Intrinsic variation in the vertically transmitted core virome of the mosquito Aedes aegypti
Handling Editor: Camille Bonneaud
Abstract
Virome studies among metazoans have revealed the ubiquity of RNA viruses in animals, contributing to a fundamental rethinking of the relationships between organisms and their microbiota. Mosquito viromes, often scrutinized due to their public health relevance, may also provide insight into broadly applicable concepts, such as a “core virome,” a set of viruses consistently associated with a host species or population that may fundamentally impact its basic biology. A subset of mosquito-associated viruses (MAVs) could comprise such a core, and MAVs can be categorized as (i) arboviruses, which alternate between mosquito and vertebrate hosts, (ii) insect-specific viruses, which cannot replicate in vertebrate cells, and (iii) viruses with unknown specificity. MAVs have been widely characterized in the disease vector Aedes aegypti, and the occurrence of a core virome in this species has been proposed but remains unclear. Using a wild population previously surveyed for MAVs and a common laboratory strain, we investigated viromes in reproductive tissue via metagenomic RNA sequencing. Virome composition varied across samples, but four groups comprised >97% of virus sequences: a novel partiti-like virus (Partitiviridae), a toti-like virus (Totiviridae), unclassified Riboviria, and four orthomyxo-like viruses (Orthormyxoviridae). Whole or partial genomes for the partiti-like virus, toti-like virus, and one orthomyxo-like virus were assembled and analysed phylogenetically. Multigenerational maintenance of these MAVs was confirmed by RT-PCR, indicating vertical transmission as a mechanism for persistence. This study provides fundamental information regarding MAV ecology and variability in A. aegypti and the potential for vertically maintained core viromes at the population level.
1 INTRODUCTION
The application of high-throughput sequencing to investigate RNA virus biodiversity has led to an enormous increase in the discovery of new taxa (Shi et al., 2016). Until recently, most knowledge on RNA viruses was derived from those that can be cultured or that act as agents of human disease. It is now clear, however, that RNA viruses are ubiquitous in animals with a wide range of effects, contributing to a fundamental shift in how we perceive relationships between multicellular organisms and their microbiota. Nevertheless, the viromes of some arthropod groups, such as mosquitoes (Order Diptera, family Culicidae), have received relatively greater scrutiny due to their implications for human and animal health. Collectively, analyses of mosquito viromes may provide insight into broadly applicable principles, including the concept of “core viromes,” which refers to a set of viruses consistently associated with a host group at the species or population level, which in combination with other components of the microbiome, may fundamentally influence host biology.
From previous studies, mosquito-associated viruses (MAVs) may be broken down into at least three categories: (i) arboviruses, which alternate between mosquito and vertebrate hosts and include well-known pathogenic agents in humans and domestic animals (Weaver & Reisen, 2010), (ii) insect-specific viruses (ISVs), which are incapable of replicating within vertebrate cells and therefore limited to mosquito hosts (Junglen et al., 2017; Nasar et al., 2012; Sang et al., 2003), and (iii) viruses for which host specificity is unknown (Agboli et al., 2019; Atoni et al., 2019). Although the first group has received much greater attention, it is now clear that the latter two are more common in virome data sets and more likely to contribute to a “core virome.” However, distinguishing between viruses in these categories is challenging, as many have only been described through sequencing, and experimental data on their routes of transmission and persistence across generations are limited (Atoni et al., 2019). Presumably, ISVs are maintained by transmission from mother to offspring (i.e., vertical transmission), although horizontal transmission between individuals may certainly occur. Even less in known about MAVs with unknown specificity, as these viruses may infect multiple hosts and/or occur only transiently in mosquitoes.
Interest in mosquito viromes has coincided with the spread of mosquito-borne viral diseases into new areas, as over half the world's population is now at risk of infection with arboviruses that cause dengue, chikungunya, or Zika (Brady & Hay, 2020; Kraemer et al., 2015; Weaver, 2014). This increase in risk is largely correlated with the global range expansion of Aedes aegypti mosquitoes, the primary vector for these viruses (Brady & Hay, 2020; Messina et al., 2019). In Florida, USA, A. aegypti populations thrive in most of the state's large population centers due to their urban niche (Britch et al., 2008; Reiskind & Lounibos, 2013; Wilke et al., 2019). Locally transmitted infections of dengue virus (DENV) serotypes 1–3 have occurred in 11 of the last 12 years in South Florida (CDC, 2010; Rey, 2014; https://www.cdc.gov/dengue/statistics-maps/index.html), while in 2014 and 2016, outbreaks of chikungunya (Kendrick et al., 2014) and Zika (Grubaugh et al., 2017; Likos et al., 2016), respectively, occurred exclusively in counties with A. aegypti (Hahn et al., 2017). Moreover, a recent study of MAVs in a Floridian A. aegypti population detected and fully sequenced DENV serotype 4 (DENV-4) despite the absence of any reported human cases (Boyles et al., 2020), raising the question of whether MAV coinfections influence vertical transmission of this DENV serotype.
It is widely appreciated that the ability of a mosquito to harbour and transmit arboviruses (i.e., its vector competence) varies based on the genetic backgrounds of the vector and virus, external environmental variables, and the composition of the mosquito's microbiome (Tabachnick, 2013). The latter is complex and includes nonarboviral MAVs. Studies of MAV effects on mosquito biology have emphasized “classic” ISVs, which are congeneric to arboviruses in a handful of genera such as Flavivirus (Flaviviridae) and Alphavirus (Togoviridae) (Junglen et al., 2017; Nasar et al., 2012; Sang et al., 2003). Dual infections of some arbovirus/ISV combinations in mosquitoes or cell lines are known to influence arbovirus dynamics in vitro and vector competence in vivo, although the directions of the effects have varied (Baidaliuk et al., 2019; Bolling et al., 2012; Nasar et al., 2015; Zhang et al., 2017). Data on potential underlying mechanisms are limited, but negative effects may stem from competitive interactions for cellular resources or when an antiviral response triggered by one virus impacts the other. On the other hand, positive effects may occur through upregulation of shared host factors or when suppression of host defences by one virus benefits the other (Zhang et al., 2017; Baidaliuk et al., 2019).
Many recently discovered ISVs and MAVs of unknown specificity belong to virus families more distantly related to arboviruses than the classic ISVs described above. Given their pervasiveness, a better understanding of their ecology and impact on host biology is needed. The term “core virome” was recently coined to describe the set of MAVs common to the majority of individuals in a mosquito population (Shi et al., 2019), and has since been divided into two components: vertical (passed from mother to offspring) and environmental (acquired horizontally from the environment) (Shi et al., 2020). The distinction may have implications for long-term effects on host biology at the population level, as the vertical core is more likely to infect all developmental stages and have temporally stable relationships with the host that are buffered from environmental heterogeneity. In contrast, environmentally acquired viruses probably infect only a subset of developmental stages, depending on the timing of infection and environmental source (e.g., acquisition by immature stages in an aquatic environment vs. acquisition by adults from plant nectar when sugar feeding), and these may form less consistent associations with the host over time due to environmental change.
To date, limited research on Aedes mosquitoes suggests that at the population level, viromes are consistent across developmental stages (Shi et al., 2020), and over short time scales (~ 1 year) (Boyles et al., 2020; Shi et al., 2020). However, it is unknown how well vertical core viromes are sustained over longer periods of time, or the degree to which environmental core viromes are transient. Moreover, most laboratory mosquito colonies used in arbovirus research have yet to be examined, and these could be useful models to address questions about the impact of core viromes on mosquito biology.
To begin filling these gaps in knowledge, we used tissue-specific RNA metagenomics and reverse-transcription PCR to follow up on previous virome profiles characterized for A. aegypti from central Florida (Boyles et al., 2020). Our objectives were to (i) define the vertically transmitted virome in ovary pools from field-derived (G0) and laboratory-derived A. aegypti, (ii) examine the persistence of specific MAVs over time in ovaries of generation 7 (G7) descendants of field-derived females, (iii) assess variability among the sexes for MAVs maintained in a field-derived colony, and (iv) use phylogenetics to investigate evolutionary relationships between ovary virome members and previously described MAVs. We hypothesized that elements of the vertical core virome would be present in both our field-derived A. aegypti and the laboratory-derived “Orlando” (ORL) strain due to both originating from central Florida, but that field-derived viromes would have higher MAV diversity due to greater environmental variability, as the ORL strain was colonized in the late 1940s (Kuno, 2010). We further predicted that male MAV infection status would mimic that of their female counterparts because of efficient vertical transmission, and that A. aegypti MAVs would cluster phylogenetically with MAVs from other mosquito taxa.
2 MATERIALS AND METHODS
2.1 Mosquito collections and sample preparation
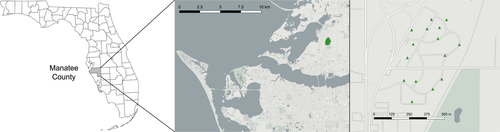
Mosquito eggs were sampled from Palmetto, Florida, in July and August of 2018 in collaboration with the Manatee County Mosquito Control District (MCMCD) using ovijars. These were 500 ml sampling containers approximately half filled with water and lined with moist seed-germination paper as an egg-laying substrate. Fifteen ovijars were placed throughout a periurban area of approximately 1 square kilometer, and ovijar locations were the same as those previously described to sample Aedes spp. eggs in 2016–2017 (Boyles et al., 2020) (Figure 1). Egg papers were collected on a weekly basis and dried at MCMCD before being returned to the insectary at the Florida Medical Entomology Laboratory (FMEL) in mid-August. These were hatched in distilled water, reared to adulthood, and sorted by species into separate cages by ovijar. Eggs from four ovijars yielded sufficient A. aegypti adults for successful mating, with most coming from a single ovijar (Table S1). On days 5–7 post-emergence, females (G0) were fed on chickens (IACUC protocol 201807682) and three days later transferred individually to 50 ml ovicages lined with moist seed germination paper. Gravid mosquitoes were allowed to oviposit over a two-day period, and eggs were dried and stored separately for 140 females.
Upon egg collection in the laboratory, each female was cold anaesthetized on ice, surface sterilized in 70% ethanol, rinsed twice with sterile PBS, and then dissected one at a time to remove pairs of ovaries. Following dissection, ovaries were immediately transferred to RNAlater solution (Invitrogen), and then combined into five pools of 24–40 pairs. Because one ovijar produced over 70% of the females, ovaries from these mosquitoes were divided among four separate pools with the fifth consisting of ovaries of females from three ovijars (Table S1). Nevertheless, because A. aegypti practices “skip oviposition” in nature (i.e., lays eggs across multiple sites), high egg numbers in a single ovijar suggests oviposition from multiple females. Therefore, each pool probably contained ovaries of descendants from multiple wild females. After pooling, tubes were stored at –80°C until processing for RNA extraction. Eggs collected from these mosquitoes were used to establish a field-derived colony from Palmetto. After seven generations of maintenance in the laboratory, ovaries were dissected from 50 G7 A. aegypti females as described above, combined into five pools of ovaries from 10 mosquitoes (one pair each), and stored at −80°C in RNAlater. Similarly, 15 G7 A. aegypti males were collected from the same cage as the females, killed by freezing at −20°C for 15 min, and stored as five pools of three individuals in RNAlater at –80°C. These same methods were used for the ORL A. aegypti with 15 females selected at random for ovary dissections.
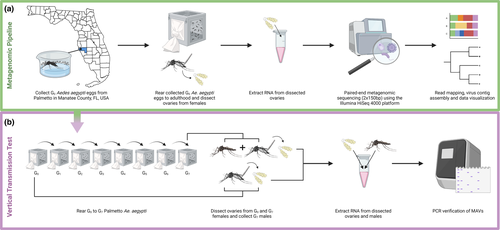
Prior to RNA extraction, ovary pools from G0 females were thawed on ice, and RNAlater was removed. Pools were homogenized manually in 0.2 ml of TRIzol reagent (Invitrogen) using a sterilized pestle. The volume of TRIzol was then brought up to 1.0 ml for each pool, and RNA was extracted following the manufacturer's instructions, followed by treatment with TURBO DNase (Invitrogen). RNA samples were shipped on dry ice to Novogene where RNA metagenomic libraries were prepared using the NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (New England Biolabs). Libraries were pair-end sequenced (2 × 150 bp) using the Illumina HiSeq 4000 platform (Figure 2a), and raw sequence reads were deposited into the NCBI Sequence Read Archive (Coatsworth et al., ; https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA765718). RNA from G7 males and from ovaries dissected from G7 and ORL females was extracted as described above and used to investigate vertical transmission by reverse transcription PCR as described below (Figure 2b).
2.2 Bioinformatic analyses of read level metagenomic data by sample
Sequence contaminants and adapters were removed using BBduk v. 38.86 (https://sourceforge.net/projects/bbmap/). Aedes aegypti reads were mapped to the A. aegypti genome (LVP_AGWG version AaegL5.3) and removed using BBmap. Local similarity searches were performed against the National Centre for Biotechnology Information's nonredundant protein database (NCBI nr) (downloaded 01/21) (NCBI Resource Coordinators, 2018) using DIAMOND v 2.0.7 (Buchfink et al., 2015). MEGAN6 v 6.19.2 (Huson et al., 2007) was used to assign reads to each lowest common ancestor (LCA) using the default naive LCA settings (in read count mode), mapping against NCBI nr (megan-map-Jan2021.db.zip from the MEGAN6 website (https://software-ab.informatik.uni-tuebingen.de/download/megan6)). A detailed summary of sequencing results can be found in Table S2.
2.3 Viral genome assembly on concatenated sample reads
Due to issues with small incomplete viral contigs arising from individual sample viral genome assembly, reads not mapping to the A. aegypti genome from field samples 1–5 were concatenated, and contigs were assembled de novo using SPAdes v 3.14.1 (Bankevich et al., 2012) in metagenomics mode. Reads found to align to Atrato partiti-like virus 3 were separately parsed into contigs using the same pipeline (subdivided due to low similarity with known database matches). Local similarity searches were performed against NCBI nr (NCBI Resource Coordinators, 2018) using DIAMOND v 2.0.7 (Buchfink et al., 2015). MEGAN6 v 6.19.2 (Huson et al., 2007), was used to assign each LCA (using the long-read import option), and to visualize and extract viral contigs. NCBI’s open reading frame (ORF) finder (https://www.ncbi.nlm.nih.gov/orffinder/) was used to identify ORFs in each assembled viral contig. ORFs >300 nucleotides in length were searched using BLAST (Zhang et al., 2000) against the nr protein sequence database through NCBI.
2.4 Reverse transcription PCR
For a subset of MAVs identified through metagenomics, mapped viral reads from each sample were aligned to create consensus sequences using the Velvet Optimiser (v2.2.6) (Zerbino & Birney, 2008) to enable primer design for reverse transcription PCR to confirm viral RNA in the original pools and screen tissues from subsequent generations. Consensus sequences were then searched against the NCBI nr database (NCBI Resource Coordinators, 2018) using Blastx to confirm the virus and protein match. Primers were designed using Primer3 with default parameters to yield amplicons ranging from 150–500 bp (Table S3).
For reverse transcription PCR, we performed a two-step procedure: cDNA first-strand synthesis using an RNA template followed by traditional PCR. For cDNA synthesis, 5 µg of total RNA from each ovary pool (field- and colony-derived) was used to generate cDNA with the RevertAid First Strand cDNA Synthesis kit (Thermo Scientific) following the manufacturer's instructions for random hexamers. PCR amplifications were performed on each pool with all primer sets in 25 µl reactions, each containing dNTPs at a concentration of 0.2 mM, primers at a concentration of 0.2 µM, 1.0 units of Taq DNA polymerase (DreamTaq, Thermo Scientific), 1x Taq polymerase buffer, and 1 µl of cDNA from the first-strand synthesis reaction diluted 1:1 with ultrapure water. All PCR amplifications were performed with an initial melt step of 95°C for 5 min followed by 35 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s, followed by a final extension step of 72°C for 10 min. Amplicons were electrophoresed on 2% agarose gels, stained with GelRed nucleic acid stain (Biotium), and visualized on a gel documentation system (Azure Biosystems c200). For samples yielding a PCR amplicon, products were purified using the GeneJET PCR Purification Kit (Thermo Scientific). Purified amplicons were ligated into plasmids using the CloneJET PCR Cloning Kit (Thermo Scientific) and then incubated with chemically competent E. coli (DH5α) (New England Biolabs) following the manufacturer's instructions. Transformed E. coli were grown overnight on selective LB agar plates with carbenicillin (100 µg/ml) at 37°C. Colonies carrying the plasmid and amplicon were transferred to liquid LB media with carbenicillin (100 µg/ml) and grown overnight, shaking at 220 rpm at 37°C. Cultures were harvested by centrifugation in microcentrifuge tubes at 17,000 × g for 5 min, and plasmid DNA was extracted using the GeneJET plasmid miniprep kit (Thermo Scientific). Plasmid DNA was sent to Eurofins Genomics for sequencing using a plasmid-specific primer included with the CloneJET kit.
2.5 Viral gene phylogenetics
Alignments were created from resultant viral RdRp and capsid sequence BLAST results using MUSCLE (Edgar, 2004) in MEGA X 10.0.3 (Kumar et al., 2018) (Files S1–S7). PhyML v 3.0 (Guindon et al., 2010) and smart model selection (SMS) (Lefort et al., 2017) were used to create an optimized maximum likelihood (ML) phylogenetic tree for each alignment. Branch supports were computed by an approximate likelihood ratio test (aLRT) with SH-like support as implemented in PhyML. Trees were annotated and coloured in FigTree v1.4.4 (https://github.com/rambaut/figtree/releases).
3 RESULTS
3.1 Partiti- and toti-like insect-specific viruses dominate the Aedes aegypti virome
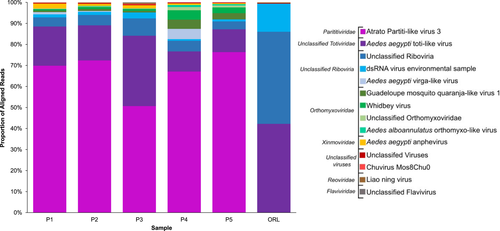
To identify potential core virome MAVs and compare field versus laboratory A. aegypti virome diversity, viral reads from each ovary pool were assigned to their lowest common ancestor (LCA) in MEGAN6 v 6.19.2 (Huson et al., 2007), an algorithm that matches sequencing reads to their lowest level of taxonomic conservation and sums each taxonomic category per sample based on read count. Partitiviridae reads comprised between 50%–76% of all viral reads in the field-derived Manatee County (Palmetto, P) samples, while the general class of unclassified Riboviria reads made up >57% of the viral reads in the ORL sample and between 5%–11% of the p-samples (Figure 3). Viral reads aligning best with Atrato partiti-like virus 3 were the most prevalent across all the P samples, making up 70, 72, 51, 67 and 76% of the viral reads (P1–P5 ovary pools respectively), but were completely absent from the ORL sample. Reads aligning to A. aegypti toti-like virus accounted for 19, 17, 33, 10, and 11% of the reads for the P samples, and represented 42% of the ORL sequences. Together, 75%–90% of all viral reads from field-derived samples aligned to these two groups (Partitiviridae and Totiviridae). In addition, 42% of the ORL reads and ~5% of reads in each of the P samples aligned with unclassified Riboviria virus(es), followed by 13% matching to a dsRNA virus environmental sample (a single virus as per NCBI taxonomy ID: 1075826) in ORL and ~1.5% in all P samples. Sequences matching to four viruses in Orthomyxoviridae (Guadeloupe mosquito quaranja-like virus 1, Whidbey virus, unclassified Orthomyxoviridae, and Aedes alboannulatus orthomyxo-like virus) were restricted to field-derived samples and collectively accounted for 1.7, 2.4, 2.0, 11.4, and 7.2% of the reads from P1–P5, respectively. The remaining viral sequences in these samples each represented less than one percent of the total viral sequence pool, and in order of read abundance, matched to A. aegypti virga-like virus, A. aegypti anphevirus, unclassified viruses, Chuvirus Mos8Chu0, Liao ning virus, and an unclassified flavivirus (Figure 3). The field-derived P samples were more diverse than the ORL sample, as the P samples had an average of 12 different viral assignments (range of 10–13), while the ORL sample only contained 5. However, the ORL sample contained ovaries from 15 females compared to an average of 28 females for the P samples (range = 24–40, Table S1).
3.2 Genome assembly of Palmetto toti-, partiti- and orthomyxo-like viruses
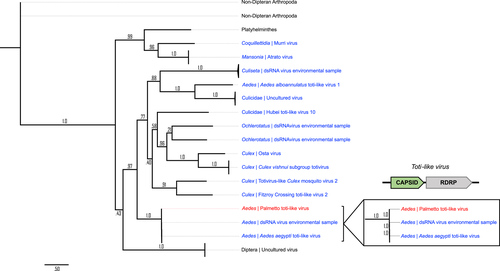
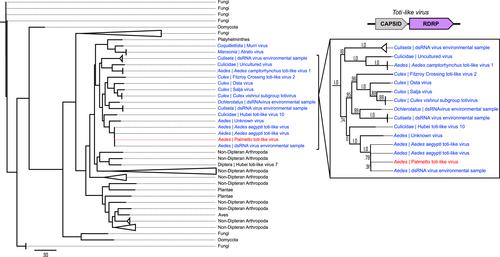
Whole or partial genome assembly was completed for our Palmetto toti-, partiti- and orthomyxo-like viruses to compare the overall similarity between the viruses found here and those reported previously. Eight open reading frames (ORFs) were predicted based on the assembled Palmetto toti-like virus contigs. Only two of these ORFs (ORF 2 [1033aa] and ORF 3 [1021aa]) assembled in a linear format akin to the typical capsid-RdRp Totiviridae structure (Figures 4, 5) and created products with known sequence similarity. The 1033aa long ORF of the Palmetto toti-like virus showed high amino acid similarity to other A. aegypti RNA-dependent RNA polymerase (RdRp) genes in toti-like viruses (>98% identity), with high query coverage (~90%) to 926aa RdRp sequence isolated from individual Aedes aegypti mosquitoes from Guadeloupe, a Caribbean island (QEM39131.1 and QEM39133.1) (Shi et al., 2019). The 1021aa sequence showed high similarity and query coverage to the 1008aa long Guadeloupe Aedes aegypti toti-like capsid sequences (>99% and 98%, respectively) isolated from the samples. Together, these sequences represent the RdRp and capsid genes of the Palmetto toti-like virus identified herein, and because of their near identity with nucleotide sequences of the Guadeloupe Aedes aegypti toti-like virus, these viruses are considered the same species.
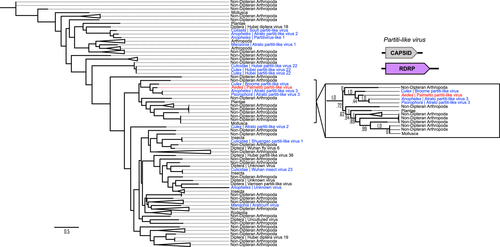
In contrast, only three ORFs were predicted for the Palmetto partiti-like virus, which probably has a simple two-segmented genomic structure characteristic of the Partitiviridae (Figure 6). Only one of these ORFs (ORF 1 [446aa]) had sequence similarity to known sequences, with 78% identity and 95% query coverage to a 472aa RdRp Atrato partiti-like virus sequence (QHA33899.1) isolated from Psorophora albipes mosquitoes in Colombia, and 79% identity and 86% query coverage to another RdRp Atrato partiti-like virus sequence (QHA33901.1, 387aa) isolated from Anopheles darlingi mosquitoes in Colombia. Therefore, this sequence was classified as the RdRp for the Palmetto partiti-like virus.
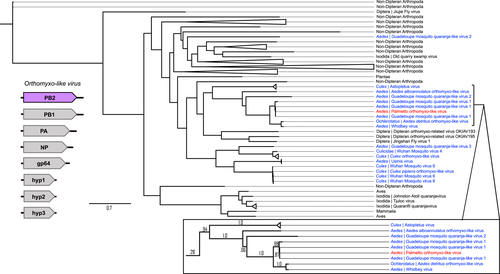
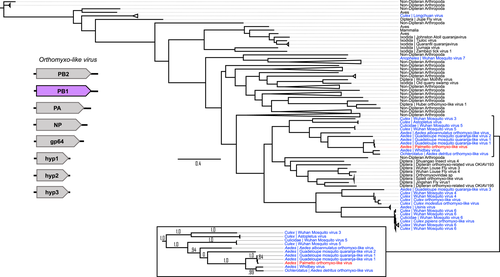
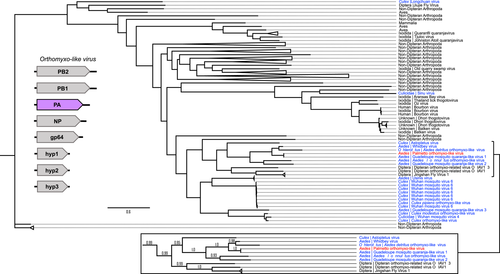
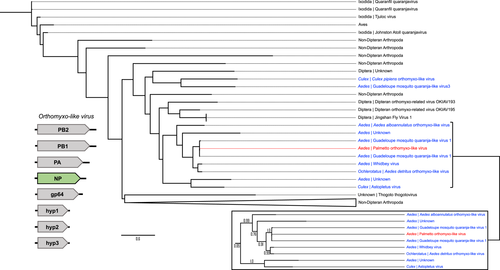
Twenty ORFs were predicted from the Orthomyxoviridae contigs, and six of these ORFs had known sequence similarity based on nonredundant protein blast searches. Four of these ORFs appeared to represent one virus (Palmetto orthomyxo-like virus), while the remaining two ORFs are probably from two other orthomyxoviruses: ORF 4 (229aa) with 33% identity and 65% query coverage to an Atrato Chu-like virus 5 hypothetical protein 2 (QHA33674.1) identified in Psorophora albipes from Colombia, and ORF 5 (294aa) with 66% identity and 87% query coverage with the nucleoprotein of Wuhan Mosquito Virus 6 (QRW42410.1) isolated from Culex tarsalis mosquitoes from California. The four Palmetto orthomyxo-like virus ORFs had highest sequence similarity to Guadeloupe mosquito quaranja-like virus 1 (GMQLV1), isolated from A. aegypti collected in San Diego County, California (Batson et al., 2021). Like other viruses in the Orthomyxoviridae, quaranjaviruses have a segmented genome, and GMQLV1 was originally described as probably having six or seven segments (Shi et al., 2019). However, recently reported data (Batson et al., 2021) indicate that eight segments are more likely (Figures 7-10). ORF7 (800aa) had 100% identity and 98% query coverage to GMQLV1 PB2 (QRW42587.1); ORF 10 (797aa) had 99.75% identity and 98% query coverage to GMQLV1 PB1 (QRW42591.1); ORF 11 (716aa) had 99.86% identity and 98% query coverage to GMQLV1 PA (QRW42581.1); and ORF 16 (584aa) had 99.64% identity and 95% query coverage to GMQLV1 NP (QRW42580.1). These four ORFs represent the nucleoprotein (NP - ORF 16), and the heterotrimeric RdRp complex (PB2 – ORF7, PB1 – ORF10, PA – ORF11) for our Palmetto orthomyxo-like virus. Given their nearly identical nucleotide sequences, the Palmeetto orthomyxo-like virus is considered the same species as GMQLV1.
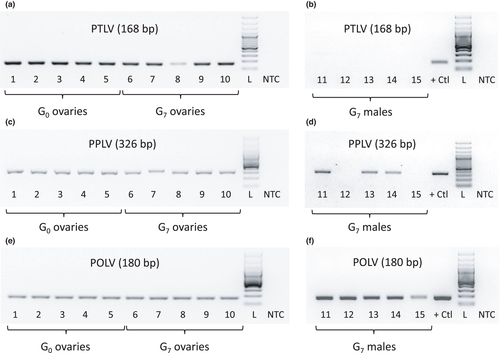
3.3 Reverse transcription PCR confirmation supports virus vertical transmission
To assess vertical transmission success of these three MAVs, reverse transcription PCR with primers designed from metagenomic data for the Palmetto toti-, partiti- and orthomyxo-like viruses (Table S1) was used to rescreen RNA remaining from the original G0 pools, as well as RNA from ovaries of G7 females and from G7 males. This approach yielded amplicons for the toti-like virus capsid gene, the partiti-like virus RdRp gene, and the orthomyxo-like virus PA gene (Figure 11). Amplicons were sequenced and the presence of all three viruses was confirmed in both G0 and G7 ovary pools (Figure 11a, c, e). The Palmetto orthomyxo-like virus was present in all G7 adult-male pools (Figure 11b, d, f), while the Palmetto partiti-like virus was found only in a subset of these samples, occurring in three of five pools. Intriguingly, no males were positive for Palmetto toti-like virus. Although the occurrence and frequency of environmental acquisition and venereal transmission of these viruses remain open questions, the ovary-positive data and presence across multiple generations suggests viral persistence in the population by vertical transmission.
3.4 Viral gene phylogenetics
Phylogenetics on virus gene segments was completed to investigate the evolutionary relationships of each of the MAVs to infer their ecological history. Sequences were downloaded from Genbank, and protein alignments resulted in 25 sequences spanning 1008 sites for the toti-like capsid tree, 101 sequences (1032 sites) for the toti-like RdRp tree, 101 sequences (447 sites) for the partiti-like RdRp tree, 78 sequences (1668 sites) for the orthomyxo-like PB2 tree, 101 sequences (1041 sites) for the orthomyxo-like PB1 tree, 101 sequences (1388 sites) for the orthomyxo-like PA tree, and 63 sequences (932 sites) for the orthomyxo-like NP tree. After smart model selection (SMS), five trees resulted in an LG optimal model with Gamma rates (G), invariable sites (I), and empirical frequencies (F) (LG+G+I+F). Log-likelihood ratios for the toti-like trees were −53142.30 and −23019.63 (RdRp and capsid, respectively), −44320.2 for the partiti-like RdRp tree, and −77638.40 and −94785.91 for the orthomyxo-like PB1 and PA trees, respectively. The orthomyxo-like PB2 and NP trees resulted in a LG+G+F optimal model with log-likelihood ratios of –86514.82 and −49892.72, respectively. Both the toti-like capsid and RdRp gene trees showed distinct mosquito clusters (Figures 4 and 5) with strong branch support. The Palmetto toti-like virus identified herein clustered with other Aedes aegypti toti-like viruses. Culex derived toti-like viruses made up a distinct clade in the RdRp tree, while in the capsid tree two of the Culex-derived viruses appeared more closely related to viruses from Ochlerotatus, a subgenus of Aedes according to traditional Culicidae systematics (Wilkerson et al., 2015). Because of better representation of RdRp sequences in databases, we were able to analyse a broader range of totivirus sequences in the RdRp virus tree revealing distant relatedness to totiviruses from numerous nondipteran arthropods, fungi and oomycota.
In contrast, the partiti-like RdRp phylogenetic tree showed no obvious mosquito clustering, as mosquito-derived partiti-like virus sequences were dispersed throughout the tree (Figure 6). Our Palmetto partiti-like virus formed a small but well-supported clade with other mosquito partiti-like viruses (Atrato partiti-like virus 3 and Broome partiti-like virus), as well as partiti-like viruses from other nondipteran arthropods, plants, and molluscs. Virus sequences from nondipteran arthropods, dipterans, and insects made up most of the remaining branches in the tree.
All the orthomyxo-like trees (PB2, PB1, PA, and NP) displayed similar trends to one another, with two distinct mosquito clusters, loosely organized into Culex- and Aedes-derived viruses, separated by MAVs from primarily haematophagous Dipterans (Figures 7-10). In all the orthomyxo-like gene segment trees, our Palmetto orthomyxo-like virus was part of the well-supported Aedes-based mosquito clade, directly clustering with Guadeloupe mosquito quaranja-like virus 1, Whidbey virus, and Aedes detritus orthomyxo-like virus.
4 DISCUSSION
4.1 Conservation of vertically transmitted core viruses in Floridian Aedes aegypti
Five of the viral taxa detected through our sequence analysis were present in both field- and laboratory-derived samples originating from central Florida, despite the source of the latter being maintained in the laboratory since the early 1940s (Kuno, 2010). Of the sequences common to all samples, the most abundant corresponded to Aedes aegypti toti-like virus, although dsRNA virus environmental sample and unclassified Riboviria virus(es) were also well represented (Figure 3). The Palmetto toti-like virus identified also occurred in descendants of the field-collected mosquitoes seven generations after sampling, indicating that vertical transmission is a potential mechanism for long-term persistence. This virus showed striking similarity to Aedes aegypti toti-like viruses from Guadaloupe and an unnamed virus identified in A. aegypti from Thailand. Totiviridae and toti-like viruses have been found in several mosquito genera with widespread global prevalence, including Anopheles in Liberia (Fauver et al., 2016), Armigeres in China (Zhai et al., 2010), Culex in Belgium (Wang et al., 2020), Culex in California (Batson et al., 2021), Culex in Australia (Williams et al., 2020), Culex in Japan (Isawa et al., 2011), and Mansonia in Brazil (de Lara Pinto et al., 2017), suggesting that toti-like viruses may be common components of core viromes among mosquito populations. Metagenomic analyses of mosquitoes from our Palmetto site collected in 2016 and 2017 also identified a toti-like virus, as well as dsRNA virus environmental sample (Boyles et al., 2020), further supporting that A. aegypti mosquitoes with similar genetic backgrounds (here, representative of central Florida-based populations) share many of the same viruses, as previously reported (Konstantinidis et al., 2021; Öhlund et al., 2019; Shi et al., 2019; Wang et al., 2020).
4.2 The field A. aegypti mosquito-associated viral landscape is more diverse
Despite the pooled nature of our samples, there were clear differences in the general diversity of the virus communities, as 12 eukaryotic viral taxa were found on average in the field-derived samples compared to only five in the ORL sample. This greater virome diversity of field-derived samples was also noted in the 2016–2017 samples from our Palmetto site (Boyles et al., 2020), as were numerous other partiti-like viruses (Hubei partiti-like viruses 29, 32, 33, 34, Wenling partiti-like virus 2) and two viruses in Orthomyxoviridae (Whidbey virus, Aedes alboanulatus orthomyxo-like virus). The Palmetto partiti-like virus (Partitiviridae) and the four taxa belonging to the family Orthomyxoviridae (Guadeloupe mosquito quaranja-like virus 1, Whidbey virus, Aedes alboannulatus orthomyxo-like virus, and an unclassified Orthomyxoviridae virus) (Figure 3) were solely found in the field samples. However, these differences should be interpreted with caution since only one laboratory-derived sample was examined compared to five from the field and consisted of ovaries from fewer females. It is also possible that our hatching of field-collected eggs in the lab and rearing to adults could have altered the virome profiles of the field samples in unknown ways (e.g., loss of viruses, shift in dominant taxa). Nevertheless, the variation between lab and field samples is intriguing, as the ORL colony has been maintained for decades under standardized conditions that are undoubtedly more stable than those experienced by A. aegypti in nature. Further, a similar finding was reported for comparisons of field versus lab strains of the congeneric mosquito species Aedes albopictus (Shi et al., 2020), and an in-depth comparison of wild-caught Drosophila and laboratory stocks revealed strikingly different viral communities (Webster et al., 2015). Together, these data suggest that an organism's environment, whether laboratory or field, serves as a source of new viral infections and greatly impacts virome diversity over time. Although nonvertical routes of MAV acquisition in mosquitoes (i.e., horizontal transmission) are not well studied, the data to date suggest that some MAVs establish new infections through a wide range of routes involving environmental sources or interactions with other organisms. Potential routes of horizontal transmission include plant or detritus feeding by larvae, directly from water in the larval environment, from the meconium upon adult eclosion, from fungal or parasitic infection, via parasitoids, through nectar feeding by adults, and from one mosquito to another during mating (Agboli et al., 2019; Dolja & Koonin, 2018; Vasilakis & Tesh, 2015; Xu et al., 2020). As the ORL strain A. aegypti have been raised in laboratory settings for over 70 years (Kuno, 2010), these diverse viral acquisition routes would be limited.
4.3 Totiviridae and Partiviridae phylogenies point to shared plant- and fungal-based lineages
The clear mosquito-associated clade present in the toti-virus like sequences (Figures 4 and 5) could represent widespread concurrent viral movement or long host coevolution (Wang et al., 2020), as is thought to be the case for Bunyaviridae, Flaviviridae, Rhabdoviridae, and Togoviridae ISVs, which are assumed to have evolved and diversified alongside their host (Vasilakis & Tesh, 2015). Historically, totiviruses were primarily fungal associated, and probably represent a viral taxon with an ancient origin (Dolja & Koonin, 2018). This historic basis is mirrored in our toti-like virus RdRp tree (Figure 5), as mosquito-associated sequences matched to dipteran and nondipteran arthropod virus RdRps, followed by distant clades of fungal toti-like virus RdRps. The jump of these toti-like viruses into invertebrate systems is probably due to horizontal virus transfer (Dolja & Koonin, 2018), and may have occurred within the mosquito itself, as fungi are common inhabitants of the mosquito microbiome. Similarly, the Partitiviridae and partiti-like viruses have been historically associated with plants and fungi (Nibert et al., 2014), and their associations with arthropods have only recently been discovered, largely from high-throughput sequencing data. Partitiviridae and partiti-like viruses have been reported across numerous mosquito genera (Culex, Culiseta, Coquilettidia, Anopheles, Aedes) with a nearly global distribution (North and South America, Africa, Asia, and Europe) (Konstantinidis et al., 2021; Öhlund et al., 2019; Shi et al., 2019; Wang et al., 2020). The low congruence between viral host phylogeny and host-range for these partiti-like viruses may suggest a recent host-switching event (Dolja & Koonin, 2018; Grubaugh et al., 2016), as the Palmetto partiti-like virus was highly divergent (<80% similarity) from other known partiti-like viruses and probably represents a novel virus. Cross-species transmission (i.e., horizontal virus transfer) is clearly common throughout the MAV landscape (Shi et al., 2018) and has probably been a major factor in the evolutionary history of the partiti-like virus described herein (Figure 6).
4.4 Orthomyxoviridae phylogenies show a distinct hematophagous arthropod clade with known human pathogenic members
The phylogenies for the Orthomyxoviridae genome segments displayed similar trends, with viruses from non-hematophagous, nondipteran arthropods as ancestral to those in mosquito-enriched clades in all four trees (Figures 7-10). Known vertebrate pathogenic viruses formed distinct and separate subclades from the mosquito and hematophagous dipteran viruses. The former largely included tick-vectored vertebrate viruses such as Bourbon virus, Johnston Atoll quaranjavirus, and Batken virus, among others. The latter was subdifferentiated loosely into Aedes and Culex MAV subclades, with our Palmetto orthomyxo-like virus falling into the Aedes clade. Our Palmetto orthomyxo-like virus appears to be nearly identical to the Guadeloupe mosquito quaranja-like virus 1 (GMQLV1) first identified in A. aegypti from the Caribbean by Shi et al. (2019), and as such, is probably a member of the genus Quaranjavirus. This genus was only recently described (Presti et al., 2009), and genomes of its viruses probably consist of eight negative-sense, single-stranded RNA segments (Batson et al., 2021) (Figures 7-10). Genome fragments recovered from our data matched to genes on four segments. Most known Quaranjavirus members differ from their influenza relatives via their surface glycoprotein (gp64), which has similarity to Baculoviridae members, and is hypothesized to have been the catalyst for virus entry and fusion in ticks (Allison et al., 2015). These quaranjaviruses have demonstrated horizontal transmission cycles akin to arboviruses between ticks and tropical and subtropical birds (Presti et al., 2009), which is probably why viruses from birds and ticks appear as distant relatives to the Palmetto orthomyxo-like virus in our gene trees (Figures 7-10). Interestingly, these tick-vectored quaranjaviruses showed a lack of replication in Aedes albopictus C6/36 cells (Allison et al., 2015; Presti et al., 2009), suggesting that host specificity may be more strictly defined for quaranjaviruses (i.e., less amenable to host switching). In turn, this supports the notion that MAVs vary in their efficiency of vertical transmission, and hence, their capacity to be maintained in a host population by this mechanism over time. The phylogenies based on gene segments for the orthomyxo-like viruses suggest high transmission fidelity, while the partiti-like virus tree suggests greater amenability to host switching. Such patterns may provide clues to MAV ecology, including their degree of specialization and relative ability to use environmental routes to infect mosquitoes horizontally.
4.5 Palmetto toti-like, partiti-like and orthomyxo-like insect specific viruses are vertically maintained in A. aegypti
Vertical maintenance of all three Palmetto MAVs investigated herein is probably occurring, as we were able to detect each virus across A. aegypti generations post-field collection through multiple generations (G0 and G7) via RT-PCR (Figure 11), a trend noted with numerous ISVs (Bolling et al., 2011; Contreras-Gutierrez et al., 2017; Frangeul et al., 2020; Haddow et al., 2013; Lutomiah et al., 2007). As all ovary pools were positive in both G0 and G7 generations for all three viruses (Figure 11a, c, e), transovarial transmission was probably the primary route of vertical transmission. As males are known to carry a wealth of MAVs (Frangeul et al., 2020), we also tested pools of adult males for each of our three viruses. Male pools were consistently positive for Palmetto orthomyxo-like virus, positive in the majority of pools for Palmetto partiti-like virus, and notably negative for Palmetto toti-like virus (Figure 11b, d, f). These discrepancies in positivity may suggest differences in transstadial transmission between the sexes during development, which could arise from sex-specific tissue tropisms as adult structures form in the pupal stage. Moreover, testes might display specificity for certain viruses in their small RNA machinery, leading to a more efficient antiviral response (Frangeul et al., 2020). Alternatively, since the toti-like virus was also discovered in our laboratory-adapted colony (ORL), it may have been acquired during blood feeding. Both G0 and G7 females were fed on chickens to enable egg production and oviposition prior to ovary dissection. This is the only environmental factor in our study that differs between the sexes, but because totiviruses are not common in domestic birds, we find this route unlikely. For the two viruses present in males, venereal transmission may complement vertical transmission as a secondary maintenance mechanism for MAV persistence, as has been described for bunyaviruses and alphaviruses in other mosquito species (Ovenden & Mahon, 1984; Schopen et al., 1991).
4.6 Metagenomic sequencing utility and relevance
Many of the virus assignments for our A. aegypti (Palmetto) mosquitoes matched to unclassified Riboviria lacking further taxonomic resolution, a trend noted in other publications (Öhlund et al., 2019; Shi et al., 2019; Williams et al., 2020). This is probably due to limitations imposed by incomplete virus databases and augmented by the difficulty of recovering segmented virus genomes from pooled samples with high virus similarity (Batson et al., 2021; Shi et al., 2019). As more studies examine viral communities and increase the overall database size, taxonomic classification of this metagenomic “viral dark matter” should drastically improve (Batson et al., 2021). It is also worth noting that Chuvirus Mos8Chu0, one of the low-abundance virus assignments in both our field-derived and laboratory samples, is probably an endogenous viral element (EVE), a sequence of viral origin that integrated into the Ae. aegypti genome in the past (Dezordi et al., 2020). Some EVEs are thought to play a role in defence against viruses and selfish genetic elements and cluster with PIWI-interacting RNA clusters in the genome (Whitfield et al., 2017). It is possible that, like Chuviruses, many of our unclassified Riboviria are actually EVEs and represent remnants of past viral infections now integrated into the genome.
Despite the presence of DENV-4 in the abdomens of Manatee County mosquitoes in 2016 and 2017 (Boyles et al., 2020), we did not identify any circulating arboviruses in the ovaries of our field-derived 2018 mosquitoes from Palmetto. The effects of the virome, particularly from the presence of various combinations of viruses, are largely still unknown. Studies have shown variation in vector competence with ISV infection (Baidaliuk et al., 2019; Bolling et al., 2012; Zhang et al., 2017) and a positive association between ISV infection and arbovirus infection in mosquitoes (Newman et al., 2011). However, these results do not hold true in all systems (Crockett et al., 2012; Kent et al., 2010). Furthermore, although potential ISV-arbovirus interaction modes, such as competitive inhibition and superinfection exclusion, have been proposed (Roundy et al., 2017; Vasilakis & Tesh, 2015), mechanistic data regarding these interactions, especially on a community scale (i.e., the whole microbiome within a mosquito), are severely lacking. ISV infection in mosquitoes is widely assumed to be commensal (Hall et al., 2016), although examples of ISV-insect interactions with outcomes that depend on the biological context can be found in the literature. For example, a partiti-like virus infection in the fall armyworm (Spodoptera frugiperda) causes negative fitness effects in S. frugiperda but renders the caterpillar more resistant to a pathogenic nucleopolyhedrovirus (Xu et al., 2020). Furthermore, the low abundance of some MAVs and co-occurrence with fungi may mean that these MAVs are more likely associated with infecting fungi than the mosquito itself (Shi et al., 2017). It remains unclear if similar symbioses are occurring or could occur with the three MAVs studied here.
4.7 Conclusions
Our data suggest that there is a set of vertically transmitted MAVs in Palmetto Aedes aegypti representing at least three virus families: Partitiviridae, Totiviridae and Orthomyxoviridae. The toti-like virus was also prevalent in the ovaries of A. aegypti derived from a colony long maintained in the laboratory and may represent a component of the core virome common to populations from central Florida. We were able to confirm that vertical maintenance of three of these viruses is likely occurring via transovarial transmission, as the Palmetto partiti-, toti- and orthomyxo-like viruses were present in all ovary pools spanning seven generations. Although the orthomyxo-like virus was also present in all male pools, occurrence of the partiti-like virus was less consistent among males, and the toti-like virus was absent from male pools altogether, suggesting potential variation in sex-specificity among MAVs. The striking differences in distant host taxa between the toti-like and partiti-like virus trees compared to the orthomyxo-like virus tree may suggest disparate routes of viral evolution or acquisition (e.g., plant/fungi-based versus vertebrate-based). However, because these data were obtained from pools of ovaries and males, we lack the individual-level resolution to determine MAV prevalence in the population or colony, assess the variability of MAV community composition among individuals, or assess within-mosquito MAV abundance. Furthermore, despite previous detection and full genome sequencing of DENV-4 in field-collected A. aegypti from 2016 and 2017 (Boyles et al., 2020), we did not detect arboviruses in our metagenomic screen. Nevertheless, this study provides ecological baseline data for MAV diversity and vertical transmission for a mosquito vector species in a state with rising local DENV transmission. Further research on MAV presence/persistence in mosquitoes and their impact on the suite of biological parameters that determine vectorial capacity could help contextualize the role MAVs play in arboviral transmission.
ACKNOWLEDGEMENTS
We would like to thank X. Wang and T. Stenn for assistance with mosquito feeding and colony maintenance, J. Crosby for animal husbandry, and C. Lesser, Director of Manatee County Mosquito Control District, for the District’s collaboration in mosquito sampling. A fellowship for J. Bozic and an internship for J. Carrillo were supported by funds through the CDC Southeastern Center of Excellence in Vector-Borne Diseases (U01CK000510).
AUTHOR CONTRIBUTIONS
Derrick K. Mathias and Rhoel R. Dinglasan designed the research plan and procured funding, Juliana Carillo and Eva A. Buckner collected the mosquitoes, Adam R. Rivers and Heather Coatsworth performed the bioinformatic analyses, Jovana Bozic and Derrick K. Mathias completed all molecular analyses, Heather Coatsworth, Jovana Bozic, Rhoel R. Dinglasan and Derrick K. Mathias wrote the manuscript, and all authors edited the final version.
Open Research
OPEN RESEARCH BADGES
This article has earned an Open Data, for making publicly available the digitally-shareable data necessary to reproduce the reported results. The data is available under BioProject PRJNA765718 [https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA765718].
DATA AVAILABILITY STATEMENT
Metagenomic raw sequence reads have been deposited in the Sequence Read Archive at NCBI, while genomic RNA and protein sequences for all assembled contigs have been uploaded to Genbank. All data, including metadata for field-derived samples, is publicly available under BioProject PRJNA765718 [https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA765718].




