the head tissues of larvae were sampled at 0, 1, 2, 3, 4, and 5 dpi (5 heads per sample).
2.7 Construction of homozygous strains by CRISPR/Cas9 gene editing
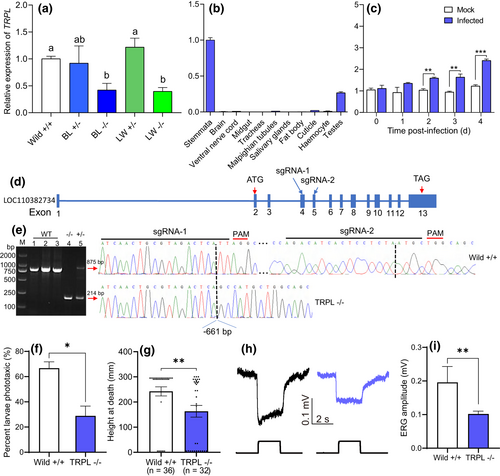
The above transcriptomics and qPCR analyses identified three light signal-related genes that were upregulated following infection with HearNPV: HaBL (blue light-sensitive opsin gene), HaLW (long wave-sensitive opsin gene) and TRPL (transient receptor potential-like channel protein). To confirm the roles of HaBL, HaLW and TRPL genes in mediating phototactic climbing behaviour, H. armigera strains with mutated HaBL, HaLW or TRPL genes were constructed using the CRISPR/Cas9 system. The sgRNA target sequence (5′-GAGGTGGTCGAGCACATGCTCGG-3′) for HaBL was selected at exon 2 of HaBL (Figure 4a), and the target sequence (5′-GCATAGCCGCCCTGCAAGCATGG-3′) for HaLW was selected at exon 2 of HaLW (Figure 4c), and the target sequences (sgRNA-1: 5′-ATCAACTGCGTAGACTCATTAGG-3′, sgRNA-2: 5′-AGACATAACTCCTCTAATGCTGG-3’) for TRPL were selected at exon 4 and 5, respectively (Figure 5d). The sgRNAs were synthesized by in vitro transcription utilizing the GeneArt Precision gRNA Synthesis Kit (Thermo Fisher Scientific) according to the manufacturer's instructions.
The Cas9 protein (GeneArt Platinum Cas9 Nuclease) was obtained from Thermo Fisher Scientific. Newly laid H. armigera eggs were collected and fixed on a microscope slide with double-sided adhesive tape. A mixture of Cas9 protein and sgRNA (200 ng/μl sgRNA + 200 ng/μl Cas9 protein) was microinjected into these eggs (n = c. 500 eggs per gene), with all operations completed within 2–3 h of oviposition. Injected eggs were incubated at 26 ± 1°C, 70 ± 10% RH, and a 14:10 (L:D) photoperiod until eclosion. When these F0 individuals became adults, we sampled one median leg from each to extract genomic DNA. A specific pair of primers for one gene sequence was designed to amplify a fragment from the genomic DNA containing the sgRNA target site. The PCR products were sequenced by TsingKe Biological Technology to determine the exact indel mutation type. In addition, we designed the primers that bound specially bind wild or mutant site sequences to enable detection of the mutant site for HaBL and HaLW by PCR and agarose gel electrophoresis. The primers for use in CRISPR/Cas9 gene editing are listed in Table S6. F0 mutant adults were mated with wild-type adults to produce F1 progeny, and heterozygous F1 mutant adults were mated with each other to produce F2 progeny. Homozygous F2 mutants were mated with each other to produce F3 homozygous mutant progeny and with wild-type adults to produce F3 heterozygous mutant progeny. These homozygous and heterozygous mutant strains were then used to determine the role of HaBL, HaLW and TRPL in phototaxis and climbing behaviour in HearNPV-infected H. armigera larvae. After HearNPV infection, the larvae were divided into two groups to test their phototaxis responses and climbing behaviour, respectively. Phototaxis was tested in the hexagonal light box at 3 dpi, as described above in Section 2.2.4, with three replicates of 30 larvae per treatment. Climbing behaviour was tested using the apparatus described above in Section 2.2.1 under a 14:10 (L:D) photoperiod, with the light source placed above the tubes. Height at death was recorded for all larvae in each treatment (n ≥ 20 larvae). ERG recordings between wild and mutants were performed on larvae at 3 dpi as described in Section 2.4. Expression levels of the TRPL gene in head tissue of wild-type and mutant (BL, LW) larvae were tested by qPCR as described above in Section 2.6.
2.8 Statistical analysis
Data were analysed using SPSS for Windows, version 21.0 (IBM Corporation). We used a single-sample Kolmogorov-Smirnov test to confirm that the data were normally distributed. Data for climbing height at death in Sections 2.2.3 and 2.3 were analysed by one-way ANOVA followed by Bonferroni test (α = 0.05), as were the phototaxic responses, climbing height and ERG amplitude data for wild, BL mutant and LW mutant larvae, and expression levels of the TRPL gene in wild-type and mutant larvae (Section 2.7). Data for the distribution of larval cadavers between upper and lower parts of plant in treatments with different light source locations (Section 2.2.3), were analysed by independent t test, as were phototaxic data for mock versus infected larvae (Section 2.2.4), the phototaxic data for sham versus blind mock (or infected) larvae and mock-sham versus Infected-sham (Section 2.4), the ERG responses of mock versus infected larvae (Section 2.5), the qPCR data for mock versus infected larvae (Section 2.6), and the climbing height deaths and ERG responses of wild-type versus TRPL mutant larvae (Section 2.7).
3 RESULTS
3.1 Climbing and phototaxic behaviour
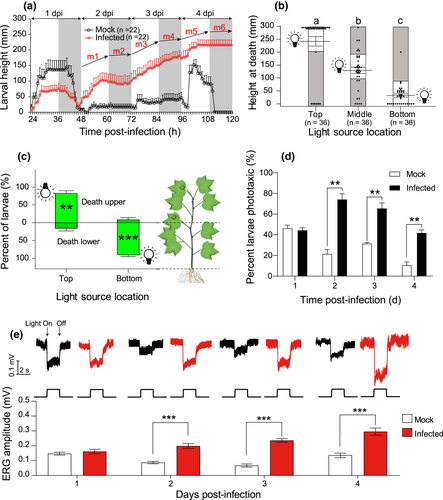
Mock-treated H. armigera larvae climbed to a mean (±SE) height of 136 (±30) mm in the tubes prior to the fourth molt (24–36 hpi), and to a mean (±SE) height of 152 (±16) mm during the wandering period (96–108 hpi, prior to pupation), but eventually returned to the base of the tubes to pupate (Figure 1a). Infected caterpillars remained at a mean (±SE) height of 75 (±17) mm during molting, then returned to the base of the tubes, and began to climb again at about 48 hpi; this continued at 2, 3, and 4 dpi, until all larvae finally died at elevated heights. The slope (m) of trend lines during photophase (m(day) = 4.56 ± 0.82; m1 = 5.12, m3 = 3.62, m5 = 4.95; Figure 1a) were significantly higher than those observed during scotophase (m(night) = 0.79 ± 1.21; m2 = 0.19, m4 = 2.19, m6 = 0; Figure 1a; t = 4.457, p = .011), so vertical movement occurred mostly during daylight hours, with but little movement in the dark.
Climbing behaviour in response to light source position was assayed in vertical orientation (Figure S1A). The location of the light source affected the height at which infected larvae died varied (F = 55.73, df = 2,105; p < .001; Figure 1b). Similarly, placement of the light source at the top of the plant resulted in more larvae dying on upper plant parts (t = 6.794, p = .002), whereas light placement at the bottom resulted in more larvae dying near the plant base (t = −12.004, p < .001; Figure 1c). In addition, experiments with the cylinders in horizontal or inclined positions confirmed that larvae were responding to light, not gravity, as most infected larvae remained in the lighted part of the cylinders, whereas mock larvae remained in the dark parts (Chi-square test: *p < .05; **p < .01; Figure S2). In complete darkness, larvae were evenly distributed throughout the cylinder (Figure S2D).
Significantly more infected larvae than mock larvae displayed positive phototaxis in the hexagonal light box (Figure S1C,D) at 2, 3 and 4 dpi (2 dpi, t = 7.68, p = .002; 3 dpi, t = 6.20, p = .003; 4 dpi, t = 7.14, p = .002; Figure 1d). In addition, the ERG responses of infected larvae were significantly higher than those of mock-infected larvae at 2, 3 and 4 dpi (2 dpi, t = 6.17; 3 dpi, t = 10.56; 4 dpi, t = 5.62; p < .0001 in all cases, Figure 1e).
3.2 The role of stemmata in phototaxis and climbing behaviour of HearNPV-infected larvae
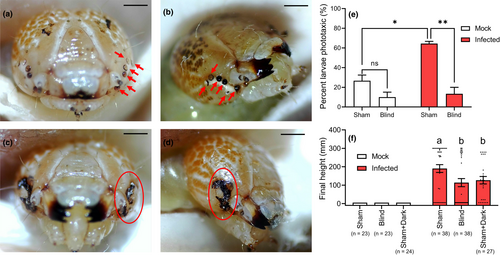
The phototaxis of sham-infected larvae was significantly higher than sham-mock larvae (sham-infected vs. sham-mock: t = 6.107, p = .004; Figure 2e). Surgical destruction of the stemmata virtually eliminated phototaxic behaviour of infected larvae (infected: sham vs. blind, t = 7.273, p = .0019; Figure 2e). Blind larvae were not significantly less phototaxic than sham larvae in mock larvae (mock: sham vs. blind, t = 2.165, p = .096; Figure 2e). Moreover, the height at death of blind infected larvae was lower than that of sham infected larvae under 14:10 (L:D) condition, and not significantly different from sham infected larvae placed in continuous darkness (F = 5.842, df = 2,100, p = .004; Figures 2f and S3). All mock larvae (sham, blind, and sham + dark groups) pupated at the bottom of the tubes (Figures 2f and S3).
3.3 Transcriptome analysis of DEGs induced by infection
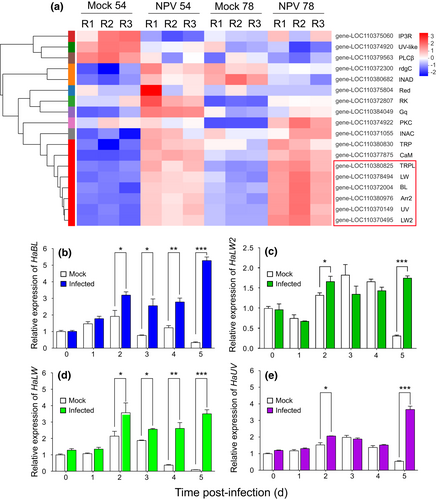
Quality control data of the transcriptome are reported in Table S1 and mapping rates to the genome are reported in Table S2. A total of 3484 and 2715 genes were differentially expressed in infected larvae when compared with mock larvae at 54 and 78 hpi, respectively (padj < 0.05 based on degseq; Figure S4). The DEGs were analysed by GO and KEGG enrichments (Figures S5 and S6). Six genes related to the light transduction pathway were significantly upregulated following HearNPV infection at both 54 and 78 hpi using transcripts per million (TPM) values, including four opsin genes (the long wave-sensitive opsin gene, HaLW; the long wave-sensitive opsin like gene, HaLW2; the blue light-sensitive opsin gene, HaBL; the UV-sensitive opsin gene, HaUV), one opsin regulatory gene (arrestin-2, Arr2), and the gene encoding transient-receptor-potential-like protein (TRPL; Figure 3a). Other DEGs involved in circadian rhythm, hormone, and immune-related pathways are listed in Table S3. The DEGs were verified by qPCR (Table S5). By qPCR analysis, changes in expression of the four opsin genes in the head tissue of mock and infected larvae at different days post-infection were examined. Expression of HaBL (Figure 3b) and HaLW (Figure 3d) were significantly upregulated in infected larvae compared to mock larvae at 2, 3, 4 and 5 dpi, whereas expression of HaLW2 (Figure 3c) and HaUV (Figure 3e) were upregulated only at 2 and 5 dpi.
3.4 The roles of opsin genes in virus-induced phototactic climbing behaviour
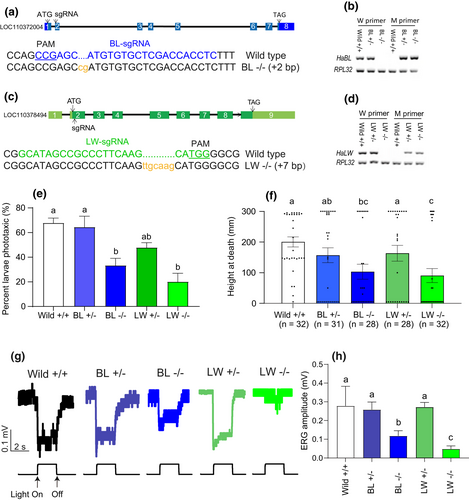
The qPCR analysis revealed that three opsin genes (HaBL, HaLW, HaUV) were mainly expressed in the larval stemmata and were more highly expressed in earlier life stages (end of the egg stage, and first and second instars; Figure S7). HaBL and HaLW were both upregulated after HearNPV-infection, verifying amplification of their expression by the virus. In HaBL and HaLW mutants constructed using CRISPR/Cas9 gene editing technology, the BL homozygous mutant strain had a 2 bp (CG) nucleotide sequence added into exon 2 of the HaBL gene sequence (Figures 4a and S8A), and the LW homozygous mutant line had a 7 bp (TTGCAAG) nucleotide sequence added into exon 2 of the HaLW gene sequence (Figures 4c and S8B), resulting in frameshift mutations in both cases. The primers were designed so that they would bind specifically to the sequences of the mutant sites in mutant and wild-type larvae, respectively. In both cases, primers specific to the wild-type genome did not recognize the homozygous mutant genome, and primers specific to the homozygous mutant did not recognize the wild-type genome (Figure 4b,d). There was downregulation of the phototactic response of infected larvae to white light in the case of HaBL and HaLW knockout (F = 10.72; df = 4, 10; p < .01; Figure 4e), and a slight decrease in mutants compared to uninfected wild larvae (F = 0.247; df = 2, 6; p = .789; Figure S9). Height at death was significantly reduced for infected HaBL and HaLW homozygous mutant larvae (F = 3.97; df = 4, 146; p = .0043; Figure 4f). Knockout of HaBL or HaLW significantly inhibited the responsiveness of infected larvae to light, as reflected in ERG waveforms (Figure 4g) and the reduced amplitudes of these waveforms (F = 19.37; df = 4, 20; p < .0001; Figure 4h). ERG amplitude in the LW −/− mutant was more reduced than in the BL −/− mutant (Figure 4h).
3.5 The role of the TRPL gene in virus-induced phototactic climbing behaviour
Knockout of either HaBL or HaLW using CRISPR/Cas9 significantly reduced expression of the TRPL gene (F = 4.43; df = 4,10; p = .026; Figure 5a). In healthy larvae, the TRPL gene was primarily expressed in the stemmata (Figure 5b). Although the TRP gene was also expressed primarily in the stemmata, expression of TRPL was c. 14 times greater (Figure S10). Expression of the TRPL gene in infected larvae was significantly higher than in mock larvae at 2, 3, and 4 dpi (Figure 5c). TRPL homozygous mutant larvae constructed using CRISPR/Cas9 technology with two sgRNAs (Figure 5d) had a 661 bp nucleotide sequence deleted from the TRPL gene sequence (Figure 5e). The primers designed to detect the mutant showed up as an 875 bp single band in wild-type, a 224 bp single band in homozygous mutant, and a double band in heterozygous mutants (Figure 5e). Knockout of the TRPL gene suppressed phototaxis in infected mutant larvae when compared to infected wild larvae (t = 4.06, p = .015; Figure 5f), and height at death was significantly reduced (t = 2.72, p = .008; Figure 5g). Knockout of TRPL also significantly inhibited the responsiveness of infected larvae to light, as reflected in ERG waveforms (Figure 5h) and the reduced amplitudes of these waveforms (t = 4.38, p = .0023; Figure 5l).
4 DISCUSSION
The mechanisms by which parasites and pathogens manipulate host behaviour are of broad interest, but few studies have definitively characterized them (Hoover, 2019). Although some studies have previously investigated the molecular mechanisms underlying phototaxis in adult Lepidoptera (Johansen et al., 2011; Park & Lee, 2017), phototaxis by larvae, which have stemmata as opposed compound eyes (Gilbert, 1994; Kim et al., 2019), has received less attention. Here, we illustrate how HearNPV induces enhanced phototaxis in H. armigera larvae by hijacking the host's visual perception and triggering climbing behaviour, causing infected larvae to die at an elevated height.
The climbing behaviour of infected larvae occurred almost exclusively during daylight hours, and the relative position of the light source, whether the assay was conducted in tubes or on plants, determined the height at death of infected larvae, without the involvement of any negative gravitropism. In addition, the phototaxis response of larvae was significantly enhanced after NPV-infection. Since sunlight shines on plants from above, positive phototaxis is probably a reliable mechanism to ensure that infected larvae die at high elevations on host plants.
Ablation of the stemmata in infected H. armigera larvae eliminated phototaxic responses, which demonstrate that intact stemmata are required for the virus to induce climbing behaviour. High-throughput transcriptomic analysis and qPCR revealed that the opsin genes HaBL and HaLW were significantly upregulated post-infection, specifically during the period of larval phototaxis. When expression of these two opsin genes was blocked using the CRISPR/Cas9 system, phototaxic behaviour was largely eliminated, probably via reduced light sensitivity as reflected in diminished ERG responses. Phototransduction, the process whereby light energy is converted into an electrical signal perceived as visual information, has rarely been studied in insects other than Drosophila melanogaster (Fain et al., 2010; Hardie & Raghu, 2001; Macias-Muñoz et al., 2019), and our study is the first to examine phototransduction in lepidopteran larvae. In adult Drosophila, opsin genes affect the activity of TRP and TRPL channels, which are involved in the conversion of light into electric signals (Hardie & Minke, 1992; Phillips et al., 1992). In healthy larvae, the TRPL gene had 14-fold greater expression than the TRP gene, mainly in tissues of the stemmata, and its expression was also affected by expression of HaBL and HaLW. The TRPL gene was significantly upregulated in larvae following HearNPV infection, and its knockout reduced phototaxis and climbing height at death in infected larvae. These results implicate the involvement of TRPL in the phototaxic responses of HearNPV-infected larvae and suggest that the virus amplifies the expression of genes controlling phototransduction in the host visual perception pathway, in addition to those mediating opsin production and photoreception.
The induction of host phototaxic responses has been reported in various parasite-host interactions other than baculovirus-caterpillar systems. For example, crickets exhibit strong positive phototaxis after infection with Gordian worms (Ponton et al., 2011), as do many Gammaridae when infected by their acanthocephalan parasites (Fisher et al., 2014). Similarly, positive phototaxis is induced in Succinea putris snails when infected by Leucochloridium spp. flatworms (Wesolowska & Wesolowski, 2014) and in Dolichoderus thoracicus ants infected with the parasitic fungus Ophiocordyceps pseudolloydii (Chung et al., 2017). It is possible that these systems utilize similar regulatory mechanisms to enhances host phototaxis, despite their broad taxonomic disparity. In this study, our demonstration that HearNPV hijacks host visual perception pathways to induce positive phototaxis may be a key insight that helps elucidate mechanisms by which parasites manipulate host phototaxic behaviour in other host-parasite systems.
The death of infected caterpillars at elevated positions on plants should be favoured by natural selection acting on the virus. Eggs and early instar larvae of H. armigera tend to occur primarily on the upper parts of plant, where the foliage is most nutritious and reproductive structures are present (Yang et al., 1998; Yu et al., 2004). Therefore, the niche of young (early instar) larvae is similar to that of the infected cadavers which are on the upper parts of plant. Young larvae are nucleopolyhedroviruses' suitable and vulnerable hosts, whose infection need a lower dose of virus, as late stage larvae require a higher dose of virus to be infected successfully. Thus, cadavers that form at high elevations on a plant are more likely to easily infect other caterpillars via consumption of infected foliage or necrophagy (Rebolledo et al., 2015), while occlusion bodies serve as a form of packaging that extends the viability of virus particles exposed to solar radiation (Elnagar & Abulnasr, 1980).
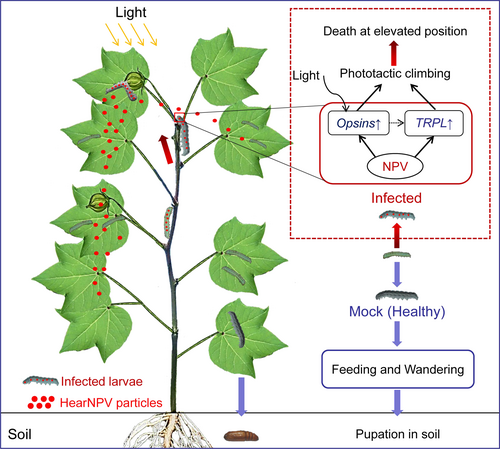
In conclusion, HearNPV alters the visual perception of its host by affecting the expression of genes involved in photoreception and phototransduction in order to alter host behaviour for its benefit. At maturity, healthy larvae do not display phototaxis but rather undergo an ontogenetic niche shift; they descend the plant and enter the soil to pupate. In contrast, opsin and TRPL genes are significantly upregulated in infected larvae, causing positive phototaxis and climbing behaviour, so that these larvae die on upper plant parts, which is conducive to viral consumption by conspecific larvae, and the wider dispersal of HearNPV OBs in the environment (Figure 6). Our present study not only confirms that baculoviruses induce host phototactic responses in the system of HearNPV and H. armigera larvae, but also provides valuable information to further understand the molecular mechanism of this behavioral change. One challenge for future studies would be the identification of viral genes responsible for the increased expression of visual pathway genes in the host. In addition, since host behaviour is ultimately controlled at the level of the central nervous system (CNS), the ways in which baculoviruses might regulate neuronal activity of the host CNS warrant exploration.
ACKNOWLEDGEMENTS
We thank Chenzhu Wang, Guirong Wang, Shiheng An, Yunhe Li, Tao Wang, Zhangwu Zhao, Jie Shen, Xin Zhou, Pei Liang, Chuan Cao, Tao Zhou, Wei Zhang and Jun Xie for suggestions that improved the manuscript. We are grateful to Lihua Liang for assistance in rearing the insects. This study was supported by the National Natural Science Foundation of China (grant no. 31972275) and the National Key Research and Development program of China (2017YFD0201900), and the Special Research Projects of China for Developing Transgenic Plants (2016ZX08011002).
CONFLICT OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Xiaoming Liu and Xiaoxia Liu designed the experiments; Xiaoming Liu, Zhiqiang Tian, Limei Cai, Zhongjian Shen and Lin Zhu conducted the experiments; Xiaoming Liu, J. P. Michaud, and Zhiqiang Tian contributed new reagents/analytic tools; Xiaoming Liu, J. P. Michaud, Songdou Zhang, and Zhen Li analysed the data; and Xiaoming Liu, J. P. Michaud, Vera I. D. Ros, Zhen Li, Kelli Hoover, Shuo Yan, and Xiaoxia Liu wrote the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
All the gene sequences in this study were from genomic sequence of Helicoverpa armigera (GenBank Accession: GCA_002156985.1). DEGs of transcriptome sequencing: uploaded as Tables S1, S2 and S3.




