A novel microRNA regulates cooperation between symbiont and a laterally acquired gene in the regulation of pantothenate biosynthesis within Bemisia tabaci whiteflies
Xiang Sun and Bing-Qi Liu contributed equally to this work.
Handling Editor: Jacob Russell
Abstract
Horizontally transferred genes (HTGs) play a key role in animal symbiosis, and some horizontally transferred genes or proteins are highly expressed in specialized host cells (bacteriocytes). However, it is not clear how HTGs are regulated, but microRNAs (miRNAs) are prime candidates given their previously demonstrated roles in symbiosis and impacts on the expression of host genes. A horizontally acquired PanBC that is highly expressed in whitefly bacteriocytes can cooperate with an obligate symbiont Portiera for pantothenate production, facilitating whitefly performance and Portiera titre. Here, we found that a whitefly miRNA, novel-m0780-5p, was up-regulated and its target panBC was down-regulated in Portiera-eliminated whiteflies. This miRNA was located in the cytoplasmic region of whitefly bacteriocytes. Injection of novel-m0780-5p agomir reduced the expression of PanBC in whitefly bacteriocytes, while injection of novel-m0780-5p antagomir enhanced PanBC expression. Agomir injection also reduced the pantothenate level, Portiera titre and whitefly performance. Supplementation with pantothenate restored Portiera titre and the fitness of agomir-injected whiteflies. Thus, we demonstrate that a whitefly miRNA regulates panBC-mediated host–symbiont collaboration required for pantothenate synthesis, benefiting the whitefly–Portiera symbiosis. Both panBC and novel-m0780-5p are present in the genomes of six Bemisia tabaci species. The expression of a novel miRNA in multiple B. tabaci species suggests that the miRNA evolved after panBC acquisition, and allowed this gene to be more tightly regulated. Our discovery provides the first account of a HTG being regulated by a miRNA from the host genome, and suggests key roles for interactions between miRNAs and HTGs in the functioning of symbiosis.
1 INTRODUCTION
Obligate intracellular symbionts are present in more than 10% of insect species and can promote insect fitness by providing nutrients such as essential amino acids (EAAs), B vitamins and more (Douglas, 2015; Moran et al., 2008). Such symbionts, particularly those restricted to specialized host cells (bacteriocytes), have reduced genomes, limiting their capacities for nutrient biosynthesis in several cases (Moran et al., 2008; Shigenobu et al., 2000; Wernegreen, 2002). Horizontal gene transfer is one important way to provide the raw material for host adaptation that supports and controls insect symbionts (Bublitz et al., 2019; Chung et al., 2018; Husnik et al., 2013; Luan et al., 2015; Moran & Bennett, 2014; Nakabachi et al., 2014; Ren et al., 2020, 2021; Sloan et al., 2014; Wybouw et al., 2016). Importantly, such gene transfer may allow hosts to compensate for missing/degraded symbiont function (Moran & Bennett, 2014). In one such instance, horizontally transferred murF collaborates with the symbiont Moranella for peptidoglycan synthesis in mealybugs (Bublitz et al., 2019). Laterally transferred lysA cooperates with the symbionts Portiera and Rickettsia for lysine synthesis in whiteflies (Bao et al., 2021). Similarly, and of relevance to our study, the horizontally acquired panBC gene cooperates with the symbiont Portiera for pantothenate synthesis in whiteflies (Ren et al., 2021). Many horizontally transferred genes (HTGs) are highly expressed in bacteriocytes (Husnik et al., 2013; Luan et al., 2015; Moran & Bennett, 2014; Sloan et al., 2014), but how they are transcriptionally regulated in insect genomes remains unclear.
Symbioses in whiteflies provide a useful model system for studying gene regulatory mechanisms of laterally transferred, symbiosis-impacting genes. Bemisia tabaci provides one well-studied example. While currently possessing just a single species name, this group of whiteflies consists of a complex of multiple, cryptic species (De Barro et al., 2011). All B. tabaci species harbour the obligate symbiont ‘Candidatus Portiera aleyrodidarum’ (hereafter Portiera) in specialized insect cells called bacteriocytes and harbour up to four facultative symbiont lineages (Gottlieb et al., 2008; Skaljac et al., 2010). B. tabaci cryptic species MEAM1 is a globally important and invasive agricultural pest (Liu et al., 2007; Luan et al., 2013; Zhang et al., 2012). In China, B. tabaci MEAM1 bears Portiera and ‘Candidatus Hamiltonella defensa’ (hereafter Hamiltonella) in the same bacteriocytes and Rickettsia sp. (hereafter Rickettsia) in the whole-body cavity (Li et al., 2022; Ren et al., 2020; Wang et al., 2020). Portiera and Hamiltonella are vertically transmitted via the bacteriocytes (Luan et al., 2016, 2018). The highly degenerate genome of Portiera mainly retains most genes involved in the synthesis of 10 EAAs and, also, a single B-vitamin: pantothenate (Chen et al., 2016; Douglas, 2017; Sloan & Moran, 2012). Curiously, this symbiont's genome lacks genes encoding 3-methyl-2-oxobutanoate hydroxymethyltransferase (PanB) and pantoate-beta-alanine ligase (PanC), required for pantothenate synthesis. The genome of B. tabaci MEAM1 contains a fused gene-panBC-horizontally transferred from Pseudomonas-associated bacteria from the order Pseudomonadales (Chen et al., 2016). Previously, we demonstrated that panBC cooperates with Portiera for the synthesis of pantothenate, facilitating whitefly fecundity and symbiont titre (Ren et al., 2021). However, how the expression of panBC in whitefly bacteriocytes is regulated for pantothenate synthesis, and thereby influences whitefly performance and symbiont fitness, remains unknown.
Micro RNAs (miRNAs) impact gene expression, RNA stability, or translation and function as important post-transcriptional regulatory factors in eukaryotes (Kim et al., 2009). Eukaryotic miRNAs regulate almost all cellular pathways and affect biological processes such as development, immunity and host–pathogen/symbiont interactions (Hussain & Asgari, 2014; Hussain et al., 2011; Kim et al., 2016). Aphid miRNAs that target aphid mRNA for amino acid transport and metabolism and signal transduction have been identified (Feng et al., 2018). In mosquitoes, Wolbachia infection leads to induction of the miRNA aae-miR-2940, which targets the mosquito metalloprotease gene in promoting this symbiont's maintenance (Hussain et al., 2011). However, how miRNAs regulate host protein expression in bacteriocytes, and thereby impact host–symbiont interactions, remains unclear.
Whitefly PanBC is highly expressed in bacteriocytes (Ren et al., 2021). We hypothesized that PanBC in whitefly bacteriocytes is regulated by specific miRNAs, and that such regulation shapes the important trait of pantothenate biosynthesis (Douglas, 2017). To test this hypothesis, we first analysed differentially expressed B. tabaci miRNAs in symbiont-infected and symbiont-cured whiteflies. We then investigated whether PanBC protein expression, in bacteriocytes, is regulated by any such miRNAs. Through pantothenate measurement, fitness experiments, dietary manipulations and symbiont quantification, we finally studied the impact of PanBC protein-regulating miRNAs on both whitefly fitness and the whitefly symbiosis. We found that a whitefly miRNA regulates the cooperation of PanBC and Portiera for pantothenate synthesis by impacting PanBC expression in bacteriocytes of whiteflies.
2 MATERIALS AND METHODS
2.1 Insect rearing and plants
The whitefly Bemisia tabaci MEAM1 colony (mtCO1 GenBank accession no. GQ332577) was maintained on cotton plants (Gossypium hirsutum, cv. Shiyuan 321) as previously described (Ren et al., 2020, 2021; Wang et al., 2020). Cotton plants were cultivated to the six- to- seven true-leaf stage for use in experiments.
2.2 Symbiont elimination by antibiotic treatment
To eliminate Portiera, hundreds of adult B. tabaci (F0, 0–7 days after emergence) were released into each feeding chamber and fed a 25% sucrose solution (w/v) supplemented with 30 µg ml–1 of rifampicin (BBI Life Sciences) for 2 days as previously described (Ren et al., 2021; Shan et al., 2016; Zhang et al., 2015). Control whiteflies were fed on a sucrose solution without antibiotic supplementation. Following antibiotic treatment, B. tabaci were transferred to cotton plants. After symbiont quantification by qPCR (quantitative polymerase chain reaction), Portiera-cured F1 B. tabaci (−PBt) obtained by antibiotic treatment and control Portiera-infected F1 B. tabaci (+PBt) were identified.
2.3 Library construction and sequencing of whitefly miRNAs
DNA was extracted from four biological replicates, with four female adult whiteflies per replicate (at 3–7 days after eclosion) and used for symbiont quantification by qPCR in whiteflies after antibiotic treatment. Then, total RNA was extracted from three biological replicates of +PBt and −PBt whiteflies with TRIzol reagent (Sigma-Aldrich) and treated with DNase I (Invitrogen) following the manufacturer's instructions. The concentration and purity of total RNA were assessed using a NanoDrop 2000 spectrophotometer. RNA integrity was assessed using an RNA Nano 6000 assay kit on an Agilent Bioanalyzer 2100 system (Agilent Technologies).
Next, miRNA libraries were constructed using a NEBNext Multiplex Small RNA Library Prep kit (New England Biolabs) following the manufacturer's instructions. RNA molecules within the size range of 18–30 nt were enriched by polyacrylamide gel electrophoresis. The bands of that range were excised from a gel and purified by the Universal DNA Purification and Recovery Kit (TIANGEN). Subsequently, the 3′ adapters were added, and the 36–44-nt RNAs were enriched. The 5′ adapters were ligated to the RNAs. The ligation products were reverse transcribed by PCR, and the 140–160-bp PCR products were enriched to generate a cDNA library and sequenced using the Illumina HiSeq 2500 platform with single-end 50-base reads by Gene Denovo Biotechnology.
2.4 Strand-specific library construction and sequencing for whitefly mRNAs
After extraction of total RNA, whitefly rRNAs were removed to retain mRNAs using an Epicentre Ribo-zero rRNA Kit (Epicentre). The enriched mRNAs were broken into short fragments using fragmentation buffer and reverse transcribed into cDNA with random primers. Second-strand cDNA was synthesized by using DNA polymerase I, RNase H, dNTP (dUTP instead of dTTP) and buffer. Next, the cDNA fragments were purified with a QiaQuick PCR extraction kit (Qiagen), end repaired, poly(A) added and ligated to Illumina sequencing adapters. UNG (uracil-N-glycosylase) was used to digest the second-strand cDNA. The digested products between 150 and 400 bp were selected by agarose gel electrophoresis, purified with the Universal DNA Purification and Recovery Kit (TIANGEN), PCR-amplified, and sequenced using the Illumina Novaseq 6000 platform with paired-end 150-base reads by Gene Denovo Biotechnology.
2.5 Analysis of differentially expressed whitefly miRNAs
The raw reads from sequencing data were filtered by removing low-quality reads, adaptor reads, and reads <18 nt in length. All clean tags were aligned with small RNAs in GenBank (Release 209.0) (Benson et al., 2009) and Rfam (Release 11.0) (Griffiths-Jones et al., 2003) to identify and remove rRNAs, small conditional RNAs, small nucleolar RNAs, small nuclear RNAs and tRNAs. The remaining clean reads of small RNAs were aligned to the reference genome of B. tabaci MEAM1 using bowtie2 (version 2.2.8) (Chen et al., 2016; Langmead & Salzberg, 2012). The sequences that corresponded to known miRNAs were determined by matching them to the miRNA database (miRBase 22.0). The unmapped miRNA sequences were aligned to the genome of B. tabaci to identify novel miRNAs. Based on genome positions and hairpin structures, novel miRNAs were predicted by mireap (version 0.2) using default parameters (Robinson et al., 2010; Wang et al., 2015). Levels of miRNA expression were normalized using transcripts per million (TPM) values. The edgeR package (Robinson et al., 2010) was used to analyse differentially expressed miRNAs with the absolute values of log2 ratio ≥1 and p < .01.
2.6 Analysis of differentially expressed whitefly mRNAs
Raw data were filtered by removing low-quality reads and adaptor reads. The short read alignment tool bowtie2 (version 2.2.8) (Langmead & Salzberg, 2012) was used for mapping reads to the rRNA database (NCGB) (version 209). Then, the rRNA mapped reads were removed. The remaining reads were used in the assembly and analysis of the transcriptome. All of the clean tags were mapped to the reference genome of the whitefly with hisat2 (v2.1.0) allowing no mismatches (Chen et al., 2016; Kim et al., 2015). Each assembled transcript was searched against the NCBI non-redundant (nr) database (r20200419) and the KEGG database (r94) using the Basic Local Alignment Search Tool (blast) version 2.6.0+ with a maximum E value of 1.0E−5 (Stephen et al., 1990). Transcript abundance was quantified with stringtie software using a reference-based approach (Mihaela et al., 2015). Gene expression levels were normalized using the fragments per kilobase of transcript per million (FPKM) method. The edgeR package (Robinson et al., 2010) was used to identify differentially expressed genes across samples with the absolute value of log2 ratio ≥1 and a false discovery rate-adjusted p (FDR value) <.05.
2.7 miRNA target prediction and construction of an miRNA-target network
Three software packages—rnahybrid (version 2.1.2) (Krüger & Rehmsmeier, 2006) +svm_light (version 6.01) (Joachims, 1999), miranda (version 3.3a) (Turner, 1985) and targetscan (version 7.0) (Lewis et al., 2005)—were used to predict miRNA targets. The default parameters used for these prediction tools are listed in Table S1. After prediction of miRNA targets, correlation of the expression between differentially expressed miRNAs and targets was evaluated using the Pearson correlation coefficient (PCC). For Portiera-infected and Portiera-cured whiteflies, pairs with the absolute value of PCC >0.7 and p < .05 were selected as co-expressed miRNA–target pairs.
2.8 miRNA expression pattern clustering analysis
The heat maps of known miRNAs and novel miRNAs were drawn to display miRNA expression levels in +PBt and −PBt whiteflies and to cluster miRNAs with similar expression pattern. The heat maps were plotted using omicshare tools (www.omicshare.com/tools). The relative expression of miRNAs was clustered based on z-scores from low to high value (with a scale from −2 to 2) among three biological replicates. Euclidean distance with complete linkage was adopted for heat map plotting.
2.9 qPCR and quantitative reverse transcription PCR (qRT-PCR)
Total DNA was extracted following the nonidet-P40-based protocol as previously described (Luan et al., 2018). Symbiont DNA was quantified by qPCR using the CFX96 Real-Time PCR Detection System (Bio-Rad) with 2×SYBR Green Master Mix (Bimake) following a previously described protocol (Bao et al., 2021; Ren et al., 2021). Portiera, Hamiltonella and Rickettsia were quantified using the copy numbers of 16S rRNA, 16S rRNA and gltA genes, respectively, with the B. tabaci β-actin gene as an internal standard for normalization. Two technical replicates were performed for each biological replicate. Relative symbiont density was calculated using the 2−ΔCt method (Schmittgen & Livak, 2008).
Total RNA was extracted as previously described (Ren et al., 2020). cDNAs were synthesized from the total RNA using an All-in-One cDNA Synthesis SuperMix Kit (Bimake) as previously described (Bao et al., 2021; Ren et al., 2021). The qRT-PCRs were performed using the CFX96 Real-Time PCR Detection System (Bio-Rad) with 2×SYBR Green master Mix (Bimake).
Our miRNA sequencing showed that one miRNA, novel-m0780-5p, that targets the whitefly panBC gene was up-regulated in −PBt whiteflies compared with +PBt whiteflies. To compare the expression of miRNA novel-m0780-5p in +PBt and −PBt whiteflies, cDNAs for miRNAs were reverse transcribed by using an miRcute miRNA First-Strand cDNA Synthesis Kit (TIANGEN), and qRT-PCRs were performed using an miRcute miRNA qPCR detection kit (TIANGEN) according to the manufacturer's protocol. The housekeeping gene RPS5 was used as endogenous control for whitefly miRNA. The primers used for novel-m0780-5p and RPS5 were obtained from Tiangen Biotech. All primers used in this study are listed in Table S2.
2.10 Fluorescence in situ hybridization (FISH) analysis for symbionts and miRNA
To localize Portiera in the bacteriocytes of female adult whiteflies, FISH was conducted following a previously described protocol (Kim et al., 2009; Ren et al., 2021; Shan et al., 2021; Wang et al., 2020). To determine the location of novel-m0780-5p in the bacteriocytes of female adult whiteflies, bacteriocytes were fixed in 4% (v/v) paraformaldehyde at 4°C overnight, permeabilized with 0.2% Triton-X-100 for 2 h, and hybridized overnight in hybridization buffer with the fluorescent probe Bt-novel-m0780-5p-Cy3 (5′-Cy3-TTTCCTTTGAACTATTCTTGAAA-3′), using methodologies described in a previous study (Guo, Ma, et al., 2018). Samples were stained with DAPI (1 µg ml–1 in PBS, Sigma) for 30 min at room temperature.
2.11 Immunofluorescence microscopy
Bacteriocytes from Portiera-infected and Portiera-cured adult female whiteflies or miRNA agomir- and antagomir-injected adult female whiteflies were dissected, fixed, permeabilized and incubated with Alexa-Fluor 488-labelled anti-PanBC antibodies following a previously described protocol (Ren et al., 2021). Three biological replicates were conducted. Images were captured and analysed using an FV3000 confocal microscope (Olympus).
2.12 Effects of miRNA agomir and antagomir injection on PanBC localization in whiteflies
The miRNA agomir is the double-stranded miRNA mimic, and its in vivo delivery results in the activation of endogenous miRNA (Guo, Ma, et al., 2018). The miRNA antagomir is the chemically modified single-stranded miRNA that complements the miRNAs and can specifically silence the endogenous miRNAs (Krutzfeldt et al., 2005). To investigate whether overexpression or repression of novel-m0780-5p influences PanBC localization in whiteflies, ~800 female adult whiteflies infected with Portiera within 3–4 days after emergence were injected with 3 µm agomir or antagomir using an Eppendorf microinjection system following a previously described method (Bao et al., 2021; Li et al., 2022; Ren et al., 2021; Wang et al., 2022). The whiteflies that were injected with 3 µm negative control agomir were used as a negative control for agomir injection. The whiteflies that were injected with 3 µm negative control antagomir with modification of methylation were used as a negative control for injection of antagomir with modification of 2'Ome. The novel-m0780-5p agomir or antagomir and their controls were designed and synthesized by GenePharma, and their sequences are listed in Table S2. After injection, whiteflies were transferred onto cotton leaf discs and kept on 1.5% agar plates in the incubator at 26 ± 2°C, with a 14:10-hr (light–dark) photoperiod and 60–80% relative humidity. To examine the effects of miRNA agomir injection on PanBC localization in bacteriocytes, whiteflies were collected on days 1, 3 and 5 after microinjection, and bacteriocytes from miRNA-injected adult female whiteflies were dissected for immunofluorescence microscopy as described above. To examine the effects of miRNA antagomir injection on PanBC localization in bacteriocytes, whiteflies were collected on day 3 after microinjection, and bacteriocytes from antagomir-injected adult female whiteflies were dissected for immunofluorescence microscopy.
2.13 Effects of miRNA agomir injection on pantothenate level, symbiont titre and whitefly performance
To investigate whether overexpression of novel-m0780-5p influences pantothenate titre, symbiont titre or whitefly fitness, ~1500 female adult whiteflies infected with Portiera within 3–4 days after emergence were injected with 3 µm agomir. Control whiteflies were injected with 3 µm negative control agomir. After injection, whiteflies were incubated on cotton leaf discs as described above. To assess whether induction of novel-m0780-5p influences pantothenate titre, a microbiological assay was used for pantothenate quantification in whiteflies on day 3 after agomir injection using Lactobacillus plantarum ATCC 8014 (Beijing Landbridge Technology) as previously described (Ren et al., 2021). Briefly, the homogenized whitefly samples were mixed with vitamin B5-deficient pantothenate assay medium (Beijing Landbridge Technology) and inoculated with log-phase L. plantarum ATCC 8014. The growth of pantothenate-dependent L. plantarum is proportional to the amount of pantothenate in the medium and growth can be measured by assessing the change in the medium's turbidity. To test whether induction of novel-m0780-5p influences symbiont abundance, DNA was extracted from individual female adult whiteflies for each of eight biological replicates on day 3 after whiteflies were microinjected with agomir. Then, qPCR was performed as described above.
To further determine whether miRNA agomir injection influences whitefly mortality, 25 female adults per replicate of agomir-injected and negative control agomir-injected whiteflies were incubated on cotton leaf discs as described above. The mortality of injected female whiteflies was recorded on day 3. Ten biological replicates were conducted. To determine whether miRNA agomir injection influences whitefly fecundity, after injection, individual whiteflies were transferred onto cotton leaf discs as described above. The number of eggs of living whiteflies was recorded with 15 biological replicates of individuals on day 3 post-injection.
2.14 Effects of pantothenate supplementation on PanBC level, symbiont titre and whitefly performance in agomir-injected whiteflies
To investigate whether pantothenate supplementation restores the PanBC level, symbiont titre and whitefly performance of agomir-injected whiteflies, ~2000 female adult whiteflies within 3–4 days after emergence were microinjected with agomir. After recovery on a cotton leaf disc for 12 h, these whiteflies were fed a 30% (w/v) sucrose solution supplemented with or without pantothenate at a final concentration of 250 ng ml−1 for 2 days based on previous studies (Duron et al., 2018; Hosokawa et al., 2010; Pant & Fraenkel, 1950; Ren et al., 2021). The controls were negative control agomir-injected whiteflies fed with 30% (w/v) sucrose solution. Then, bacteriocytes were dissected for examination of PanBC level by immunofluorescence microscopy and for localization of Portiera in bacteriocytes of female adult whiteflies by FISH following the protocol described above. Three biological replicates were conducted. Additionally, DNA was extracted from bacteriocytes of eight female adult whiteflies from each of the five biological replicates on day 3 after whiteflies were microinjected with agomir. Then, qPCR was performed as described above. After agomir injection and pantothenate supplementation treatment as described above, the mortality of injected female whiteflies was recorded. Three biological replicates with 80 female adult whiteflies per replicate were conducted. Moreover, individual agomir-injected female adult whiteflies were released on cotton leaf discs. After 3 days, the number of eggs was counted. Ten replicates were conducted for each treatment.
2.15 Evolutionary analysis for novel-m0780-5p in whiteflies
Mature miRNAs represent single‑stranded RNAs ~22 nt in length that are generated from hairpin shaped transcripts or pre-miRNAs (Kim et al., 2009). Sequences of PanBC and hairpin structure sequence of novel-m0780-5p (Data S1) of the whitefly B. tabaci MEAM1 were subjected to tblastx and blastn, respectively, against the genome of the six B. tabaci species, MEAM1, MED, SSA1, SSA2, SSA3 and Uganda (GenBank accession Nos.: 9, GCA_003994315.1, GCA_902825415.1, GCA_903994125.1, GCA_903994115.1 and GCA_903994095.1, respectively), and the greenhouse whitefly Trialeurodes vaporariorum (GenBank accession No.: GCA_011764245.1). Nucleotide sequence alignments for sequences of pre-novel-m0780-5p containing mature novel-m0780-5p were conducted using bioedit version 7.1.3.0 among six B. tabaci species. The hairpin structure of novel-m0780-5p in six B. tabaci species, MEAM1, MED, SSA1, SSA2, SSA3 and Uganda, was predicted using mireap version 0.2.
2.16 Statistical analyses
For miRNA expression level, pantothenate amount, symbiont titre, female mortality and number of eggs, statistical differences were evaluated using one-way ANOVA at a significance threshold of 0.05. Percentage data were transformed to arcsine square roots before analysis. All data analyses were conducted using statistica version 12 software (StatSoft).
3 RESULTS
3.1 Many B. tabaci miRNAs are differentially expressed between symbiont-infected and symbiont-cured whiteflies
To detect which Bemisia tabaci miRNAs may regulate PanBC expression, Portiera, Hamiltonella and Rickettsia were eliminated in female adult whiteflies, and differentially expressed B. tabaci miRNAs were analysed in symbiont-infected and symbiont-cured whiteflies. After F0 whiteflies were treated with rifampicin, the abundance of Portiera, Hamiltonella and Rickettsia was reduced by 99.9% in F1 female adult whiteflies (Figure S1a; p < .01). Portiera had a significantly reduced titre in the bacteriocytes of symbiont-cured (−PBt) compared with symbiont-infected (+PBt) female whiteflies (Figure S1b). Then, miRNA sequencing was performed for +PBt and −PBt female whiteflies with the same genetic background. To examine the differentially expressed B. tabaci miRNAs, miRNA expression levels were compared between +PBt and −PBt whiteflies as previously described (Hussain et al., 2011). In total, 36,994,381 and 45,098,300 whitefly genome mapped reads that were 18–35 nt in length were retained in the +PBt whiteflies and −PBt whiteflies, respectively (Table S3). The miRNA annotations identified 1180 known miRNAs and 1,633 novel miRNAs in the genome of B. tabaci MEAM1 (Data S1). Among these, 47 whitefly miRNAs (31 novel miRNAs and 16 known miRNAs) were up-regulated, and 48 whitefly miRNAs (40 novel miRNAs and eight known miRNAs) were down-regulated in −PBt whiteflies compared with +PBt whiteflies (Figure S2a,b; Data S2a). It appears that the majority of differentially expressed whitefly miRNAs between −PBt and +PBt whiteflies are novel (Figure S2a,b; Data S2a). We found 220 whitefly mRNAs were up-regulated and 732 whitefly mRNAs were down-regulated in −PBt whiteflies compared with +PBt whiteflies (Data S2b).
To investigate whether whitefly miRNAs target whitefly genes, three stringent target prediction software packages (rnahybrid, miranda and targetscan) were used to predict potential targets in the genome of B. tabaci. After miRNA targets were predicted, correlations between miRNA–mRNA targets were evaluated. Co-expressed miRNA–mRNA pairs with differential expression of all RNAs were determined. We identified a total of 1865 miRNA–mRNA pairs (Data S3a).
3.2 B. tabaci miRNAs that target whitefly HTGs are differentially expressed in symbiont-infected and symbiont-cured whiteflies
A total of 142 HTGs from bacteria or fungi have been identified in the genome of B. tabaci MEAM1 (Chen et al., 2016). Intriguingly, we found that 10 of these HTGs were targeted by 10 whitefly miRNAs with differential expression between −PBt and +PBt whiteflies (Figure 1; Data S3b). Among these, five whitefly miRNAs that were differentially expressed between −PBt and +PBt whiteflies targeted four whitefly HTGs of bacterial origin, and six whitefly miRNAs that were differentially expressed between −PBt and +PBt whiteflies targeted six whitefly HTGs of fungal origin. In addition to novel-m0117-5p, nine other whitefly miRNAs were up-regulated in −PBt whiteflies compared with +PBt whiteflies (Figure 1; Data S3b). Additionally, both novel-m0328-5p and miR-7230-y targeted the same gene, frr, and miR-7230-y targeted two genes, frr and MRJPs-1.
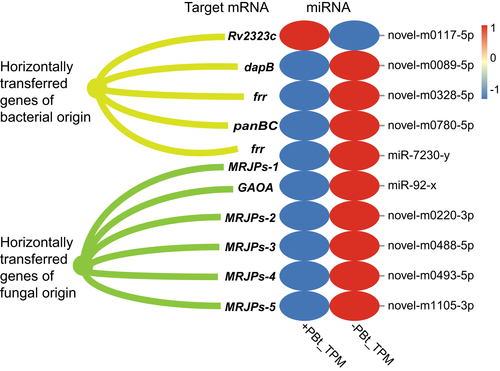
Among these 10 targeted whitefly HTGs, dapB is involved in lysine synthesis and, as mentioned previously, panBC is involved in pantothenate synthesis. Both DapB and PanBC have been demonstrated to have high protein expression levels in whitefly bacteriocytes (Bao et al., 2021; Ren et al., 2021). We found that the miRNA novel-m0089-5p was up-regulated and its target dapB was down-regulated in −PBt whiteflies compared with +PBt whiteflies (Data S3b). Likewise, the miRNA novel-m0780-5p was up-regulated and its target panBC was down-regulated in −PBt whiteflies compared with +PBt whiteflies (Data S3b). These data suggest that dapB, panBC and other HTGs expressed in whitefly bacteriocytes could be regulated by miRNAs.
3.3 A B. tabaci miRNA, novel-m0780-5p, targets the whitefly PanBC gene and regulates whitefly PanBC expression in bacteriocytes
The novel-m0780-5p is predicted to target the 3′ untranslated region of the PanBC gene (Figure 2a). Matching the above results from our shotgun transcriptomics, qRT-PCR verified the up-regulated expression of whitefly novel-m0780-5p in −PBt whiteflies compared to +PBt whiteflies (Figure 2b; p = .046). To detect this miRNA in whiteflies, we performed FISH microscopy, showing that novel-m0780-5p miRNA localized to the cytoplasmic region of whitefly bacteriocytes, and that bacteriocytes of −PBt whiteflies emitted stronger fluorescence when probing for this miRNA than did those of +PBt whiteflies (Figure 2c). We further verified that after Portiera was cured, PanBC protein expression significantly decreased in whitefly bacteriocytes (Figure 2d), as reported previously (Ren et al., 2021). This correlation remained consistent with our expectation that novel-m0780-5p regulates PanBC expression in bacteriocytes.
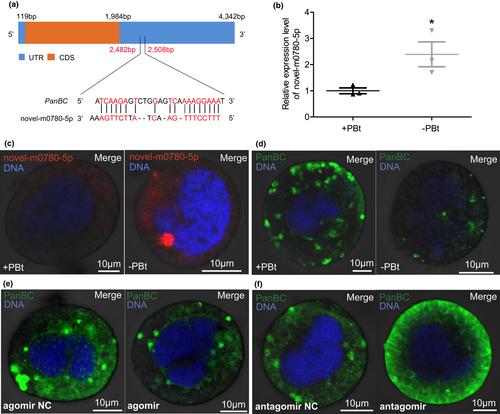
To confirm the regulation of PanBC expression by B. tabaci miRNA, female adult whiteflies were injected with novel-m0780-5p agomir, the double-stranded miRNA, and novel-m0780-5p antagomir, the chemically modified single-stranded miRNA, and PanBC expression in bacteriocytes was examined by immunofluorescence microscopy. The expression of PanBC was significantly reduced on days 1, 3 and 5 post-injection in agomir-injected whiteflies compared with the negative controls (Figure 2e; Figure S3a,b). In contrast, the expression of PanBC was significantly enhanced on day 3 post-injection in antagomir-injected whiteflies compared with the negative controls (Figure 2f). Collectively, these results demonstrated that novel-m0780-5p negatively regulates PanBC expression in bacteriocytes.
3.4 A B. tabaci miRNA regulates pantothenate level, symbiont titre and whitefly performance
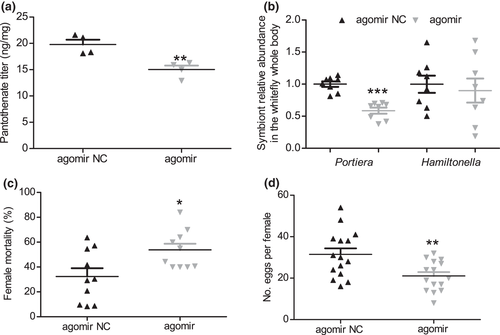
As we have previously demonstrated, silencing panBC reduced pantothenate levels, increased the mortality and decreased the fecundity of female adult whiteflies (Ren et al., 2021). After novel-m0780-5p agomir injection, the amount of pantothenate, symbiont titre, and the mortality and fecundity of female adult whiteflies were examined. A microbiological assay showed that pantothenate had significantly lower levels on day 3 post-injection in agomir-injected whiteflies compared with negative controls (Figure 3a; p = .0071). In addition, qPCR analysis showed that the titre of Portiera was significantly decreased on day 3 after agomir injection treatment (Figure 3b; p < .0001). In contrast, the titre of Hamiltonella remained unchanged on day 3 after agomir injection (Figure 3b; p = .67). We also observed that whitefly mortality was significantly increased and female fecundity was significantly reduced on day 3 post-injection in agomir-injected whiteflies compared with negative controls (Figure 3c; p = .015 for mortality and Figure 3d; p = .0056 for fecundity). Overall, these data demonstrated that novel-m0780-5p negatively regulates pantothenate levels. They also suggest that strong down-regulation of pantothenate biosynthesis can have negative impacts on symbiont titres and whitefly fitness.
3.5 Supplementation with pantothenate restores PanBC level, symbiont titre and fitness of agomir-injected whiteflies
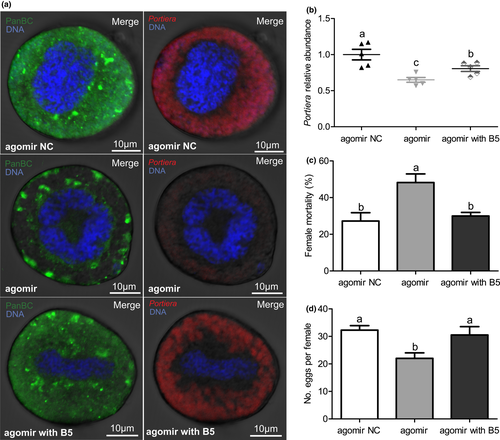
To examine whether pantothenate supplementation can alter the PanBC level, titre of Portiera and fitness of agomir-injected whiteflies, novel-m0780-5p agomir-injected whiteflies were fed an artificial diet supplemented with pantothenate for 2 days. The level of PanBC in bacteriocytes of agomir-injected whiteflies recovered after pantothenate supplementation treatment (Figure 4a). As reported for our earlier described experiments, the titre of Portiera in bacteriocytes of agomir-injected whiteflies was significantly decreased compared to agomir negative control (NC)-injected whiteflies; after pantothenate supplementation, the titre of Portiera in bacteriocytes of agomir-injected whiteflies significantly increased, although it was lower than that of the control agomir NC-injected whiteflies (Figure 4a,b; p < .01). As seen in the previous section, the mortality of agomir-injected whiteflies was significantly increased compared to agomir NC-injected whiteflies; after pantothenate supplementation, the mortality of agomir-injected whiteflies was decreased, approaching levels similar to those seen for agomir NC-injected whiteflies (Figure 4c; p < .05). When measuring fecundity as a separate proxy for whitefly fitness, we found that the fecundity of agomir-injected whiteflies was significantly decreased compared to agomir NC-injected whiteflies; but as seen in our mortality assays, pantothenate supplementation restored the fitness of agomir-injected whiteflies, boosting fecundity to levels comparable to those of agomir NC-injected whiteflies (Figure 4d; p < .01).
3.6 Evolutionary analysis for novel-m0780-5p in whiteflies
Mature miRNAs are single‑stranded RNAs of ~22 nt in length that are generated from hairpin-shaped transcripts or pre-miRNAs. Across these lengths, six “seed” nucleotides are key to base pairing with target mRNAs (Kim et al., 2009). We detected panBC and novel-m0780-5p in the genomes of six B. tabaci species, MEAM1, MED, SSA1, SSA2, SSA3 and Uganda, but not in the genome of Trialeurodes vaporariorum, and the hairpin structure was predicted for pre-novel-m0780-5p in all six B. tabaci genomes (Figure S4). Homologous copies of pre-novel-m0780-5p and mature novel-m0780-5p exhibited a minimum of 98.4% and 100% sequence identity, respectively, when compared across these six genomes (Figure 5), indicating novel-m0780-5p is highly conserved in B. tabaci.

4 DISCUSSION
Both HTGs and miRNAs are key players in mediating insect symbiosis (Bublitz et al., 2019; Chung et al., 2018; Feng et al., 2018; Husnik et al., 2013; Hussain & Asgari, 2014; Hussain et al., 2011; Kim et al., 2016; Luan et al., 2015; Moran & Bennett, 2014; Nakabachi et al., 2014; Ren et al., 2020, 2021; Sloan et al., 2014; Wybouw et al., 2016). Here, we show that insect bacteriocytes have evolved to express HTG and regulatory miRNA, which work together, jointly impacting levels of pantothenate biosynthesis. As we have further shown here, expression of this gene regulates the persistence of the obligate whitefly endosymbiont, Portiera. These findings suggest a need for future studies on interactions between miRNAs and HTGs in more insect symbiosis systems.
Some HTGs or their encoded proteins are highly expressed in insect bacteriocytes (Bao et al., 2021; Husnik et al., 2013; Luan et al., 2015; Moran & Bennett, 2014; Ren et al., 2021; Sloan et al., 2014). However, the means by which the expression of host HTGs or their proteins is regulated, and thereby impact host–symbiont interactions, remain unknown. A fused gene panBC horizontally transferred from bacteria in the genome of Bemisia tabaci is speculated to complement the missing genes PanB and PanC in Portiera for pantothenate synthesis (Chen et al., 2016). Previously, we revealed that PanBC cooperates with Portiera in pantothenate synthesis, which mediates the coordination of whitefly and symbiont fitness (Ren et al., 2021). In this study, we found that a B. tabaci miRNA, novel-m0780-5p, located in the cytoplasmic regions of whitefly bacteriocytes negatively regulates expression of the PanBC protein in bacteriocytes. Pantothenate level was significantly decreased and survival and fecundity were significantly reduced in novel-m0780-5p agomir-injected whiteflies compared with the negative controls. This result corroborates our previous findings that silencing panBC reduces pantothenate levels and whitefly fitness (Ren et al., 2021). To the best of our knowledge, this is the first example of an HTG being regulated by an miRNA generated from the host genome.
miRNAs are key players for host–pathogen/symbiont interactions (Hussain et al., 2011; Hussain & Asgari, 2014; Kim et al., 2016). For example, aae-miR-2940 targets the mosquito metalloprotease gene for Wolbachia maintenance (Hussain et al., 2011). Additionally, 14 miRNAs were either highly expressed in the aphid bacteriome, the Buchnera-housing tissue or differentially expressed in bacteriome vs. gut, a non-Buchnera-housing tissue (Feng et al., 2018). These aphid miRNAs can target aphid mRNAs for amino acid transport and metabolism (Feng et al., 2018). Metabolic complementation, particularly for the biosynthesis of amino acids and B vitamins, is widespread between sap-feeding Hemiptera and their symbionts (Moran & Bennett, 2014; Wilson & Duncan, 2015). However, how miRNAs impact metabolic collaboration between host and symbiont remains unclear. Here, we reveal that a novel miRNA regulates panBC-mediated cooperation between B. tabaci and Portiera for pantothenate synthesis. Our findings will advance study of the miRNA-mediated metabolic collaboration between host and symbionts.
Progressive hypervacuolation, autophagy or apoptosis and transcriptional factors are involved in bacteriocyte development or death, which can control the changes in obligate symbiont populations, in the pea aphid Acyrthosiphon pisum (Simonet et al., 2018), the cereal weevils Sitophilus spp. (Vigneron et al., 2014), the seed bug Nysius plebeius (Matsuura et al., 2015) and whiteflies (Li et al., 2022; Wang et al., 2022). Because novel-m0780-5p agomir-injection reduced the titre of Portiera in whiteflies, the miRNA could be used as another way for the host to control their symbiont population.
The mechanism by which pantothenate regulates the whitefly–symbiont association is intriguing but is currently unknown. B. tabaci miRNA novel-m0780-5p negatively regulates the expression of the PanBC protein in bacteriocytes. Pantothenate levels, the titre of Portiera but not Hamiltonella, and whitefly performance were reduced in PanBC RNA interference (RNAi) or novel-m0780-5p agomir-injected whiteflies (Ren et al., 2021, and this study). Supplementation with pantothenate restores PanBC levels, Portiera titre, and fitness of PanBC RNAi or agomir-injected whiteflies (Ren et al., 2021, and this study). These results suggest that pantothenate mediates the coordination of whitefly and Portiera fitness by some molecular mechanisms. Pantothenate is a precursor of CoA and acetyl-CoA (Douglas, 2017). Acetyl-CoA is a precursor for juvenile hormone (JH) synthesis (Bellés et al., 2005), and JH regulates the level of vitellogenin (Vg) in many insects (Bell and Barth, 1971; Santos et al., 2019; Tufail et al., 2014). Thus, pantothenate may impact Vg levels in whiteflies. Vg can provide critical nutrients for oogenesis and embryogenesis of insects (Raikhel & Dhadialla, 1992; Sappington & Raikhel, 1998; Tufail & Takeda, 2008), and it is also a pathogen pattern recognition receptor (Li et al., 2009; Salmela et al., 2015). Additionally, Vg plays a key role in insect–symbiont interactions (Guo, Hoffmann, et al., 2018; Herren et al., 2013; Brumin et al., 2020). Therefore, pantothenate could regulate whitefly fitness and symbiont abundance by influencing Vg levels in whiteflies, suggesting another hypothesis for future investigation.
Recruitment of an miRNA to regulate a newly acquired HTG in insects is of great interest. There are more than 1900 species of whiteflies (Ouvrard & Martin, 2019), with over 40 cryptic species in the B. tabaci complex, alone (De Barro et al., 2011; Mugerwa et al., 2021). Both panBC and novel-m0780-5p are present in the genomes of the six B. tabaci species studied here. Given the absence of this miRNA—and the panBC gene—from at least one other whitefly species (T. vaporariorum), it is possible that the laterally transferred panBC and the host-derived novel-m0780-5p have coevolved in a more limited range of whiteflies, perhaps solely in the B. tabaci complex. The expression of this whitefly miRNA in bacteriocytes of B. tabaci is an adaptation of bacteriocytes to regulate the expression of the foreign gene panBC. It is our hope that the present findings on the association between horizontally transferred panBC and a novel miRNA in B. tabaci will promote investigation of the coevolution of HTGs and miRNAs in other insect symbiosis systems. It will be valuable to study whether the mechanisms revealed in this study also take place in other insect symbioses.
Insect–microbe symbiosis can be used for pest control (Douglas, 2015). For example, stable introduction of a life-shortening Wolbachia into the mosquito Aedes aegypti halved adult lifespan (McMeniman et al., 2009). In the process of such study, it will be valuable to identify the variety of miRNAs that have evolved to target HTGs, and the broader impacts of such gene regulatory mechanisms in shaping symbiosis. Through an understanding of such mechanisms may come future opportunities for symbiosis-based pest control strategies, as alternatives to the widespread use of harmful pesticides.
ACKNOWLEDGEMENTS
We thank Professor Liu Shu-Sheng from Zhejiang University for providing the B. tabaci MEAM1 culture, and Dr Ren Fei-Rong for help with pantothenate assays. This work was supported by the National Natural Science Foundation of China (No. 31871967), and High-Level Talent Support Foundation from Liaoning and Shenyang Agricultural University (Projects XLYC1902104 and 880418001).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
J.B.L. conceived the study. X.S. performed symbiont elimination, pantothenate assays, qRT-PCR, qPCR and immunofluorescence experiments with Z.B.C. and ecology and FISH with C.Q.L. B.Q.L., C.Q.L., and Z.B.C carried out microinjection and ecology experiments. X.R.X. conducted symbiont elimination and RNA-sequencing data analyses. X.S., X.R.X. and J.B.L. analysed the data. J.B.L. wrote the manuscript. All of the authors edited and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The raw reads for whitefly miRNAs and mRNAs are available at the National Center for Biotechnology Information (NCBI) Short Read Archive (SRA), with accession nos. PRJNA682258 and PRJNA683123, respectively. The data that support the findings of this study are available in the Supporting Information and in the Dryad repository (https://datadryad.org/stash/share/9yqwcSXqzTO9pveE2Ju8VDWPwPBvGshSDq8Y_kK19yA).




