How bacterioplankton community can go with cascade damming in the highly regulated Lancang–Mekong River Basin
Abstract
Rivers make vital contributions to the transport of water, sediment and nutrients from terrestrial to marine ecosystems. However, many large rivers worldwide are suffering from dam regulation. Increasing attention has been paid to bacterioplankton communities since they are highly responsive to river alterations and may influence biogeochemical processes. Here, a comprehensive study was conducted in the highly regulated Lancang–Mekong River Basin to address the question of how bacterioplankton communities respond to cascade damming. The results showed that dam constructions increased nutrient concentrations and threatened water quality in cascade reservoirs. Bacterioplankton cell abundance was reduced by damming, and α-diversity was inhibited in cascade reservoirs. Fortunately, however, river ecosystems were resilient after the remarkable disturbance caused by damming. Moreover, bacterioplankton community composition was significantly altered by cascade dams, including a shift in the dominant phylum from r-strategists to k-strategists. Meanwhile, according to GeoChip analysis, the functional composition of bacterioplankton was less affected than taxonomic composition. In addition, geographic and environmental features both followed a distance–decay relationship with community and functional composition, but the local environment condition was the dominant driver in the Lancang River. Therefore, the impoundments of cascade dams had significant impacts on bacterioplankton communities and more attention should be paid to the potential ecological consequences of river regulation.
1 INTRODUCTION
Rivers play an important role in the transport of water, sediments and nutrients from terrestrial to marine ecosystems (Fan, He, & Wang, 2015; Savio et al., 2015). Compared with oceans, rivers are thought to process and store significantly large amounts of terrestrial organic and inorganic compounds and thereby be essential participants in global biogeochemical cycles (Jackson, Millar, Payne, & Ochs, 2014; Read et al., 2015). In addition, bacterioplankton are often considered vital players in aquatic ecosystems due to their important functions in biogeochemical nutrient cycling (Meyer, 1994) and food chains (Rubin & Leff, 2007). Given that different bacterioplankton groups exhibit various functional roles, a shift in the bacterioplankton community may have implications for biogeochemical processes (Jackson et al., 2014). According to previous studies, the detection of diverse bacterial communities within watersheds revealed that bacterioplankton communities are highly responsive to environmental disturbances and stresses as a result of their small size and rapid growth rates (Ibekwe, Ma, & Murinda, 2016; Zeglin, 2015). Moreover, as indicated by the classic dictum “everything is everywhere, but the environment selects,” environmental and geographic factors have both been identified as influencers of bacterioplankton communities (Eid, Arab, & Greenwood, 2017; Staley et al., 2015). Since assembly of microbial communities in natural systems is seldom exposed to static environments, examining their responses to physicochemical environmental and geographic variations can help to understand the processes driving biogeochemical cycles (Fan et al., 2015; Zwart, Crump, Agterveld, Hagen, & Han, 2002) and to integrate assembly of microbial communities into existing biogeochemical predictive models. However, while the significance of the bacterioplankton community to ecosystems and the potential influence of environmental disturbances on this community are major focuses in various aquatic ecosystems, river bacterioplankton have received less attention than those in oceans and lakes (Baxter, Johnson, Edgerton, Royer, & Leff, 2012; Berdjeb, Ghiglione, Domaizon, & Jacquet, 2011; Eiler, Heinrich, & Bertilsson, 2012).
In recent decades, many large rivers worldwide have suffered from increasing anthropogenic disturbances as a result of social and economic development (Grumbine & Xu, 2011); and river damming is one of the largest anthropogenic disturbances (Winemiller et al., 2016). Dam constructions, especially integrated cascade development along large rivers, coordinate hydropower generation, water supply, flood control, shipping and irrigation within the catchment (Zhang, 2017). However, highly regulated rivers have generated inevitable hydrological alterations through space (Kummu & Varis, 2007). The presence of reservoirs leads to a discontinuity in a river system as it modulates water circulation and affects nutrient loadings through the retention of large fractions of suspended particles and sediments transported by the river (Baxter et al., 2012). Consequently, the sites upstream and downstream of the dams differ remarkably in their physical and chemical properties, which results in variation in bacterioplankton communities in rivers (Ruiz-González, Proia, Ferrera, Gasol, & Sabater, 2013) and further influences biogeochemical cycles (Zeglin, 2015). Nevertheless, different results were reported by the limited available studies comparing bacterioplankton communities upstream and downstream of reservoirs. The impoundment in the Yarlung Tsangpo River had undiscernible influences on bacterioplankton communities (Wang, Wang, et al., 2017), while a significant alteration of the bacterial community was reported to have resulted from the construction of a dam at the small Sinnamary River (Dumestre, Casamayor, Massana, & Pedrós-Alió, 2002). As a result, how river damming governs community patterns remains a key issue in aquatic microbial ecology, and predicting the response of bacteria to aquatic environment change is thus a need for science and political management (Rousk, Brookes, & Bååth, 2009).
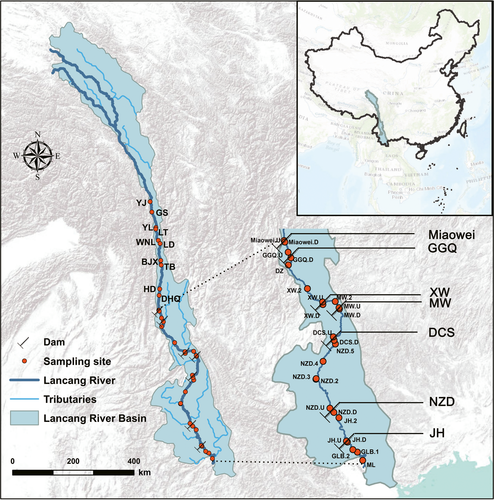
The Lancang River is the upper reaches of the Lancang–Mekong River, with a length of 2,161 km and a catchment of 167,487 km2 (Zhang, 2017), and it exhibits potentially abundant hydropower as the elevation drop along the river is more than 4,700 m (Liu et al., 2015). At present, a 21-cascade damming scheme has been planned for the mainstream of the Lancang River, along which seven dams have already been constructed and are in operation: Miaowei, Gongguoqiao (GGQ), Xiaowan (XW), Manwan (MW), Dachaoshan (DCS), Nuozhadu (NZD) and Jinghong (JH) (Winemiller et al., 2016). Although the cascade dams would provide substantial hydropower, they might also reduce the biodiversity and ecosystem services of the river basin, while undercutting the livelihood of millions of people (Eid et al., 2017). In addition, the Lancang–Mekong River Basin is a key area for biodiversity and is one of the world's largest international rivers, linking China with five other countries, indicating its great ecological, economic and sociological importance (Nilsson, Reidy, Dynesius, & Revenga, 2005).
The zoobenthic community has been altered in the regulated Lancang River, where the dominant species switched from lotic taxa to aquatic earthworms (Fan et al., 2015). Moreover, significant decreases in aboriginal fish species were also observed after cascade dam constructions in the Lancang River (Fan et al., 2015), further confirming the adverse effects of river damming. However, compared with studies on variation at higher trophic levels, fewer studies have focused on variation in bacterioplankton in the Lancang River, which are basic participants at the bottom of the food chain and also the major drivers of water quality. Since the large-scale construction of cascade dams, which is occurring at an unprecedented rate to satisfy the increase in economic growth worldwide, implies great complexity and uncertainty in the scale and scope of potential environmental and ecological influences (Grumbine & Xu, 2011), it is urgent to obtain a comprehensive understanding of cascade dam effects on river ecosystems and biogeochemical cycling.
In our study, next-generation sequencing of the 16S rRNA gene and a GeoChip functional gene microarray analysis with various multivariate statistical methods was applied to explore the variation in the bacterioplankton community along the highly regulated Lancang River. We hypothesized that the upstream and downstream areas partitioned by the dams would have distinct bacterioplankton communities with potential diverse biogeochemical roles. The difference in environmental conditions between the two areas of the reservoirs provides a good opportunity to identify relationships between the dynamics of these bacterioplankton groups and their geographic and environmental features. As a result, the main aims of the present study were to (a) describe the distribution patterns of bacterioplankton abundance, diversity, composition and function in the Lancang River; (b) determine the impacts of cascade dam regulation on the bacterioplankton community in the river; and (c) uncover the critical variables influencing the bacterioplankton community in the Lancang River.
2 MATERIALS AND METHODS
2.1 Study area and field sample collection
The Lancang River is the upper reaches of the Lancang–Mekong River, with a length of 2,161 km and a catchment of 167,487 km2 (Fan et al., 2015). There are seven dams in the mainstream that have been constructed and are in operation: the Miaowei, GGQ, XW, MW, DCS, NZD and JH. Detailed information on the cascade dams can be found in Supporting Information Table S1. Among the cascade reservoirs, the XW and NZD reservoirs are multiyear regulating storage reservoirs with the largest reservoir capacities. The field sampling was conducted in February 2017, covering over 1,200 km of river and an elevation gradient from 420 to 2,600 m. During the sampling, 35 sites were sampled along the Lancang River (Figure 1). The longitude, latitude and elevation of each site and the distance between sampling sites were measured using a GPSMAP 62s device (Garmin, KS, USA) and are presented in Supporting Information Table S2. Water samples were collected at a depth of 0.5 m by a Hydrobios Ruttner sampler (Altenholz, Germany). In each sampling site, three 1 L water samples were collected for the triplicate laboratory physical and biogeochemical analyses described below. Three 2 L water samples were also taken in each site and were filtered through 0.22 μm Millipore membrane filters (MA, USA). The filters were then stored at −80°C until the extraction of deoxyribonucleic acid (DNA).
2.2 Physical and biogeochemical analysis of water samples
Water temperature (T), electrical conductivity (EC), dissolved oxygen (DO) and pH were measured in the field by an HQ40d multiparameter water quality analyser (Hach, CO, USA). Turbidity was also determined in situ by a Hach 2100P turbidimeter (CO, USA). In the laboratory, water samples were filtered through precombusted 0.45 μm Whatman filters (NJ, USA) and analysed by a Liquid-TOC analyser (Elementar, Frankfurt, Germany) for the determination of dissolved organic carbon (DOC) concentration. Dissolved silicate (DSi) concentration was measured by the molybdate blue spectrophotometric method according to a previous study (Mortlock & Froelich, 1989). Total nitrogen (TN) and total phosphorus (TP) were determined colorimetrically after acid hydrolysis with persulfate digestion. Nitrate (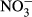 ), nitrite (
), nitrite (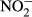 ), ammonium (
), ammonium (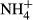 ) and soluble reactive phosphorus (SRP) were all estimated by an AA3 Seal Auto Analyzer (Norderstedt, Germany) with a detection limit of 0.003, 0.001, 0.003 and 0.004 mg/L, respectively.
) and soluble reactive phosphorus (SRP) were all estimated by an AA3 Seal Auto Analyzer (Norderstedt, Germany) with a detection limit of 0.003, 0.001, 0.003 and 0.004 mg/L, respectively.
2.3 Determination of bacterioplankton 16S rRNA copy numbers by quantitative polymerase chain reaction and GeoChip 5.0 analysis
The bacterioplankton DNA of samples was extracted from the prefrozen 0.22 μm filters using the MoBio PowerWater DNA extraction kit (CA, USA) according to the manufacturer's instructions. DNA quality and quantity were verified using agarose gel electrophoresis (1%) and spectrophotometry (NanoDrop ND 2000; Thermo Scientific, DE, USA). Bacterioplankton 16S rRNA copy number was determined by quantitative polymerase chain reaction (qPCR) amplification (7500 PCR system; Applied Biosystems, Darmstadt, Germany). The details of thermal cycling conditions and primer sets can be found in a previous study (Wang, Wang, et al., 2017). In brief, amplification was initiated at 95°C for 5 min and followed by 40 cycles of 95°C for 30 s, 60°C for 30 s and 72°C for 30 s. All PCRs were performed in triplicate. The 16S rRNA gene copy number has been applied with appropriate corrections to investigate variation in bacterial cell abundance in many studies (Chen, Wang, Shen, Gao, & Zheng, 2017; Yong et al., 2011), but it should also be noted that it is the approximation of cell abundance rather than the accurate bacterial biomass.
GeoChip 5.0, which is a comprehensive functional gene microarray with 167,044 probes (Xue et al., 2016), was used to examine functional variation in the bacterioplankton community in the Lancang River. A total of 51 replicate samples from 17 selected sites (two sites in the natural upstream reaches, 14 sites in the 7-cascade dam reaches and one site in the natural downstream reaches) were chosen for the GeoChip 5.0 analysis (Figure 1 and Supporting Information Table S2). In each sample, a total of 1.5 μg extracted DNA was labelled with fluorescent Cy3 dye (GE Healthcare, CA, USA) by random primers and the Klenow fragment of DNA polymerase. The labelled DNA was then purified using a QIA quick purification kit (Qiagen, CA, USA) and dried at 45°C for 45 min with a SpeedVac (Thermo Savant, MA, USA). Subsequently, the dried DNA was rehydrated; the details of the rehydration can be found in a previous study (Yan et al., 2015). The rehydrated solution was then incubated at 95°C for 3 min and 37°C for 30 min, and hybridized with GeoChip 5.0 arrays (180 K) at 42°C for 16 hr. The microarray was then scanned by a Roche NimbleGen MS 200 Scanner (WI, USA) at 532 and 635 nm. The images were extracted by agilent feature extraction 11.5 software (Agilent Technologies, CA, USA), and the signal intensities were quantified. The signal intensity information was analysed on the website (http://ieg.ou.edu/microarray) using the data analysis pipeline. In brief, the poor-quality spots, the signal intensity of which were less than 1,000, were removed, and the genes which were detected in only one of the three replicate samples were also discarded. The normalization of signal intensity included the division of the mean value to the total signal intensity. Then, the data were transformed to natural logarithmic form. Detailed descriptions can be referred to previous studies (Zhang et al., 2017).
2.4 Illumina HiSeq 16S rRNA high-throughput sequencing of the bacterioplankton community
The extracted DNA was also used for 16S rRNA gene high-throughput sequencing. In our study, the V4 hypervariable region of 16S rRNA gene was amplified with the 515F/806R primer set in a 25 μl reaction, containing 60 ng purified template DNA (Wang, Wang, et al., 2017). Thermal cycling was initiated by denaturation at 94°C for 5 min followed by 31 cycles at 94°C for 30 s, 52°C for 30 s and 72°C for 45 s and ended with a final extension at 72°C for 10 min. All PCRs were conducted in triplicate to minimize potential PCR bias, and the triplicate PCR products were combined equally for each sample and verified by a dsDNA Assay Kit (PicoGreen, Invitrogen, CA, USA). The combined PCR products in each sample were mixed with other samples in equimolar concentrations. Prior to sequencing runs, the mixture was purified and recollected with an EZNA Gel Extraction Kit (Omega, USA). More details on the amplification conditions can be found in a previous study (Wang, Lü, et al., 2017). The obtained DNA library was then sequenced with the Illumina HiSeq 2000 platform following the manufacturer's protocols.
2.5 Data preprocessing and statistical analysis
Detailed information on the preprocessing of the 16S rRNA high-throughput sequencing data can be found in previous studies (Li et al., 2015; Yan et al., 2015). Briefly, the raw sequenced reads were trimmed to remove low-quality reads (trimmomatic, version 0.33). Forward- and reverse-end reads were attributed to the corresponding samples using mothur (version 1.35.1) and were matched by flash (version 1.2.11). The matched reads that contained an “N” or were shorter than 200 bp were discarded. And the chimeras were also removed using the UCHIME algorithm in usearch (version 8.0.1517). Among filtered reads, reads with 97% similarity were clustered into operational taxonomic units (OTUs) using the UPARSE algorithm in usearch. Taxonomic results were obtained by aligning selected representative sequences to the greengenes (version 13.5) database with Ribosomal Database Project Classifier.
Spearman correlation analysis was conducted to reveal correlations between monitored geographic and environmental parameters. Generalized additive models (GAMs) were used to determine the important variables influencing bacterioplankton cell abundance and α-diversity. A principal coordinate analysis (PCoA) was implemented to cluster sampling sites using the bacterioplankton community composition and functional gene composition data. An analysis of similarity (ANOSIM) was conducted to reveal significant differences among clusters. A redundancy analysis (RDA), a variable partitioning analysis (VPA) and a Mantel test were applied to identify the significant drivers and to determine the correlations between the bacterioplankton community, functional compositions and variables. All above-mentioned statistical analyses were conducted with the vegan and mgcv packages in r (version 3.2.1, Vienna, Austria). In addition, the average value and standard deviation of each triplicate were calculated. One-way ANOVA followed by Tukey's test was also conducted to identify significant differences at a level of p < 0.05.
3 RESULTS
3.1 Physicochemical characteristics of water samples in the Lancang River
The physicochemical parameters of water samples from the 35 sites of the Lancang River are presented in Figure 2 and Supporting Information Table S2. The EC of the river decreased from upstream to downstream sites, and significant decreases were observed in the XW and NZD reservoirs (p < 0.05, Figure 2a). A similar decreasing pattern was also detected for DOC, specifically, it remained high in upstream sites but remarkably decreased in the XW reservoir (p < 0.05) and remained stable in downstream cascade reservoirs (Figure 2b). Meanwhile, the DSi concentration showed a statistically insignificant increase along the Lancang River but a reduction in the XW reservoir (Figure 2b).
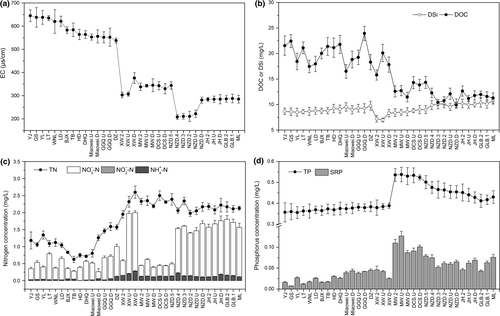
In contrast to the carbon and silicon concentrations, the TN concentration ranged from 0.63 to 1.34 mg/L in upstream sites, peaked at 2.35 mg/L in the XW reservoir and remained stable in downstream cascade reservoirs (Figure 2c). Comparable patterns were also observed for dissolved inorganic nitrogen (DIN), which was dominated by 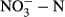 . However, the DIN concentration decreased sharply in the MW and DCS reservoirs and increased again in the NZD reservoir (Figure 2c). According to the Spearman correlation analysis (Supporting Information Figure S1), the TP and SRP concentrations varied synchronously along the Lancang River (r = 0.923, p < 0.001), increasing slightly in upstream sites and upstream cascade reservoirs (Miaowei, GGQ and XW) and ranging from 0.36 to 0.39 and 0.01 to 0.05 mg/L, respectively (Figure 2d). A pronounced stimulation of phosphorus was observed in the MW reservoir, but phosphorus continued to decrease in downstream cascade reservoirs.
. However, the DIN concentration decreased sharply in the MW and DCS reservoirs and increased again in the NZD reservoir (Figure 2c). According to the Spearman correlation analysis (Supporting Information Figure S1), the TP and SRP concentrations varied synchronously along the Lancang River (r = 0.923, p < 0.001), increasing slightly in upstream sites and upstream cascade reservoirs (Miaowei, GGQ and XW) and ranging from 0.36 to 0.39 and 0.01 to 0.05 mg/L, respectively (Figure 2d). A pronounced stimulation of phosphorus was observed in the MW reservoir, but phosphorus continued to decrease in downstream cascade reservoirs.
3.2 Variation in bacterioplankton cell abundance and its important drivers
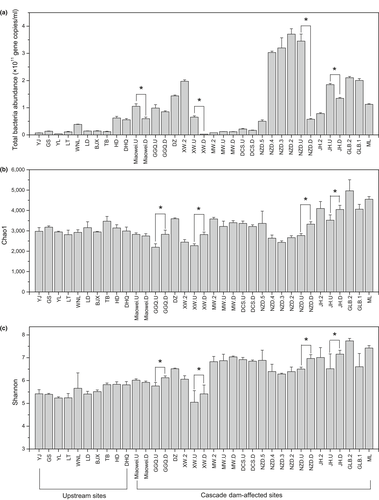
The total number of bacterial 16S rRNA gene copies in the 105 samples from 35 sites along the Lancang River ranged from 2.85 × 109 to 3.71 × 1011 gene copies/ml (Figure 3a). Bacterioplankton cell abundance was lower in upstream sites than in downstream cascade reservoirs. Moreover, the cell abundance decreased downstream of all the dams, especially in Miaowei, XW and NZD (p < 0.05), while it increased and recovered after the inhibition by each dam. Notably, in the MW and DCS reservoirs, the bacterioplankton cell abundance remained low downstream of the dam, which was in contrast to the tendency for higher cell abundance to occur in cascade reservoirs than in upstream natural sites.
A GAM was applied to determine the important drivers of bacterioplankton cell abundance. The statistical results showed that most of the measured spatial and environmental parameters were significantly correlated with bacterioplankton cell abundance, and DSi and SRP were the most important variables explaining cell abundance variation in the Lancang River (Table 1). In addition, nonlinear relationship occurred between bacterioplankton cell abundance and DSi and between bacterioplankton cell abundance and SRP, with degrees of freedom of 2.96 and 2.69, respectively (Supporting Information Figure S2). The GAM also revealed that the relationship between bacterioplankton cell abundance and SRP formed a single-peak curve with a peak value of 0.06 mg/L, and the curve formed between cell abundance and DSi peaked at 10 mg/L.
| Variable | Total bacteria abundance | Chao1 | Shannon | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Deviance explained | R 2 | p | Deviance explained | R 2 | p | Deviance explained | R 2 | p | |
| Elevation | 0.232 | 0.224 | <0.001 | 0.479 | 0.464 | <0.001 | 0.679 | 0.673 | <0.001 |
| Distance | 0.309 | 0.290 | <0.001 | 0.433 | 0.417 | <0.001 | 0.628 | 0.625 | <0.001 |
| DSi | 0.498 | 0.483 | <0.001 | 0.420 | 0.403 | <0.001 | 0.394 | 0.382 | <0.001 |
| DO | 0.084 | 0.065 | 0.038 | 0.227 | 0.210 | <0.001 | 0.411 | 0.395 | <0.001 |
 |
0.079 | 0.071 | <0.001 | 0.208 | 0.191 | <0.001 | 0.137 | 0.114 | <0.001 |
| pH | 0.062 | 0.038 | 0.133 | 0.207 | 0.190 | <0.001 | 0.522 | 0.508 | <0.001 |
| DOC | 0.128 | 0.290 | <0.001 | 0.198 | 0.175 | <0.001 | 0.614 | 0.604 | <0.001 |
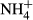
|
0.228 | 0.206 | <0.001 | 0.181 | 0.160 | <0.001 | 0.571 | 0.56 | <0.001 |
| EC | 0.354 | 0.336 | <0.001 | 0.176 | 0.152 | <0.001 | 0.493 | 0.482 | <0.001 |
| TP | 0.356 | 0.340 | <0.001 | 0.171 | 0.147 | <0.001 | 0.493 | 0.482 | <0.001 |
| SRP | 0.379 | 0.362 | <0.001 | 0.102 | 0.078 | 0.019 | 0.584 | 0.574 | <0.001 |
| T | 0.368 | 0.349 | <0.001 | 0.079 | 0.055 | 0.060 | 0.478 | 0.468 | <0.001 |
| Turbidity | 0.172 | 0.164 | <0.001 | 0.066 | 0.049 | 0.062 | 0.532 | 0.522 | <0.001 |
| TN | 0.222 | 0.199 | <0.001 | 0.066 | 0.056 | 0.013 | 0.500 | 0.486 | <0.001 |
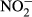
|
0.054 | 0.045 | 0.017 | 0.006 | −0.004 | 0.440 | 0.075 | 0.051 | 0.084 |
-
DSi, dissolved silicate; DO, dissolved oxygen;
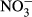 , nitrate; DOC, dissolved organic carbon;
, nitrate; DOC, dissolved organic carbon; 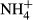 , ammonium; EC, electrical conductivity; SRP, soluble reactive phosphorus; TP, total phosphorus; T, water temperature; TN, total nitrogen;
, ammonium; EC, electrical conductivity; SRP, soluble reactive phosphorus; TP, total phosphorus; T, water temperature; TN, total nitrogen; 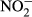 , nitrite.
, nitrite.
3.3 Variation in river bacterioplankton α-diversity and its influential drivers
According to the sequencing results, the number of valid sequences after quality filtering ranged from 36,791 to 100,760 reads in 105 samples from 35 sites, which can be assigned to a total of 25,631 OTUs. In general, the α-diversity results indicated that the Chao1 and Shannon index increased from upstream to downstream sites in the Lancang River, varying from 2,195 to 4,966 and from 5.05 to 7.74, respectively (Figure 3b,c). Interestingly, in contrast to bacterioplankton cell abundance, bacterial α-diversity decreased in reservoirs and increased downstream of dams. The GGQ, XW, NZD and JH dams in particular significantly enhanced the richness and evenness of bacterioplankton diversity.
A GAM was also used to determine the important drivers of bacterioplankton α-diversity. Elevation and distance from the source were the most influential factors explaining α-diversity variation, explaining over 40% of richness variation and over 60% of evenness variation (Table 1). The GAM also showed that elevation was negatively correlated with bacterioplankton α-diversity, while distance exhibited a positive link with α-diversity, including a positive linear relationship with the Shannon index (degree of freedom = 1, Supporting Information Figures S3 and S4).
3.4 Variation in river bacterioplankton β-diversity and functions, along with their spatial and environmental drivers
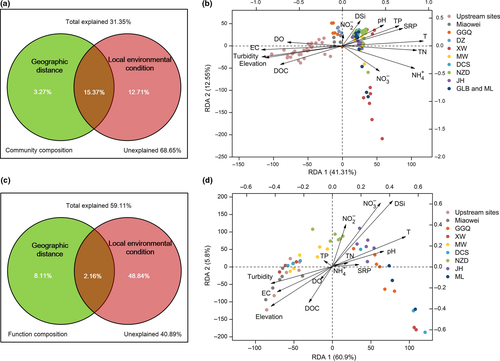
PCoA revealed spatial variation in bacterioplankton community composition (Figure 4a). The first two principal components explained 52.10% (PC1) and 12.52% (PC2) of the variation in bacterioplankton community composition, respectively. The 105 samples from 35 sites clustered into three groups, including upstream natural sites, upstream dam sites (Miaowei, GGQ and DZ) and downstream dam sites (XW, MW, DCS, NZD, JH, GLB and ML) (Figure 4a and Supporting Information Figure S5). According to the ANOSIM results, the three groups were significantly different from each other (upstream natural sites and upstream dam sites: r = 0.61, p = 0.017; upstream natural sites and downstream dam sites: r = 0.87, p < 0.001; upstream dam sites and downstream dam sites: r = 0.92, p < 0.001).
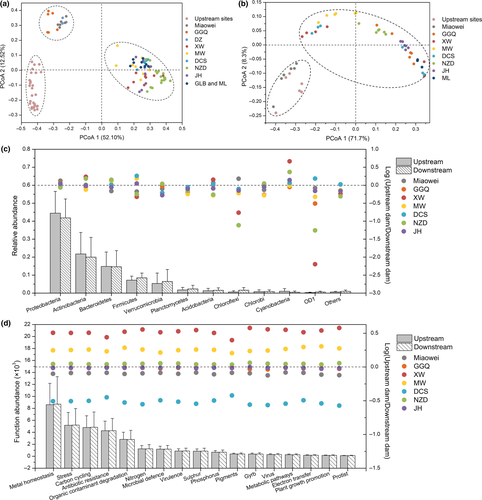
To reveal the functional variation in the bacterioplankton community, 51 replicate samples from 17 sampling sites were chosen for GeoChip 5.0 analysis. According to the PCoA of functional genes (Figure 4b), PC1 and PC2 explained 71.7% and 8.3% of the variation in bacterioplankton functional composition, respectively. All samples can be divided into two groups, including upstream sites (upstream natural sites and upstream dam sites of Miaowei) and other dam sites (GGQ, XW, MW, DCS, NZD, JH and ML), and the two groups were significantly different according to the ANOSIM (r = 0.39, p = 0.041).
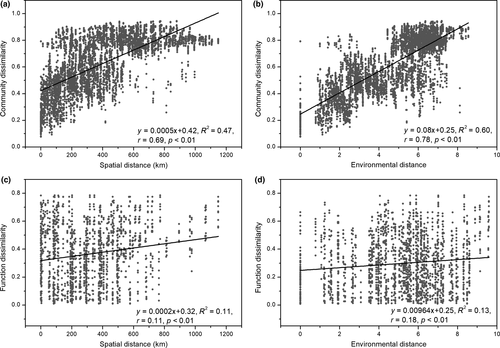
In our study, the spatial distance between two sampling sites and the environmental parameters of each site were measured to investigate the important factors shaping the bacterioplankton community in the Lancang River. Figure 5a,b shows that community dissimilarity increased with increasing spatial and environmental distances, and local environmental conditions had a stronger influence on community composition than spatial distance ( and
and  ). Moreover, according to the VPA results, local environmental conditions explained more variation in community composition than spatial distance (Figure 6a). Since the local environmental conditions comprised 14 parameters, an RDA and a Mantel test were carried out to determine the correlations between each of these conditions and community composition (Figure 6b and Table 2). The results demonstrated that EC and T, rather than nutrient concentrations, were the variables most correlated with variation in community composition.
). Moreover, according to the VPA results, local environmental conditions explained more variation in community composition than spatial distance (Figure 6a). Since the local environmental conditions comprised 14 parameters, an RDA and a Mantel test were carried out to determine the correlations between each of these conditions and community composition (Figure 6b and Table 2). The results demonstrated that EC and T, rather than nutrient concentrations, were the variables most correlated with variation in community composition.
| Environmental variable | Community composition | Function composition | ||
|---|---|---|---|---|
| r | p | r | p | |
| EC | 0.8550 | <0.001 | 0.0778 | 0.035 |
| T | 0.8000 | <0.001 | 0.1723 | 0.003 |
| Elevation | 0.6933 | <0.001 | 0.0860 | 0.019 |
| Turbidity | 0.6726 | <0.001 | 0.1368 | 0.011 |
| TN | 0.6564 | <0.001 | 0.0229 | 0.291 |
| DOC | 0.6153 | <0.001 | 0.0228 | 0.294 |
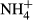
|
0.6138 | <0.001 | 0.0322 | 0.235 |
| DO | 0.5284 | <0.001 | −0.1071 | 0.994 |
| TP | 0.4394 | <0.001 | 0.1255 | 0.004 |
| pH | 0.4020 | <0.001 | 0.0668 | 0.039 |
| SRP | 0.3923 | <0.001 | 0.1414 | 0.009 |
| DSi | 0.1915 | <0.001 | 0.2464 | <0.001 |
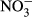 |
0.1588 | <0.001 | 0.2132 | <0.001 |
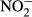 |
0.04776 | 0.019 | −0.0907 | 0.974 |
-
DOC, dissolved organic carbon; DO, dissolved oxygen; EC, electrical conductivity;
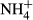 , ammonium; T, water temperature; TN, total nitrogen; TP, total phosphorus; SRP, soluble reactive phosphorus; DSi, dissolved silicate;
, ammonium; T, water temperature; TN, total nitrogen; TP, total phosphorus; SRP, soluble reactive phosphorus; DSi, dissolved silicate; 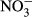 , nitrate;
, nitrate; 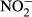 , nitrite.
, nitrite.
Similar to community composition, local environmental conditions also exhibited a greater correlation than spatial distance with the functional composition of bacterioplankton in the Lancang River; however, this correlation between local environmental conditions and functional composition was weaker than that between local environmental conditions and community composition (Figure 5c,d). The VPA results also revealed that local environmental conditions shaped variation in functional composition more significantly than they did variation in community composition (Figure 6c). In addition, according to the RDA and Mantel test, among the measured environmental variables, DSi and 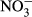 were the most important drivers of the variation in functional composition (Figure 6d and Table 2).
were the most important drivers of the variation in functional composition (Figure 6d and Table 2).
3.5 Variation in typical river bacterial phyla and functions in cascade reservoirs
According to the taxonomic results, 11 top dominant bacterial phyla were detected in water samples from the Lancang River (Supporting Information Figure S6). Bacteroidetes was the dominant phylum in upstream sites, accounting for 37.01% to 50.88% of the total bacterioplankton abundance, while Proteobacteria and Actinobacteria dominated the bacterioplankton community in cascade dam sites. In cascade dam sites, the typical bacterioplankton phyla included Proteobacteria, Actinobacteria, Bacteroidetes, Firmicutes, Verrucomicrobia, Planctomycetes, Acidobacteria, Chloroflexi, Chlorobi, Cyanobacteria and OD1 (Figure 4c). Proteobacteria was the most dominant phylum and accounted for over 40% of the total community abundance in dam-associated sites, followed by Actinobacteria (over 20%) and Bacteroidetes (over 14%). Variation in the typical phyla upstream and downstream of the cascade dams was also observed. Notably, most of the phyla did not vary significantly in the seven dams, except for Chloroflexi, Cyanobacteria and OD1. The relative abundance of Chloroflexi and OD1 increased downstream of most dams, especially the XW Dam and the NZD Dam. In contrast, the relative abundance of Cyanobacteria was stimulated in upstream sites of most dams, especially the XW Dam and the NZD Dam. In terms of functions, 17 typical functional gene categories were identified in the 51 samples (Supporting Information Figure S7). The abundance of functional genes increased from upstream natural sites to downstream dam sites (Supporting Information Figure S7), but it was reduced remarkably by the XW, MW and NZD dams (Figure 4d). Among the 17 functional gene categories, metal homeostasis, stress, carbon cycling, antibiotic resistance and organic contamination degradation were the dominant functions in all sampling sites. In cascade dam-associated sites, no significant alteration of average functional gene abundance by cascade dams was observed (Figure 4d). In addition, cascade dams exhibited similar influences on all detected functional genes, while the dominant phyla responded differently in different dams.
4 DISCUSSION
4.1 Effects of cascade dams on water physicochemical characteristics
In cascade reservoirs, EC and DOC both decreased and similar results were also observed by Lu et al.; specifically, the DOC content gradually decreased from the middle to lower reach of the Lancang River (Lu et al., 2017). According to previous studies, the impoundment of dams would turn the mainstream into slow-moving reservoirs (Kummu & Varis, 2007), and the concentration of suspended particles would decrease due to enhanced sedimentation in reservoirs, leading to reduced EC and DOC concentrations. In contrast, nitrogen and phosphorus both increased significantly in reservoirs, indicating that damming can stimulate the accumulation of nutrients. It is also reported that subsequent to reservoir impoundment, the shift from a lotic system to a lentic system may decrease the self-purification capacity of the system due to velocity reduction, leading to a deterioration of water quality in reservoirs (Teodoru & Wehrli, 2005). Moreover, since the mid- and downstream reaches of the Lancang River are in densely populated and industrialized regions, anthropogenic discharges further contribute to the increase in nutrients in cascade reservoirs. Consequently, cascade dam construction in the Lancang River may increase nutrient concentrations in the water column and threaten water quality in reservoirs.
4.2 Effects of cascade dams on the bacterioplankton community
Previous studies reported that the construction of large-scale water conservancy projects can alter natural hydrological conditions and sediment transport processes downstream (Ruiz-González et al., 2013; Xia et al., 2016). In consequence, upstream and downstream sites of the dam may differ remarkably in their physicochemical properties, which may further influence their bacterioplankton communities. In the Lancang River, bacterioplankton cell abundance and α-diversity exhibited different variations between areas upstream and downstream of dams.
In our study, higher bacterioplankton cell abundance was observed in cascade dam-affected sites than in natural upstream sites. However, in the MW and DCS reservoirs, bacterioplankton cell abundance decreased significantly, which was consistent with the pattern of DIN variation. Since bacterioplankton are closely involved in nitrification, denitrification and heterotrophic nitrogen uptake (Mulholland et al., 2008), the decline of DIN might affect bacterioplankton growth in reservoirs (Ruiz-González et al., 2013). In addition, the release of low-temperature water to downstream dam sites (Supporting Information Table S2) created an unfavourable environment for bacterioplankton growth (Fan et al., 2015), resulting in a further reduction in bacterioplankton cell abundance. Interestingly, a recovery of cell abundance was also detected in the downstream reservoir after inhibition by an upstream dam, indicating the self-recovery capacity of the river ecosystem.
Generally, the α-diversity of bacterioplankton in the Lancang River was higher in cascade dam-affected sites than in natural upstream sites due to the unfavourable oligotrophic conditions in upstream sites. However, in contrast to the cell abundance pattern, the α-diversity of bacterioplankton decreased in cascade reservoirs. Since river damming may reduce habitat heterogeneity (Jordaan & Bezuidenhout, 2016; Ruiz-González et al., 2013), the bacteria, which benefit from the homogeneity, will be sorted and dominate the community, leading to a decrease in α-diversity (Drury, Rosi-Marshall, & Kelly, 2013). In our study, the increase of nutrients in the seven reservoirs in the Lancang River can accelerate the growth of bacteria that can utilize nutrients more efficiently and contribute to their dominance in the community, eventually resulting in the co-occurrence of high cell abundance and low α-diversity (Figure 3). The self-recovery of α-diversity downstream of dams was also observed due to the recurrence of heterogeneity caused by lotic conditions. Consequently, although cascade dams could have adverse effects on bacterioplankton cell abundance and α-diversity, the river ecosystem is capable of self-recovering to some extent. Therefore, more studies should be conducted to investigate the potential self-recovery ability of the bacterioplankton communities in river ecosystems and provide scientific evidence to facilitate the management of cascade dam planning in the future.
β-Diversity represents the taxonomic diversity that is due to turnover in composition between assemblages. In our study, the bacterioplankton community composition (β-diversity) in natural upstream sites was distinct from that in cascade dam-affected sites, indicating that β-diversity was altered by the construction of cascade dams in the Lancang River. According to the environmental sorting theory, since a shift in bacterioplankton community composition includes filtering for more resistant species with an associated change in overall relative abundances (Jordaan & Bezuidenhout, 2016), the adapted bacteria would be sorted and would dominate the community, ultimately leading to the variation in community composition. In addition, different responses of bacterioplankton community to different dams were observed in the Lancang River, which may be attributed to the differences in reservoir storage. According to previous studies, the upstream dams (Miaowei and GGQ) possess the smallest reservoir storages (Liu et al., 2015), resulting in a shorter water residence time that was shown to be closely correlated with the alteration of bacterioplankton community composition in a previous study (Mašín et al., 2003).
In our study, a change in bacterioplankton community composition from upstream to downstream sites in the Lancang River was detected in which the community transitioned from a Bacteroidetes- and Proteobacteria-dominated community to a Proteobacteria- and Actinobacteria-dominated community. Bacterioplankton community composition has been proposed to mainly depend on environmental sorting, which refers to the adaptation of individual bacterial species to diverse environmental conditions (Fierer et al., 2011). The upstream portion of the Lancang River is a “simple” environment with a low level of competition, favouring rapidly growing species that can utilize available resources quickly (r-strategists) (Weinbauer & Hofle, 1998), such as Bacteroidetes. However, competition may become more intense downstream, especially with the disturbance caused by cascade dam constructions, as the community of bacterioplankton increases in both abundance and complexity, leading to the dominance of k-strategist species that are more competitive and have lower growth rates and narrower niches (Weinbauer & Hofle, 1998), such as Actinobacteria. Furthermore, it should also be noted that Cyanobacteria were stimulated in most reservoirs, especially in reservoirs with large storage, such as the XW and NZD reservoirs. According to our measurements, the water temperature and nutrient concentrations in cascade reservoirs increased significantly (especially the XW and NZD reservoirs), which may stimulate an increase in cyanobacteria species in dam reservoirs (Pawlik-Skowrońska & Toporowska, 2011). In consequence, more attention should be paid to the monitoring of potential cyanobacterial blooms in reservoirs.
According to our results, the dominant bacterioplankton phyla responded differently to cascade dam construction, while synchronous response of the dominant functional genes to damming was observed. During the comparison of average functional genes between sites upstream and downstream of the dams, no significant alteration of the functional community was detected. Consequently, the compositionally different communities in dam-associated sites exhibit similar functions, which can be attributed to bacterial functional redundancy. As reported in previous studies, variation in community composition often co-occurred with consistency in functional genes (Eid et al., 2017; Rousk et al., 2009) since functional redundancy can safeguard bacterial communities by maintaining important ecosystem processes. Therefore, taxonomic variation (which was commonly used in recent studies) may not exhibit the comprehensive response of bacterioplankton community to river damming, and further validation of functional inference tools is necessary to reveal the effects of cascade damming on bacterioplankton.
4.3 Identification of critical factors influencing the bacterioplankton community in the large highly regulated river
Bacterioplankton communities can be highly diverse in natural rivers under environmental disturbances and stresses due to their small size and rapid growth rates (Zeglin, 2015). In the Lancang River, bacterioplankton cell abundance was influenced by trophic conditions, including concentrations of DSi and SRP. Many studies have also confirmed that nutrients are important factors for the growth of river microorganisms (Allen, Booth, Verity, & Frischer, 2005; Davidson et al., 2007). In contrast to cell abundance, bacterioplankton α-diversity was influenced by elevation. Since variation in elevation often co-occurs with climatic changes, such as changes in temperature and illumination intensity, which play essential roles in bacterioplankton physiological activities (Baatout, Boever, & Mergeay, 2005; Galí et al., 2013; Zhou et al., 2016), only the adapted species would survive, leading to variation in α-diversity, which is explained by the environmental sorting theory in previous studies (Niño-García, Ruizgonzález, & Del Giorgio, 2016; Savio et al., 2015).
As no agreement on which variables influence bacterioplankton taxonomic and functional composition has been reached, large numbers of variables are often measured in relevant studies (Read et al., 2015; Zeglin, 2015). In our study, spatial distance and local environmental distance were both significantly and positively correlated with bacterioplankton taxonomic and functional dissimilarity, confirming the presence of a distance–decay relationship in the Lancang River. According to previous studies, the theory of distance–decay can be applied to predict biological diversity due to the combination of dispersal ability and environmental condition in ecosystems (Staley et al., 2015), suggesting the potential for simulation and prediction of bacterioplankton variation to be incorporated into the planning of dam construction in the future. It is also observed that local environment condition had a greater influence on the bacterioplankton community than geographic distance; however, in the study of Wang, Yang, Liu, & Yu (2015), the opposite result was observed. This discrepancy may be attributed to a difference in sampling scale, as our sampling occurred over 1,200 km, whereas sampling occurred over 380 km in the study of Wang. In consequence, further investigations are still needed to reveal the mechanism of geographic and environmental effects on bacterial taxonomic and functional communities.
According to our results, electrical conductivity (EC) and water temperature (T) were the most important environmental variables shaping the bacterioplankton taxonomic community. The influence of EC on bacterial community structure is often observed (Crump, Hopkinson, Sogin, & Hobbie, 2004; Sun et al., 2014) because EC is associated with the salinity of water and considered to affect bacterial growth. Similar to EC, T was also proved to be the significant factor influencing bacterial growth (Lefort & Gasol, 2013). Consequently, as explained by the environmental sorting theory, when the ecosystem undergoes environmental alteration, which would influence bacterial growth, the species benefiting from the changes are expected to flourish, and the sensitive taxa are expected to disappear, eventually leading to a shift in community composition across environmental gradients (Bier, Voss, & Bernhardt, 2015). Different from the taxonomic community, the bacterioplankton functional community was primarily shaped by nutrients. According to functional redundancy theory, bacterial communities have adaptive strategies to survive under a range of nutrient conditions and different species may exhibit similar functions (Allison & Martiny, 2008; Zeglin, 2015). As nutrients are essential elements for bacterial physiological activities, variation in nutrients would affect the expression of bacterial functions, such as biogeochemical cycling functions (Bier et al., 2015), confirming the important role of nutrients in functional composition but not in taxonomic composition.
Although many studies have focused on river ecosystems in recent decades, the lotic bacterioplankton community remains understudied. The Lancang River is suffering from the operation of seven cascade dams and will be further affected by the planned 21-cascade damming scheme. Therefore, the data collected from the Lancang cascade dams can be used as constructive references to reduce the potentially adverse impacts of extensive hydropower development on local and downstream environments. Our study can also provide data support and theoretical basis for energy development and ecological environment protection in other large rivers worldwide, including the Danube River in Europe, the Ebro River in Spain and the Mississippi River in the United States. Overall, in the current era of rising resource demands and reduced environmental resiliencies, more attention should be paid to the planning of potential cascade dams and to the management of existing dams.
5 CONCLUSION
Our results indicated that the construction of cascade dams in the Lancang River increased nutrient concentrations and threatened water quality in cascade reservoirs. Bacterioplankton cell abundance was reduced by river damming, and the α-diversity of the bacterioplankton community was inhibited in cascade reservoirs. Fortunately, however, the river ecosystem exhibited a self-recovery capacity after the adverse disturbance caused by damming. Moreover, bacterioplankton community composition was significantly altered by cascade dams, including a shift in the dominant phylum from r-strategists, Bacteroidetes, to k-strategists, Actinobacteria, from upstream to downstream sites in the river. Meanwhile, according to GeoChip analysis, the functional composition of bacterioplankton was less affected than taxonomic composition. In addition, geographic and environmental features both exhibited a distance–decay relationship with the taxonomic and functional communities, but the local environment was the more dominant driver in the Lancang River. Therefore, the impoundments of cascade dams had significant impacts on the bacterioplankton community, and further attention should be paid to the potential ecological consequences of river regulation.
ACKNOWLEDGEMENTS
We acknowledge the help from Dr. Zhilin Liu in the field sampling, and the assistance of Guangdong Magigene Biotechnology Co., Ltd., China, in Illumina Hiseq sequencing. We are also grateful for the grants for Project supported by the Key Program of National Natural Science Foundation of China (No. 91647206), the National Key Plan for Research and Development of China (2016YFC0502203), the National Science Funds for Creative Research Groups of China (No. 51421006), the National Natural Science Foundation of China (No. 51479065, 51579073), the Fundamental Research Funds for the Central Universities (No. 2016B44114), Jiangsu Province Ordinary University Graduate Student Scientific Research Innovation Plan (No. KYLX16_0767) and PAPD.
DATA ACCESSIBILITY
The bacterial DNA sequence data have been submitted to the SRA of the NCBI database under Accession no. SRP133583.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
X.W., C.W. and P.W. designed the study; X.W., J.C., L.M., Q.Y. and S.L. performed the field sampling and the experiment; X.W. and T.F. analysed the data and wrote the manuscript.




