The endangered northern bettong, Bettongia tropica, performs a unique and potentially irreplaceable dispersal function for ectomycorrhizal truffle fungi
Abstract
Organisms that are highly connected in food webs often perform unique and vital functions within ecosystems. Understanding the unique ecological roles played by highly connected organisms and the consequences of their loss requires a comprehensive understanding of the functional redundancy amongst organisms. One important, yet poorly understood, food web is that between truffle-forming ectomycorrhizal fungi and their mammalian consumers and dispersers. Mammalian fungal specialists rely on fungi as a food source, and they consume and disperse a higher diversity and abundance of fungi than do mycophagous mammals with generalist diets. Therefore, we hypothesize that mammalian fungal specialists are functionally distinct because they disperse a set of fungal taxa not fully nested within the set consumed by the combined generalist mammalian community (i.e., functional redundancy of fungal dispersal is limited). Using high-throughput sequencing, we compared the fungal composition of 93 scats from the endangered fungal specialist northern bettong (Bettongia tropica) and 120 scats from nine co-occurring generalist mammal species across three sites and three seasons. Compared with other generalist mammals, B. tropica consumed a more diverse fungal diet with more unique taxa. This aligns with our hypothesis that B. tropica performs a unique dispersal function for ectomycorrhizal truffle fungi. Additionally, modelling of mammalian extinctions predicted rapid loss of food web connections which could result in loss of gene flow for truffle taxa. Our results suggest that this system is sensitive to the extinction of highly connected specialist species like B. tropica and their loss could have consequences for ectomycorrhizal truffle fungal diversity. This suggests that the conservation of fungal specialists is imperative to maintaining ectomycorrhizal fungal diversity and healthy plant–mycorrhizal relationships.
1 INTRODUCTION
Ecological networks can be particularly sensitive to the extinction of highly connected species (Dunne, Williams, & Martinez, 2002; Memmott, Waser, & Price, 2004). As highly connected organisms can be central within networks and often have disproportionally large number of interactions relative to their abundance, they can provide irreplaceable ecosystem services (Mello et al., 2015). Where functional redundancy is low (i.e., few taxa occupy any given niche and food web connectance is low) the loss of highly connected species and their ecological roles can induce co-extinction of interconnecting species, because their function is not replaced by other species (Dallas & Cornelius, 2015). Alternatively, such central functions may in part be substituted by other less-central organisms (Vernes & McGrath, 2009; Wood, Wilmshurst, Worthy, Holzapfel, & Cooper, 2012). Understanding when functions are maintained despite diversity loss requires a comprehensive knowledge of functional redundancy and network structure (Dunne et al., 2002; Memmott et al., 2004). Such knowledge is becoming increasingly relevant as many systems continue to lose biodiversity (Sax & Gaines, 2008; Thomas et al., 2004; Urban, 2015).
One important, yet often overlooked, interaction involves three different groups of organisms; mammals, fungi and plants. Some mammals rely on hypogeous sequestrate fungi (i.e., fungi that form fruit-bodies below ground; truffle fungi) as a major component of their diet (Claridge, 2002), while these same fungi rely on mammals for dispersal (Claridge & May, 1994; Johnson, 1996; Malajczuk, Trappe, & Molina, 1987). Mycophagy is common in many invertebrates (Anslan, Bahram, & Tedersoo, 2018; Fogel & Peck, 1975; Houston & Bougher, 2010; Nakamori & Suzuki, 2005, 2010; Reddell & Spain, 1991) but, evidence for high spore survival and the long gut-retention times necessary for long-distance dispersal is found mostly in mammalian–truffle interactions (Nuske, 2017). In addition, almost all truffle fungi are ectomycorrhizal, making them critical for ecosystem nutrient cycling (Hawkins, Jones, & Kranabetter, 2015; van der Heijden, Martin, Selosse, & Sanders, 2015), plant health (Scott, Shearer, Barber, & Hardy, 2012), and an important component of many forest systems globally (Tedersoo, May, & Smith, 2010; Tedersoo et al., 2014).
Not all mammals consume and disperse fungi equally (e.g., Meyer, North, & Kelt, 2005; Schickmann, Urban, Kräutler, Nopp-Mayr, & Hackländer, 2012; Vernes & Dunn, 2009). For example, a recent meta-analysis found that most members of the small marsupial family, Potoroidae, consume higher abundance and diversity of fungi than do other Australian mammals (Nuske et al., 2017). This suggests that Potoroid mammals perform an ecosystem function that no other mammal species do (i.e., Potoridae are not functionally redundant in terms of their fungal dispersal role compared to other mammals with generalist diets). However, the literature upon which this conclusion is based, also contains a significant taxonomic bias, with Potorous spp. and Bettongia spp. (Potoroidae) having a higher number of studies and samples than other mammal species (Nuske et al., 2017). Additionally, some generalist mammals consume a fungal diet as diverse as that of fungal specialists, indicating that, at least in some systems, functional redundancy may be higher. In this study, we aimed to remove the influence of this bias and re-examine functional redundancy in the dispersal of fungi by mammals. To do this, we compared the fungal diet of an endangered fungal specialist, Bettongia tropica (northern bettong; Potoroidae), to that of other co-occurring generalist mammal species within the same community.
Bettongia tropica is endemic to the Wet Tropics of North Queensland, Australia. The species is endangered and in decline, having already disappeared from a number of localities at the edge of its distributional range (Bateman, Abell-Davis, & Johnson, 2011). Bettongia tropica is described as an ecotonal specialist. Its distribution is restricted to wet open sclerophyll forest where the canopy is dominated by host plants that associate with ectomycorrhizal-truffle-bearing fungi that border rainforest (Abell, Gadek, Pearce, & Congdon, 2006; Vernes, Castellano, & Johnson, 2001; Vernes & Pope, 2001).
The main diet of B. tropica consists of a diversity of truffle fungi (McIlwee & Johnson, 1998; Vernes et al., 2001) and therefore is considered a fungal specialist (Nuske et al., 2017). Fungal generalists, on the other hand, are mammals that only consume fungi seasonally, or as a supplementary food source (Nuske et al., 2017). We hypothesize that the dispersal role of a fungal specialist, B. tropica, is unique and not functionally redundant within the combined dispersal roles of fungal generalists. Specifically, that fungal specialists consume (and potentially disperse) a set of fungal taxa not fully nested within the set consumed by the combined generalist mammalian community. We also examine the robustness of this system to mammalian extinction by modelling the loss of connections to truffle taxa (potentially representing loss of dispersal events) after selectively removing mammals from the data set.
2 METHODS
2.1 Field sampling
Mammal diets were examined by collecting scat samples from trapped individuals. Trapping and scat collection were carried out at three locations on the Lamb Range, North Queensland, Australia: Emu Creek (17°6′18.10″S, 145°31′47.46″E), Danbulla State Forest near Tinaroo Dam (17°9′50.30″S, 145°32′11.56″E; now part of Danbulla National Park) and Davies Creek National Park (17°1′23.28″S, 145°34′55.71″E). These sites are roughly the same locations as used by previous studies on populations of B. tropica (Pope, Estoup, & Moritz, 2000; Vernes et al., 2001). Elevation ranged from 600 to 900 m above sea level. The dominant ectomycorrhizal tree species are Eucalyptus crebra, E. tindaliae, E. mediocris, Corymbia intermedia, Allocasuarina littoralis, A. torulosa and Acacia flavescens.
Either seven or eight cage trap transects were set up in open forest at each location. Cage traps, (30 × 30 × 60 cm treadle traps) designed to capture medium-sized mammals, were spaced 100 m apart, 7–8 cages per transect totalling 53 cage trap locations per site. Three to four Elliot trap transects (10 × 10 × 30 cm aluminium box traps, designed to catch small mammals) were also set with traps 50 m apart in each transect; totalling 50 Elliot trap locations per site. All traps were set at dusk and baited with a mixture of peanut butter, rolled oats, vanilla, honey and canned sardines. Cage traps were checked from midnight until dawn, whereas Elliot traps were checked over a two-hour span at dawn. Trapping was carried out across four consecutive nights at each site. Elliot trapping occurred within three weeks of cage trapping. Traps were set in three seasons; November-December 2014 (late dry), February–March 2015 (early wet) and May–June 2015 (late wet). Due to logistical constraints of limited time and personnel, Elliot traps were only set at Tinaroo Dam and Davies Creek, in the late dry and early wet seasons. The total number of trap placements was 1,908 cage traps (53 traps × 4 nights × 3 sites × 3 seasons) and 800 Elliot traps (50 traps × 4 nights × 2 sites × 2 seasons).
Each animal was handled according to the James Cook University animal ethical guidelines (Approved ethics application A2044). Mammals were identified according to Van Dyck, Gynther, and Baker (2013) and marked by either removing a small patch of hair with scissors at the base of the tail or microchipping (B. tropica only; Minichips, Micro Products Australia, Canning Vale, WA or ISO FDX-B Microchips, OzMicrochips, Peakhurst, NSW). Scats were collected from the bottom of each Elliot trap or from plastic placed under each cage trap. All traps and plastic were initially cleaned with 70% ethanol and then re-cleaned subsequent to each animal being caught. Scats were stored on ice, or in a portable fridge (4°C) in the field and transferred to −20°C as soon as possible (≤4 days).
2.2 Laboratory
Scats were only used from the first capture of an individual per trapping session (i.e., only one sample was taken per individual per sampling season to maintain independent samples and avoid possible influence of bait). Obvious soil contamination was removed with forceps from each scat before processing. Each boluse of scat was broken in half and a small sample of faecal material removed from the inside with sterile forceps. This material was homogenized manually with a sterilized blunt probe in a weight boat and 0.25 g of homogenate taken for DNA extraction.
DNA was extracted by PowerLyser PowerSoil DNA Isolation kit following the manufacturer's instructions (Mo Bio, Carlsbad, CA USA), except that the samples were lysed using a Qiagen Tissue Lyser for 2 × 30 s at 30 Hz, swapping the position of the samples between runs. DNA was amplified with ITS3-Mix1-5 (5′CTAGACTCGTCANCGATGAAGAACGYRG-3′) and ITS4ngs (5′-TCCTSCGCTTATTGATATGC-3′) primers (Tedersoo et al., 2014). The latter primers were tagged with 10–11 base unique molecular identifiers (MIDs) to later distinguish samples with sequencing runs (Supporting Information Table S1). We included negative controls for all DNA extractions and negative and positive controls for all PCR reactions. We used FavorPrep™ GEL/PCR Purification Kit (Favorgen Biotech Corp., Taiwan, China) to purify the amplicons, following manufactures instructions, except two FAfDF Columns were used per plate, doubling the elution with milliQ water to 80 μl which we let stand for 5 mins. Normalized amplicons were subjected to ligation of Illumina adaptors using the TruSeq DNA PCR-free HT Sample Prep kit (Illumina Inc., San Diego, CA, USA) to allow DNA strands to be read by the sequencing machine. All 242 samples were sequenced in Illumina MiSeq 2 × 300 paired-end runs.
Because Australian truffle sequences are underrepresented in existing large-scale public databases, we generated a reference sequence data set by sequencing representative specimens of multiple fungal species obtained from an extensive truffle survey at Davies Creek (Abell-Davis, 2008). Two to three specimens were selected per truffle morpho-group for DNA extraction. A small section from the gleba of dried sporocarps was taken and DNA was extracted using DNeasy Plant Mini Kit (Qiagen) following the manufacturer's instructions. As some specimens were harder to lyse because of their tough texture, we performed additional lysis steps where samples were frozen in liquid N in between bead-beating. DNA was amplified using ITS1 or ITS5 forward primers and ITS4 reverse primer (White, Bruns, Lee, & Taylor, 1990). The PCR cocktail consisted of 1.0 μl DNA, 0.4 μl each of the primers (0.2 μmol each), 2.5 μl (1.25X) Kappa BufferB (Kapa Biosystems, Massachusetts, USA), 1.25 mM MgCl2, 0.2 mM dNTPs, 2% BSA, 0.12 μg/μl DMSO, 1.25 U Taq (Kapa Biosystems) and made up to 20 μl with MilliQ water. PCR was carried out using the following thermocycling conditions: an initial 1 min at 95°C, followed by 36 cycles at 94°C for 1 min, 54°C for 1 min, 72°C for 1 min, and a final cycle of 8 min at 72°C. The relative quantity of PCR products was estimated by 1% agarose gel electrophoresis. The amplicons were cleaned with Exo-AP Mix (Enzyonmics, Daejeon, Korea). Amplicons were Sanger sequenced by AGRF or Macrogen.
The truffle sequences were processed using Geneious (v8-9.2), and the quality of the ITS sequences was checked using the guidelines outlined in Nilsson et al. (2012). ITS2 sequences were extracted to construct the local database and compared against Illumina sequences generated (see below). Raw Illumina data are deposited in Sequence Read Archive (SRA; bioproject SRP150847) and Sanger data are available in GenBank (Accessions KY686200–KY686202, KY697566–KY697576, KY697578–KY697619; Supporting Information Table S2).
2.3 Bioinformatics
Bioinformatics analysis for the paired-end Illumina data was performed using pipecraft (v1.0; Anslan, Bahram, Hiiesalu, & Tedersoo, 2017) as follows. Paired-end reads were merged and quality filtered using vsearch (v1.11.1; https://github.com/torognes/vsearch) with minimum overlap = 10, allowing no ambiguous base pairs, and expected errors = 1 (allowmergestagger = T, minlen = 50). These high-quality sequences were allocated to samples (demultiplexed) based on MIDs using mothur (v1.36.1; Schloss et al., 2009), no primer or MID differences were allowed. Putative chimeric reads were detected and removed using de novo and based on reference (unite uchime reference data set v7.0; Abarenkov et al., 2010) as implemented in vsearch (v1.9.10). Fungal ITS2 sequences were verified using its extractor (v1.0.11; Bengtsson-Palme et al., 2013). Full ITS2 reads without flanking gene fragments were then clustered to Operational Taxonomic Units (OTUs) with cd-hit (v4.6; Li & Godzik, 2006) at 97% similarity threshold. Global singletons were removed from further analyses. Representative sequences were chosen using the “abundance” method of mother and compared against unite database (v7.0), GenBank ITS database and our local truffle database to obtain taxonomic affiliation using blastn (Camacho et al., 2009).
We considered OTUs accurate at kingdom level if blastn matched to known species at ˂e-50, identity >75% and coverage >70% (Tedersoo et al., 2014). Other putatively non-fungal OTUs were removed from analyses. We removed OTUs from samples if they were present in negative and positive controls. OTUs were further filtered manually based on blastn values. Taxonomic groups were assigned to functional categories using funguild (v1.0; Nguyen et al., 2015). All Glomeromycota taxa were assigned as arbuscular mycorrhizal.
2.4 Statistics
Statistics were conducted with the phyloseq package (McMurdie & Holmes, 2013) in r (R Core Team 2012). We did not rarefy our sequencing data as this can lead to a high rate of false positives for species abundances across samples (McMurdie & Holmes, 2014). Altogether 29 samples (12%) were removed from further analyses, because these comprised <500 filtered sequences (six B. tropica samples and 18 samples from 6 mammal species with fungal generalist diets). The fungal data were examined at three broad levels; at the whole OTU community level, only examining the ectomycorrhizal (ECM) fungal OTUs (4.1% of all taxa) and only examining truffle taxa (2.0% of all taxa). The ECM subset of the data included only taxa that were assigned as “highly probable” and “probable” based on FUNGuild output. Truffle taxa are listed in Supporting Information Table S4. We also defined an “ambiguous fruiting” category for secotioid taxa (fungi with enclosed fruiting bodies, which may have active spore dispersal and therefore are not solely mammal dispersed; Bougher & Lebel, 2001) and taxa listed with ambiguous fruiting habit because of uncertain taxonomic assignment (e.g., Russulaceae which includes both truffle and wind-dispersed mushroom taxa). Only three taxa were matched to OTUs at species level and had secotioid fruiting habit (Cortinarius globuliformis, C. porphyroideus and Scleroderma spB/spC; Supporting Information Table S4). All three of these taxa are “semi-hypogeous” and may also deposit spores on the surface of the soil or into the air (after erosion of the peridium for Scleroderma spB/spC). All other taxa we lumped together with secotioid species matched at genus or family level and we could not conclusively define fruiting habit (although these genera or families contain truffle or secotioid species). We excluded these taxa from the truffle analysis because mammal consumption is not the sole form of dispersal. Sporocarpic arbuscular mycorrhizal fungi were not included as truffle taxa. Only four OTUs of arbuscular mycorrhizal fungi could be distinguished to a genus or species (three matching sporocarpic Glomus macrocarpus and one matching Scutellospora sp.).
We compared B. tropica (fungal specialist) samples with other mammal species sampled (listed in Supporting Information Table S4 and Figure 1, thereafter referred to as fungal generalist mammals) within the same site and season. We also compared all B. tropica samples (n = 93) to all fungal generalist mammal species combined (nine species, n = 120) by merging OTU matrices for either fungal specialist or generalist samples by average read count per OTU. Not all mammal species were captured in all sites and seasons.
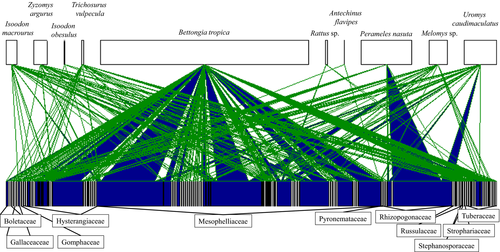
To assess the potential role of mammal species and specialization groups (fungal specialist vs. generalists) as dispersers of truffle ECM species, the fungal community data from their scats were assessed in the following ways: (a) community composition of all fungal taxa via multi-dimensional statistics, (b) raw OTU richness of all taxa, ECM taxa and truffle taxa per sample, (c) rarefaction curves of truffle OTU richness across read count (depth) per sample or specialization group and (d) unique and shared OTUs.
Distance-based Redundancy Analysis (db-RDA) with Hellinger distance was used to examine the relationships between site, season and mammal species or specialization groups on the fungal community sequenced from the scats. Constrained ordination was preferred in our application to unconstrained (such as NMDS) as it provides a good compromise between power limitations of unconstrained ordination and computational limitations of multivariate GLM extensions (ter Braak & Šmilauer, 2015). Of the four distance metrics examined—Bray–Curtis, chi-square (Canonical Correspondence Analysis), Chord and Hellinger—Hellinger distance yielded the highest fraction of explained variation by constraints (Legendre & Gallagher, 2001). Partial db-RDA was used on the community data to partition out the variation within site and season and to only examine the relationship between mammal species or specialization groups. Permutation tests were used to establish the significance of the terms in constrained ordinations using 500 permutations, equivalent in its implementation by our software to a PERMANOVA using Hellinger distance.
Fungal OTU richness per sample between B. tropica (fungal specialist) and fungal generalist mammal species were compared using Tukey HSD tests at all levels of the data (all taxa, ECM only and truffle only OTUs). To estimate the accumulation of OTUs across samples, we created rarefaction OTU accumulation curves and their 95% confidence intervals for each mammal species and all samples using estimates 9.1.0 (Colwell, 2013). We also created rarefaction curves to estimate the accumulation of OTUs across sequence read counts (depth) for each sample within each site and season using the “ggrare” function (richness.R, phyloseq extensions; https://github.com/mahendra-mariadassou/phyloseq-extended with added ggplot2 graphics). Unique fungal OTUs within B. tropica and fungal generalist mammal scats were examined by creating Venn diagrams using limma and venndiagram packages in r.
To examine the functional redundancy in this truffle-mammal dispersal network, we calculated the “secondary extinction slope” (Memmott et al., 2004) and robustness index (r; Burgos et al., 2007). The “secondary extinction slope,” which could represent loss of dispersal events for truffle taxa, was calculated using the “second.extinct” function in the bipartite r package (Dormann, Gruber, & Fründ, 2008) by deleting the most-to-least connected mammal species in the network and plotting the corresponding proportional decrease in truffle connections. Connectedness is defined as the sum of interactions divided by the number of possible interactions. The robustness index is the area under the extinction slope; values close to 0 correspond to fragile networks where small decreases in diversity have large consequences for secondary loss of network connections, whereas values close to 1 indicate more robust systems (Burgos et al., 2007; Dunne et al., 2002; Jonsson, Berg, Pimenov, Palmer, & Emmerson, 2015). Since species connectance and network structure is highly related to species abundance (Dormann, J., & Schaefer, 2017), we compared results with those found after randomly subsampling for an equal number of samples for each mammal species. We repeated this 60 times (only for mammal species with n ≥ 12 total samples) so that within each iteration the truffle diets of each mammal species were represented from 10 samples.
3 RESULTS
Fungal OTUs across all 213 scat samples totalled 7,505. Twenty-one per cent of OTUs could be assigned to functional guilds by FUNGuild; of these, the most diverse were undefined saprotrophs (728 OTUs, 9.7%; Supporting Information Table S3) followed by ectomycorrhizal taxa (323 OTUs, 4.3%). Even though just under 50% of the ECM fungal OTUs could be assigned as truffle fungi (150/323 OTUs), truffle ECM OTUs had a higher relative abundance (>50% of sequence reads) compared to other ECM OTUs for most mammal species (Supporting Information Figure S1; Table S4), except Antechinus flavipes and Isoodon obesulus. These OTUs with high relative abundance matched truffle taxa Mesophellia and Malajcukia in B. tropica, Isoodon macrourus, Uromys caudimaculatus, Trichosurus vulpecula and Zyzomys argurus scats; Chondrogaster sp. in U. caudimaculatus and Rattus sp. scats and Rhizopogon pseudoroseolus in Perameles nasuta scats (Figure 1; Supporting Information Table S4). The most OTU-rich truffle genera were Mesophellia and Malajcukia (35% of all truffle OTUs).
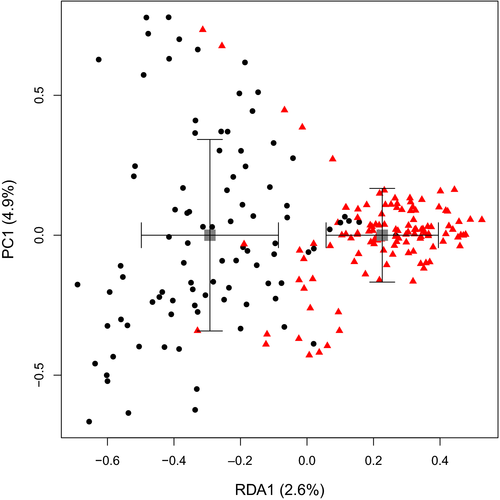
Scats of B. tropica and fungal generalist mammal species displayed distinct fungal communities (Figure 2). With site and season conditioned out using partial-RDA, specialist B. tropica fungal diets were significantly different from all generalist mammal species combined (pseudo-F1, 209 = 5.6108, p = 0.001; Figure 2). A permutation significance test on db-RDA (PERMANOVA) revealed significant interactions between site and season (Supporting Information Table S5). Variation tended to be greater in fungal communities from the specialist than generalists (Figure 2). The fungal diets of mammals grouped within families in partial RDA constraining for mammal species and conditioning out site and season (Supporting Information Figure S2).
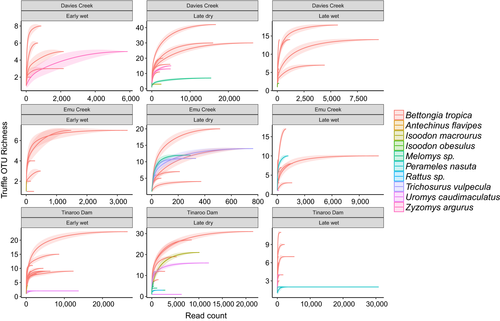
The number of OTUs obtained from the total number of sequenced samples did not reach an asymptote (Supporting Information Figure S3), suggesting that more samples would be needed to fully characterize the fungal community within the scats. This is common for high-throughput sequencing studies for fungi (e.g., Anslan, Bahram, & Tedersoo, 2016; Taylor et al., 2010; Unterseher et al., 2011). Nevertheless, for a given number of samples (e.g., 50), samples of fungal specialist mammal species contained more ECM OTUs (mean ± 95% lower and upper confidence interval: 183 ± 167, 199) than all scat samples from the fungal generalist species combined (101 ± 90, 113; Supporting Information Figure S3). This is also reflected in truffle data; B. tropica scats contained more truffle OTUs per read count than fungal generalist mammals consistently across sites and seasons (Figure 3). When OTU matrices were merged (for average read count) for fungal specialist and generalist samples, specialist samples contained a higher species richness and read count than generalists in all sites and seasons except for one (Tinaroo Dam, late dry). This latter exception was the only example where we had a much greater number of fungal generalist samples (n = 22 for six mammal species) than specialist samples (n = 7; Supporting Information Figure S4).
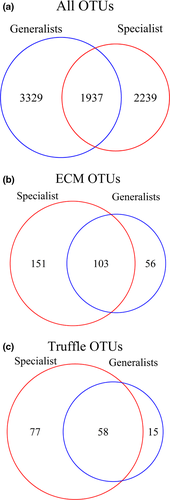
Compared to the fungal generalist mammals, specialists (B. tropica) consumed truffles of relatively greater diversity (Figure 1). The average fungal OTU richness per sample from B. tropica scats was significantly greater than in all generalist mammals combined (Table 1). This trend was most notable when only examining ECM OTUs or OTUs from truffle taxa (Table 1; Supporting Information Figure S5). The percentage of samples that contained ECM and truffle OTUs was higher in B. tropica scats (99% and 96%, respectively) compared to fungal generalist mammal species (90% and 71%, respectively). Further, B. tropica samples contained more unique ECM and truffle OTUs compared with samples from the fungal generalist mammal species (Figure 4).
| Mammal species | All OTUs | ECM OTUs | Truffle OTUs | |||
|---|---|---|---|---|---|---|
| N | Mean ± SE (total) | N | Mean ± SE (total) | N | Mean ± SE (total) | |
| Bettongia tropica (Fungal specialist) | 93 | 188.1 ± 9.34 b (4,176) | 92 | 10.0 ± 0.75 b (254) | 89 | 8.6 ± 0.80 b (135) |
| Mammals with generalist diets | 120 | 101.2 ± 8.25a (5,266) | 108 | 4.1 ± 0.32a (159) | 85 | 3.8 ± 0.44a (73) |
- a,b Different superscript letters represent significant differences in Tukey HSD comparisons between B. tropica and generalist mammal species (p < 0.05).
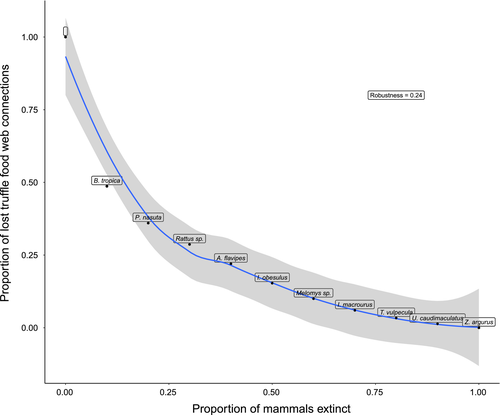
The “secondary extinction slope” showed a sharp decline in connections to unique truffle taxa for the progressive “extinction” of mammal species in the data set (Figure 5). This resulted in a robustness index value below 0.5 (0.24) indicating that this network is sensitive to mammalian extinctions. Bettongia tropica was the most connected within the food web; indeed over 50% of truffle connections were lost when B. tropica was removed from the data (Figure 5). The robustness index was similar when all data with uneven sampling was used compared to repeated subsampling for the same number of samples per mammal species (0.24 and 0.253 ± 0.003, respectively; Supporting Information Figure S6), indicating that network structure was not overly sensitive to sampling bias.
4 DISCUSSION
Our data suggest that the fungal specialist, B. tropica, potentially performs a unique ecosystem function through dispersing truffle fungi. We found that the diet of B. tropica contained a significantly different fungal community structure, higher diversity per sample and more unique ECM and truffle fungal taxa than the combined diets of nine generalist mycophagous mammal species. B. tropica diets also contained truffle fungal taxa more often and at higher relative abundances than fungal generalist mammals. The truffle diversity in fungal specialist diets exceeded the cumulative diversity consumed by generalists at all sites and seasons but one (where threefold more generalist individuals were sampled than B. tropica individuals), indicating that the dispersal role of specialists can only be matched by a greater abundance of generalists. These results are consistent with our hypothesis and previous findings that potoroid mammals (fungal specialists) consume a higher diversity of fungi than generalist mycophagists across Australia (Nuske et al., 2017).
Modelling the extinction of mammal species from this food web resulted in a rapid loss of connections for truffle fungi, representing a loss of potential dispersal events. A low (below 0.5) ecosystem robustness index indicates that networks are sensitive to loss of diversity (Burgos et al., 2007; Dunne et al., 2002; Jonsson et al., 2015). More than 50% of truffle connections were lost when only B. tropica was removed from the data set, which resulted in a robustness index of 0.24. Taken together, these results suggest that this food web is sensitive to the extinction of mammals and the fungal dispersal role of specialists is not functionally redundant within generalists. Whether the extinction of mammalian dispersers (particularly highly connected fungal specialists) results in the co-extinction of truffle taxa in the long term requires further verification.
As truffle fungi rely on zoochory, it is likely that B. tropica and other mycophagous mammals heavily influence the gene flow and possibly the population structure of these fungal taxa (e.g., Mesophellia/Malajczukia, Chondrogaster, Hysterangium, Soliocaccasus polychromus, truffle taxa of Russula and Cortinarius). It is not yet known how important the frequency of mammalian spore dispersal is for genetic structuring of truffle species amongst other local factors like compatibility and distribution mating types and mycorrhizal hosts, propensity to spread and inoculate new plant roots via extra-radial hyphae or spores and variation in abiotic soil characteristics (Douhan, Vincenot, Gryta, & Selosse, 2011; Grubisha, Bergemann, & Bruns, 2007; Molinier et al., 2016; Taschen et al., 2016). On larger scales, physical barriers like large water bodies, mountain ranges or habitat fragmentation influence mammal movement (Dietz, Büchner, Hillen, & Schulz, 2018; Pope et al., 2000) and hence are likely to also influence truffle dispersal and genetic structure. Future studies on population genetics of truffle fungi need to consider these factors as well as differing mammal communities’ to verify the strength of dispersal processes by mammals on the genetic population structure of truffle taxa.
An assumption underpinning our conclusions is fungal generalist mammals will not or cannot change their truffle diets in the absence of a fungal specialist. The extent to which generalists can increase their intake of fungi is unknown and is liable to vary between mammal groups (Claridge, Trappe, Cork, & Claridge, 1999). However, mammal species tend to consume fungi in proportion to their abundance (Johnson, 1994; North, Trappe, & Franklin, 1997), suggesting that if a fungal specialist becomes locally extinct, generalist mammal species may increase the intake of truffle fungi as they become more available. This hypothesis is yet to be tested.
If our results are representative of the situation in other truffle-mammal networks, reduced spore dispersal from mycophagous mammals may, over time, reduce the relative abundance and species richness of truffle fungi, making Australia of particular concern for truffle fungal conservation. Other members of the Potoroidae family in Australia (Bettongia spp. and Potorous spp.) and mammal species worldwide (e.g., flying squirrels, Glaucomys sabrinus in North America and bank voles, Myodes glareolus in Europe) are also considered fungal specialists (Nuske et al., 2017; Pyare & Longland, 2001; Schickmann et al., 2012). Australia's history of mammal extinction and decline, including members of Potoroidae (Claridge, Seebeck, & Rose, 2007), far exceeds other continents (Woinarski, Burbidge, & Harrison, 2015). Australia also has a high diversity of truffle species, most of which are endemic (Bougher & Lebel, 2001). In terms of biomass and carbon stocks, ECM trees (especially Eucalyptus spp.) are dominant in much of Australia's forests (Reddell, Gordon, & Hopkins, 1999; Wood, Prior, Stephens, & Bowman, 2015) and reduced ECM abundance and diversity has been linked to declines in Eucalyptus tree health (Horton et al., 2013; Ishaq, Barber, Hardy, Calver, & Dell, 2013; Scott et al., 2012). The already substantial loss of Bettongia spp. and Potorous spp. throughout much of the Australian continent (Claridge et al., 2007) could have long-lasting detrimental, yet undocumented, consequences for the diversity of truffle fungi. This raises concern about the vulnerability of these ecosystems, because consumer networks are most disrupted by the extinction of highly connected species (Bellingeri, Cassi, & Vincenzi, 2013; Burgos et al., 2007; Dunne et al., 2002).
Whether reduction of mammal diversity results in compromised plant productivity via loss of truffle diversity has not yet been empirically shown, but it has been suggested by many authors (Johnson, 1996; Malajczuk et al., 1987; Maser, Nussbaum, & Trappe, 1978; Vernes, 2007). This link between mycophagous mammals and plant productivity assumes that 1) lowered mammal diversity inhibits gene flow for truffle species, resulting in decline of truffle diversity and that 2) truffles form important and irreplaceable components of functioning ectomycorrhizal fungal communities. Neither of these assumptions have been tested; further studies are needed.
Studying how ecosystem functioning responds to diversity loss is becoming increasingly relevant in light of ever-increasing extinction rates (Morris, 2010; Thomas et al., 2004; Urban, 2015). Here, we show that an endangered mammalian fungal specialist performs a unique and potentially irreplaceable function for dispersing truffle ECM fungi. We propose that this truffle dispersal system is fragile to the loss of mammalian dispersers, and conservation of fungal specialists may be necessary for adequate gene flow of many truffle species. Further research is urgently needed to verify links between mammalian truffle dispersers and truffle ECM diversity, ECM-plant dynamics and healthy ecosystem functioning.
ACKNOWLEDGEMENTS
This research was part of a larger project, working on the population status, viability and impact of fire on Bettongia tropica in collaboration with WWF-Australia, Queensland Parks and Wildlife Service (Department of National Parks, Sport and Racing) (QPWS), Department of Environment and Heritage Protection (EHP), and James Cook University (JCU) PI Sandra Abell (Project ID: TAG14-00542). The project was funded through the Australian Government's Caring for Our Country grants program which was administered by WWF. This research was also supported by an Australian Postgraduate Award, Wet Tropics Management Authority (Student Grant 916, North Queensland Wildlife Trust [2014]) and Australasian Mycological Society Research Award (2014). This research was performed under permits granted by the Queensland Government, EHP (WISP15227914, WITK14639614 and WITK11258712). All wildlife was handled according to JCU Animal Ethics Guidelines (approval number: A2044). Sincere thanks to QPWS staff Rob Miller, Lana Little, Jack Cosgrove, Karl Goetze, Chris White, Julie Bunny and Miki Bradley, WWF staff Jessica Koleck, JCU students and staff Tegan Whitehead, Stephanie Todd, Naomi Bowie, Elise Chatterton and many field volunteers for help with field work and collection of samples.
AUTHOR CONTRIBUTION
S.J.N. designed the study, collected the data, sequenced DNA, analysed the data and wrote the manuscript. S.A. performed bioinformatics and edited the manuscript. L.T. contributed to the laboratory space, primers and reagents, provided advice on high-throughput sequencing and edited the manuscript. M.T.L.B. provided advice on the data analysis, wrote some of the R scripts and edited the manuscript. B.C.C. provided advice on the design of study and data analysis and edited the manuscript. S.E.A. provided advice on the design of study and data analysis and edited the manuscript.
DATA ACCESSIBILITY
Raw Illumina data are deposited in Sequence Read Archive (SRA; bioproject SRP150847). Sanger data are available in GenBank (Accessions KY686200–KY686202, KY697566–KY697576, KY697578–KY697619; Supporting Information Table S2).




