Genetic variation underlying local adaptation of diapause induction along a cline in a butterfly
Abstract
Diapause is a life history strategy allowing individuals to arrest development until favourable conditions return, and it is commonly induced by shortened day length that is latitude specific for local populations. Although understanding the evolutionary dynamics of a threshold trait like diapause induction provides insights into the adaptive process and adaptive potential of populations, the genetic mechanism of variation in photoperiodic induction of diapause is not well understood. Here, we investigate genetic variation underlying latitudinal variation in diapause induction and the selection dynamics acting upon it. Using a genomewide scan for divergent regions between two populations of the butterfly Pararge aegeria that differ strongly in their induction thresholds, we identified and investigated the patterns of variation in those regions. We then tested the association of these regions with diapause induction using between-population crosses, finding significant SNP associations in four genes present in two chromosomal regions, one with the gene period, and the other with the genes kinesin, carnitine O-acetyltransferase and timeless. Patterns of allele frequencies in these two regions in population samples along a latitudinal cline suggest strong selection against heterozygotes at two genes within these loci (period, timeless). Evidence for additional loci modifying the diapause decision was found in patterns of allelic change in relation to induction thresholds over the cline, as well as in backcross analyses. Taken together, population-specific adaptations of diapause induction appear to be due to a combination of alleles of larger and smaller effect size, consistent with an exponential distribution of effect sizes involved in local adaption.
1 INTRODUCTION
Numerous organisms in temperate latitudes use environmental cues to prepare for predictable seasonal stresses, such as winter, by entering a preprogrammed state of dormancy before the onset of adverse environmental challenges, where they remain until the return of favourable conditions (Danilevskii, 1965; Perry, 1971; Simpson & Dean, 2002; Tauber & Tauber, 1976). In insects, such preprogrammed dormancy is known as diapause and it is often induced by shortened photoperiod (Danilevskii, 1965; Tauber & Tauber, 1976), which sets into motion a dynamic, yet distinct series of phases (preparation, developmental arrest, resumption of development) that facilitate life cycle synchronization with optimal conditions (Koštál, 2006; Koštál, Štětina, Poupardin, Korbelová, & Bruce, 2017). Even though the developmental stage in which diapause occurs varies widely, most insects with facultative diapause exhibit strong latitudinal clines in their critical photoperiod (CPP) for diapause induction (CPP = photoperiod needed to induce diapause in 50% of a population). While these latitudinal clines demonstrate a fine-scale regional adaptation to the historically reliable relationship between photoperiod and the onset of winter (Aalberg Haugen & Gotthard, 2015; Bradshaw, 1976; Bradshaw, Quebodeaux, & Holzapfel, 2003; Danilevskii, 1965; Demont & Blanckenhorn, 2008; Hard, Bradshaw, & Holzapfel, 1993; Huang et al., 2013; Kato, 2005; Paolucci, van de Zande, & Beukeboom, 2013; Wang et al., 2011), less is known about the underlying genetic basis of CPP clines.
Understanding the evolutionary dynamics of local adaptation for a threshold trait like diapause induction provides not only insights into the adaptive process, but also reveals the adaptive potential of populations. This has particular relevance to the response of species to climate change. Presently, we lack explanations for variability among species in their response to increasing mismatches between seasonality and life cycle timing, as some are not responding (Van Dyck, Bonte, Puls, Gotthard, & Maes, 2014), while others are adapting (Bradshaw & Holzapfel, 2001). Theoretical predictions of the genomic architecture of traits that have become locally adapted are well-developed, suggesting loci with either an infinitesimal distribution (Barton & Keightley, 2002; Roff, 1996), or an exponential distribution of effect sizes (Orr, 2000). An infinitesimal distribution is made up of many loci of small effect sizes, while an exponential distribution uses a combination of large and small-effect loci, where selection initially acts upon large-effect loci and is subsequently modified by loci of smaller effect as a fitness optimum is approached.
For diapause induction, empirical insights acquired from population crosses and QTL studies are consistent with Mendelian inheritance patterns (Doležel, Vaněčková, Šauman, & Hodkova, 2005; Han & Denlinger, 2009; Suwa & Gotoh, 2006) or indicate an architecture with few loci of large effect sizes (Chen, Xiao, He, Xu, & Xue, 2014; Danilevskii, 1965; Demont & Blanckenhorn, 2008; Fu, Chen, Xiao, He, & Xue, 2015; Hagen & Scriber, 1989; Ikten, Skoda, Hunt, Molina-Ochoa, & Foster, 2011; Kim, Krafsur, Bailey, & Zhao, 1995; Kurahashi & Ohtaki, 1977; Lehmann, Margus, & Lindström, 2016; McCoy, Lloyd, & Bartlett, 1968; McWatters & Saunders, 1997; Pruisscher et al., 2017; Rockey, Hainze, & Scriber, 1987; Söderlind & Nylin, 2011; Xia, Chen, Tu, Yang, & Xue, 2012), with several reporting large sex-linked effects (Chen et al., 2014; Fu et al., 2015; Hagen & Scriber, 1989; Ikten et al., 2011; Nylin, Wickman, & Wiklund, 1994; Pruisscher et al., 2017; Rockey et al., 1987). Studies on quantitative trait loci for CPP show that effect sizes can be highly variable depending on the genetic background of the crosses involved (Bradshaw, Emerson, Catchen, Cresko, & Holzapfel, 2012). Additionally, as diapause induction is a plastic threshold trait, inheritance patterns depend on the environmental conditions used for phenotyping (Pruisscher et al., 2017). Thus, whether diapause induction thresholds arise from a few or many loci is unresolved, and further work is needed to understand how the genetic architecture of this trait varies among populations and taxa.
The identity of genes involved in insect diapause induction has been investigated using candidate gene studies with several important advances. For instance, considerable work on circadian clock genes has revealed a central role in diapause (reviewed in, e.g., Denlinger, Hahn, Merlin, Holzapfel, and Bradshaw (2017)), but their association remains controversial, as they appear to be involved in diapause induction in some studies (Ikeno, Tanaka, Numata, & Goto, 2010; Meuti, Stone, Ikeno, & Denlinger, 2015), but not in others (Emerson, Dake, Bradshaw, & Holzapfel, 2009). However, while candidate gene studies can potentially identify genes that are involved in the expression of diapause-related traits, they do not inform on which loci underlie adaptive latitudinal variation in the induction of diapause specifically, or the selection dynamics that have acted to shape these latitudinal clines. There have been several studies of genetic variation in other diapause-related traits that vary latitudinally, such as postdiapause development in Ostrinia nubilalis (Levy, Kozak, Wadsworth, Coates, & Dopman, 2015), or diapause incidence in Drosophila melanogaster (Levy et al., 2015; Schmidt et al., 2008). However, additional empirical insights into the evolutionary dynamics acting upon CPP in the wild are necessary to reconstruct the history and predict the future potential for adaptation to changing environmental conditions.
Here, we use the speckled wood butterfly Pararge aegeria in an attempt to gain an understanding of the genetic variation and the selection dynamics acting upon latitudinal variation in CPP. P. aegeria is an ecological model species for latitudinal variation in CPP. Pupal diapause in P. aegeria is induced primarily by photoperiod during larval development, where individuals establish the diapause decision prior to pupation (Friberg, Aalberg Haugen, Dahlerus, Gotthard, & Wiklund, 2011). Earlier population crosses between a nondiapausing Madeiran and a facultative diapausing Swedish population indicated a sex-linked component in the diapause induction of this species (Nylin et al., 1994). In Sweden, this species exhibits a striking latitudinal variation in CPP that gives rise to a cline in voltinism (Aalberg Haugen, Berger, & Gotthard, 2012; Aalberg Haugen & Gotthard, 2015; Nylin, 1995). Here, we use a pooled sequencing approach to conduct a genomewide scan of differentiation between two Swedish populations that differ significantly in their CPP, followed by individual-level SNP genotyping in candidate genes in population crosses along a latitudinal cline. Our goal was to identify which of the genomewide differences between populations give rise to a latitudinal cline in CPP and then evaluate the selection dynamics acting on these genes.
We do this by: (a) assessing genomic variation within two populations that differ in latitudinal origin and CPP thresholds, followed by identifying candidates for local adaptation, (b) testing the association between these candidate loci for local adaptation and diapause incidence in F2 hybrid offspring, (c) investigating genomic signatures of selection in the population samples at the loci significantly associated with diapause induction, (d) evaluating allele frequencies of the candidate genes in five Swedish populations along a cline and (e) exploring whether other genomic regions are affecting CPP thresholds for induction by assaying additional F1 population crosses and backcrosses.
2 MATERIALS AND METHODS
2.1 Genome sequencing
Genomic DNA was extracted from thoraces of 22 individuals from each of two Swedish populations collected in 2010 and 2011 for a previous study (Tison et al., 2014): samples 1-22 from Skåne (hereafter referred to as the southern population, S), 84–94; 100–110 from Sundsvall (hereafter referred to as the northern population, N), using a DNeasy blood and tissue kit (Qiagen) with an extra RNase A treatment to remove potential RNA contamination. DNA quality was checked on 2% agarose gels stained with GelRed, to ensure minimal fragmentation, and UV-Vis spectrometer (NanoDrop 8000; Thermo Scientific) to assess purity. All samples showed minimal fragmentation with an absorbance 260/280 > 1.7 and <2.0. For each population, samples were combined at equal concentration, resulting in two pools of 5 μg RNA-free gDNA. SciLifeLab (Uppsala, Sweden) performed the library preparation (Illumina TruSeq DNA PCR-free library) and sequencing (Illumina HiSeq2000, 100-bp paired-end reads, 450 bp insert size). Each library was separately sequenced on two Illumina lanes and then concatenated for analysis. Data analysis is described below, and detailed command lines are provided in SM: scripts.
2.2 Illumina read processing
Illumina fastq files were filtered for PCR duplicates using the clone_filter script of Stacks-1.21 (Catchen, Hohenlohe, Bassham, Amores, & Cresko, 2013), followed by filtering of Illumina sequencing adaptors, quality trimming to a minimum Phred base quality of 10 and discarding broken read pairs, using bbduk2 (BBMap v35.69—Bushnell B.—http://sourceforge.net/projects/bbmap/).
2.3 De novo genome assembly
Four de novo genome assemblies were generated from the combined reads of both populations, as well as an assembly for each of the two population data sets, using CLC Genomics Workbench 5.5.1 (clcbio) with differing settings (Supporting information Table S1). Each de novo assembly was rated on N50 size and gene completeness, and the assembly using the combined data sets with word size 64 and bubble size 2000 was used for further analysis as it outperformed all others. Gene completeness was assessed using mespa v1.0 (Neethiraj, Hornett, Hill, & Wheat, 2017) using default parameters, excluding xenobiotic filtering. The protein data set from three different butterfly genome projects and the silkworm genome project were compared for use in annotating the assembly. For each of these protein sets, redundancy was controlled by using the longest protein isoform of each protein cluster group based upon >90% amino acid identity using cd-hit v4.5.4 (Li & Godzik, 2006) with parameters used in making the uniref90 database (Suzek, Wang, Huang, McGarvey, & Wu, 2014). Protein numbers after clustering were as follows: 14,818 proteins for D. plexippus protein set DPOGS2 (Zhan & Reppert, 2013), 12,591 for Heliconius melpomene Ensembl protein set Hmel1.27 (Kersey et al., 2016), 16,634 for Melitaea cinxia protein set v1.0 (Ahola et al., 2014) and 18,574 for Bombyx mori protein set A, B and C (Suetsugu et al., 2013). The D. plexippus protein set generated the best results and was used to annotate and scaffold the genome assembly (Supporting information Table S2), where scaffolding occurs through joining contigs that annotate to nonoverlapping regions of the same protein. mespa outputs sequences where no genes are found, named as, for example, contig_1, or c_1, and sequences that contain genes, denoted m_scaffold_2 or ms_2. This naming convention is continued in the tables and figures of this manuscript when referring to a specific contig. We used busco v3.0.2 (Simão, Waterhouse, Ioannidis, Kriventseva, & Zdobnov, 2015) on default settings, which looks at the fraction of completely covered conserved genes, as a method to assess completeness of the genome.
2.4 Gene model order
Relative chromosomal location of the P. aegeria gene models was inferred using orthology from the high-quality D. plexippus scaffolds and B. mori chromosomes, as synteny is well-conserved within Lepidoptera (Dasmahapatra et al., 2012). As at the time of writing B. mori is the most complete and well-annotated lepidopteran genome available, it was used as chromosomal location template for D. plexippus scaffold order. Predicted P. aegeria proteins were searched against the protein sequences of B. mori using blastp (Camacho et al., 2009), taking only hits above a minimum E-value cut-off of 1e−4. B. mori chromosomal location was parsed out from the gene_ID category, of the annotation file available from these B. mori protein sets (Suetsugu et al., 2013). D. plexippus gene location was taken from the DPOGS2 gene annotations (Zhan & Reppert, 2013). The resulting gene order inferred for P. aegeria was considered “likely” when both species showed the same results. If there was no clear consensus, D. plexippus gene order was taken as correct as D. plexippus is in the same family as P. aegeria, while B. mori is much more divergent (Wheat & Wahlberg, 2012). Gene content within chromosomes is highly conserved, and the majority of genes are collinear between Lepidopteran species (Ahola et al., 2014). Thus, our comparative approach is likely sufficient to reveal whether the genomic differentiation investigated is clustered within a specific chromosome or distributed across the genome. This chromosomal-level information was not used in our outlier analysis.
2.5 Read mapping
The Pool-Seq read data were mapped to the assembly using nextgenmap 0.4.10 at 90% identity (Sedlazeck, Rescheneder, & von Haeseler, 2013), in order to minimize template mapping biases. Plotting read depth (RD) per scaffold showed a bimodal distribution where the first modus possibly represented misassembled or population-specific haploid sequences, and the second modus representing sequences that occurred at equal coverage in both populations (Supporting information Figure S1). By setting minimum RD cut-offs to >20, these first modus scaffolds were excluded from analyses. Furthermore, we only examined regions having an RD below 47 (97, 5th percentile), excluding regions with a significantly higher than average RD (indicating possible merging of similar genes, or population-specific copy number variation). Mapped reads were filtered using samtools v1.2 (Li, 2009), keeping read pairs where both reads mapped correctly.
In order to assess the possibility of population-specific genomic content, we compared the read depth per scaffold between the two populations, which revealed a strong linear relationship (R2 = 0.81, Supporting information Figure S1), with the exception of 271 sequences in the southern population, and 2,242 sequences in the northern population, where per scaffold read depth was <10 in one population (Supporting information Figure S2), but a range of read depths in the other. We performed a blastx alignment of these scaffold against the UniProt protein database, filtered on E-value <1E−5 and noted whether these sequences aligned to known proteins of other Lepidopteran species. This revealed only two scaffolds of potential Lepidopteran origin among these 2,513 population-specific scaffolds. From the 271 Sundsvall-specific scaffolds, one matched the geometrid moth Psaliodes protein, a chitinase A precursor, at a low sequence similarity of 29.6%. For the 2,242 Skåne-specific scaffolds, only one matched to a Manduca sexta protein at a low sequence similarity of 37.66%, representing trypsin alkaline B. We consider these low sequence similarities to known databases to be poor evidence of informative annotations, but nevertheless potential evidence of a very small amount of population-specific genomic content that we did not analyse further. The other scaffolds represented genomic sequences of bacteria and plants (Supporting information Table S3), reflecting potential microbiota living in and on the organism, as well as part of the gut. Our read depth filters excluded these sequences from our analyses reported below.
2.6 Variant calling
In order to quantify differentiation between the populations, SNPs were identified and FST was calculated at each base pair and using a window-based analysis of the assembly using popoolation2 v1.201 (Kofler, Pandey, & Schlötterer, 2011). The generated alignments from the two populations were converted into an mpileup file, and indels were masked with 5-bp windows in both populations, effectively excluding these from the analysis. Allele frequency differences were calculated, and FST was calculated from that using the standard equation as shown in Hartl and Clark (2007), and individual SNPs were considered for downstream analysis where Fisher's exact test P < 5*E−8, correcting for multiple testing as the genome size is approximately 5*10−8. Individual SNPs were determined to be in an exon, an intron or intergenic, by intersecting the SNP coordinates with the GFF output of mespa using bedtools-2.18.2 (Quinlan & Hall, 2010). Window-based divergence was calculated in 5-kbp (kilobase pair) consecutive nonoverlapping windows covering the entire genome, using the input file generated above. Finally, windows were kept for subsequent analyses when >50% of the window had an RD between 20 and 47 and Fisher's exact test P < 5*E−8. As each population was sequenced twice, we were able to investigate technical replicate error rate by performing independent pairwise comparisons within and between sequencing rounds. We performed analyses as described above for each combination of data sets and compared the overlap of 5-kbp outliers with an FST >0.8 between each analysis. This showed that there were no erroneous results related to technical replication, and combining the data of the two lanes enhanced the ability to detect more outliers (Supporting information Figure S3).
2.7 Nucleotide diversity and Tajima's D
To assess signals of selection in the outlier regions of divergence identified by FST, nucleotide diversity (π) and Tajima's D were calculated for both populations using PoPoolation v1.2.2 (Kofler, Orozco-terWengel et al., 2011) in 5-kbp windows. The alignments of the two populations were converted into individual pileup files, in which indels were masked with 5-bp windows. For Tajima's D, pileup files were subsampled to a depth of 20 without replacement. Both measures were calculated in 5-kbp consecutive nonoverlapping windows, and only windows that were considered in the FST -based analysis were used for further analysis. Since polymorphism is primarily determined by demographic history and mutation rate, it should in theory be moderate, variable and roughly similar across the genome. Directional selection could remove variation during a selective sweep, wherein nucleotide diversity should be greatly reduced compared to flanking regions. Tajima's D provides another measure of the action of selection, contrasting nucleotide diversity with another measure, Watterson's theta (Kofler, Orozco-terWengel et al., 2011). Genomewide estimates are expected to be centred around a mean reflecting the demographic history of the sample, with very negative outliers indicating an excess of rare variants. In order to infer significance of Tajima's D and nucleotide diversity values, we generated 10,000 means of randomly sampled Tajima's D and nucleotide diversity regions, using the mean of regions of interest identified below (i.e., representative of the size of the investigated region). We then compared the distribution of these randomly sampled means to the observed mean value in the regions of interest.
2.8 Outlier selection
To look for candidate regions having experienced strong local selection, a set of outlier regions was chosen from the windows significant for the Fisher's exact test for subsequent analysis, when conforming to at least one of four separate criteria. We used these four different criteria in order to reduce the bias of only investigating a particular type of signal. First, in the top 99.5% percentile of the 5-kbp window FST analysis the six contigs with the highest number of windows were selected. Second, the nine contigs containing the highest window-based FST values were selected. Third, the contigs with the highest number of individual fixed SNPs were selected. Finally, contigs containing fixed nonsynonymous substitutions were selected.
2.9 HRM validation
High-resolution melting (HRM) was used on each individual of the Pool-Seq libraries to validate the allele frequencies in several candidate genes that were identified in the genome scan. Primers were designed using primer express 3.0.1 (Applied Biosystems) using default settings. The SNP with the highest FST in each outlier region was selected, and to make sure downstream, HRM results were as unambiguous as possible. Primers were located ±25 bp before and after the SNP of interest containing no other polymorphic sites in the region. For the four genes showing nonsynonymous substitutions, primers were designed around that particular SNP. Supporting information Table S7 lists the generated primers. The HRM reaction, consisting of 5 μl MeltDoctor™ HRM Master Mix (Thermo Fisher Scientific, cat no: 4415440), 2.8 μl ddH2O, 0.6 μl 5 μM of the forward and 0.6 μl 5 μM reverse primer, and 1 μl of 5 ng/μl DNA, was added to each well of a 96-well reaction plate. On each plate, at least three known northern homozygote, three known southern homozygote and three known heterozygote samples were used as controls. In a Veriti® 96-Well Fast Thermal Cycler, the reaction was heat-activated at 95°C for 10 min, amplified at 95°C for 15 s and 60°C for 1 min for 40 cycles. After this, a melt curve was generated on a StepOnePlus™ Real-Time PCR System by heating the reaction to 95°C for 10 s, and 60°C for 1 min after which temperature was increased to 95°C at 1% ramp speed, measuring fluorescence levels at every 0.1°C increase. Raw melt curves were analysed using high resolution melt Software v3.0.1 (Applied Biosystems). Markers were placed at each tail end of the derivative melt curve and then the software estimated allelic states by associating the melt curves of the samples to the controls. In <2.8% of all samples, the calls of the programme were inconclusive, but visual inspection clearly revealed the most probable allelic state, and these were called manually. Ambiguous reactions were performed again only once. Samples were classified as being either homozygous for the southern allele (SS), heterozygote (SN) or homozygous for the northern allele (NN) for each locus. We validated the allele frequencies in the Pool-Seq data sets, and even though these data sets were generated using a sample size considered “low” for Pool-Seq (n = 22 individuals), the HRM validation confirmed a 93% overlap between the allele frequency estimates and the true genotypes of each individual (Supporting information Table S8).
2.10 Phenotyping crosses
In order to investigate the inheritance of diapause, we produced population crosses that were assessed for their diapause incidence (Supporting information Figure S4). In 2012, six mated females from southern Sweden (Ålstorp (55.82 N, 12.97E, hereafter denoted S) and 16 mated females from a northern population in the Sundsvall area (Alnö 62.41N, 17.46E, hereafter denoted N) were collected, and their offspring were reared individually in 0.5-l plastic cups on the grass Poa annua. They were given a photoperiod that leads to 100% direct development in both populations (L:D 21:3). The resulting adults (85 N and 61 S butterflies) were used to produce F1 offspring for the two populations SS and NN, as well as reciprocal F1 population hybrids NS and SN (female first). F1 offspring were assayed for the proportion of direct development in conditions where the SS population shows 100% direct development and the NN population shows 0%, that is, L:D 18:6 at 17°C (Aalberg Haugen & Gotthard, 2015). A second set of larvae were reared in conditions that produce 100% diapause development in both populations: L:D 13:11, 17°C. The resulting diapausing pupae were overwintered in outdoor conditions.
In spring 2013, a male informative backcross SS × NS, a female informative backcross NS × SS, as well as one hybrid cross SN × NS were produced with these overwintered individuals. The resulting offspring were assayed for the proportion of direct development in the same intermediate photoperiod of L:D 18:6 and 17°C as used for the F1 cross. Larvae were reared in family cages with ad libitum access to the grass Dactylis glomerata in three climate rooms at Tovetorp Zoological Research Station. Cages were monitored daily for newly formed pupae. Each individual was sexed and weighed at pupation, scored for larval development time, subsequently frozen in liquid nitrogen, and stored at −80°C. In all rearing experiments, family structure, defined as all offspring from a mated female, was recorded for each individual. Several previous studies of P. aegeria show that nondiapause development (i.e., direct development) is strongly associated with very short larval development times, whereas individuals entering diapause have considerably longer larval periods (Aalberg Haugen & Gotthard, 2015; Aalberg Haugen et al., 2012; Nylin, Wickman, & Wiklund, 1989). This prolonged larval development time leads to pupation later in the season when photoperiods are shorter and the risks of entering direct development are lower. This reduction in larval development rate in individuals that do not develop directly is a central component of pupal diapause induction in this species (Aalberg Haugen & Gotthard, 2015; Aalberg Haugen et al., 2012; Nylin et al., 1989). Therefore, in order to increase the size of the experiment we used larval development time as a proxy of direct or diapause development, respectively. Indeed, also in this study larval development time showed a clear bimodal distribution in all crosses except the backcross (Supporting information Figure S5), where larvae in the first modus were classified as direct development (<40 days), and larvae in the second modus (>40 days) as diapause development. For the pure S and N crosses and the F1 crosses, we directly recorded the induction of diapause in the pupae, which supported this classification.
2.11 SNP genotyping in the crosses
In order to reveal the association between the candidate loci and diapause induction, genotypes were assessed using HRM in the F1 and F2 crosses generated in the constant conditions L:D 18:6, 17°C. A total of 115 individuals were randomly selected from the tail ends of the developmental distribution of the reciprocal F1 hybrid crosses, with 61 individuals from SN cross and 54 individuals from NS cross. Moreover, 65 individuals of the F2 SN × NS cross were selected. For the backcross SS × NS, 139 individuals were randomly selected from the tail ends of the distribution of developmental times, excluding animals that were 40 ± 5 days. DNA was extracted from 4 mm of the posterior end of each pupa using an E-Z 96® Tissue DNA Kit (Omega Bio-tek, cat no: D1196-01), including RNase A treatment. HRM results were analysed in plink v1.07 for the F2 hybrid cross (Purcell et al., 2007). Allelic states were given (1,1 for SS; 1,2 for heterozygotes; and 2,2 for NN). As virtually all individuals in the family were sampled, association of loci with diapause was tested under an additive model (logistic), using family structure information. A distribution of 100,000 permutations was then tested against the result using the added options of label swapping and gene dropping. SM: scripts contain the exact commands used. The association test compares allele frequencies between the direct and diapause groups, and permutation-adjusted p-values were used to infer significance. HRM results of the F1 hybrid females and backcrosses were analysed in jmp 12.0.1 (SAS) using a generalized linear mixed model (GLMM) assuming a binary response for larval development (diapause >40 days, or direct <40 days), and a logit-link function using Firth bias-adjusted estimates. GLMM model selection was performed using stepwise backward elimination, starting with all five candidates and sex as dependent variables. A GLMM was performed here instead of a permutation test, since the individuals were chosen from the tail ends of the distribution, rather than sampling the full crosses.
2.12 SNP genotyping in a cline
The candidate loci were also assessed using the same HRM protocol in three additional Swedish populations to investigate any possible latitudinal patterns. The DNA of these samples is the same as was used in a microsatellite study of population variation (Tison et al., 2014), where all individuals of these populations were collected in 2010 and 2011. Sample numbers below refer to those used in the previous study: 24–39 for Öland, 41–56 for Gotland and 64–79 for Stockholm (N = 16 per population).
2.13 F1 hybrid pool-seq
In order to investigate whether any loci that were not tested in the HRM genotyping were segregating between diapausing and directly developing F1 hybrids, in the absence of a hemizygous Z chromosome background, a genome scan was performed on F1 hybrid males. DNA was extracted from the thoraces of 22 directly developed, and 16 diapause generation adult F1 NS males, pooled at equal concentrations, and sequenced as described above. Read mapping, variant calling and FST calculation in 5-kb windows were performed as described for the pure populations above, with the amendment that RD filtering was performed between RD 20 and 70, corresponding to the 97.5% percentile for these data sets.
3 RESULTS
3.1 Genome assembly
We first generated a 501-Mbp draft genome for P. aegeria with an N50 of 15,077. This was then scaffolded and annotated using the proteome from the monarch butterfly, D. plexippus (Zhan & Reppert, 2013), increasing the N50 to 32,208, and producing 11,538 gene models (78% of the D. plexippus genes; Supporting information Tables S1 and S2). busco analysis revealed 66.1% complete arthropod busco genes in a single copy and complete state, which is similar to other butterfly genomes that have been published (B. mori score = 54%, D. plexippus score = 67%, H. melpomene score = 55% (recently tabulated by (Talla et al., 2017)). We then placed >90% of the contigs containing gene models, encompassing 168.7 Mbp of the assembly, in a chromosomal context by exploiting the high level of gene-order conservation among Lepidoptera, using the chromosome-level assembly of the silk moth B. mori (Suetsugu et al., 2013).
3.2 Genomic differentiation between populations
Assessing genomic divergence between two populations allows us to identify regions of differentiation as candidates for local adaptation, which we can query for associations with variation in diapause induction in subsequent population crosses. A pooled sequencing approach was used to quantify the genomic variation in two Swedish populations that differ significantly in their CPP, Skåne (southern) and Sundsvall (northern) (Aalberg Haugen & Gotthard, 2015). This identified 116,809 SNPs (Supporting information Figure S6). Genomewide differentiation between the populations was low, with a mean FST of 0.085 (Figure 1a). Divergent regions (FST >0.9) were found to be scattered across the genome, with a subset of ten regions showing an aggregation of FST outliers, each representing >20 kbp in length (Supporting information Table S4). Note that the identification of the FST outliers does not rely upon the synteny analysis, which is only used here to assess whether or not outliers appear in close proximity to each other or are spread over the genome. Gene-level analysis identified four genes with nonsynonymous substitutions from the SNP analysis that were highly differentiated (FST >0.9), reflecting potential functional divergence (Supporting informationTable S5). We selected 15 candidate loci for further testing based on four different criteria: the number of co-occurring windows with high FST values, absolute FST values, number of SNPs and genes with fixed nonsynonymous substitutions. This was done in order to reduce the bias of only investigating a particular type of signal (Supporting information Table S6). For validation of these divergent regions, we genotyped a representative SNP for each of 15 candidate loci showing the highest differentiation (Supporting information Tables S6 and S7), using all individuals included in the pooled data sets, finding concordance for 93% of the allele frequencies observed in the Pool-Seq analysis (Supporting information Table S8).
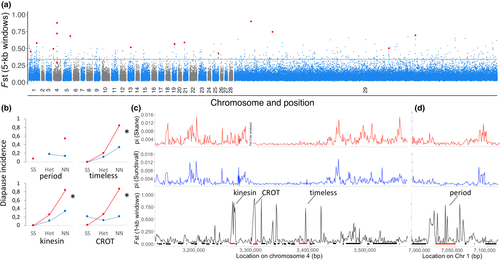
3.3 Association between candidate loci and diapause induction
In order to test whether there was a causal relationship between the 15 outlier loci and diapause incidence, we evaluated the inheritance of diapause induction by generating an F2 cross between the two populations. These crosses were assayed in a controlled environment of 17°C and L:D 18:6, in which the northern population showed a near 100% diapause incidence, while the southern population showed <2% (Table 1, Supporting information Figure S5). The crosses revealed female-specific inheritance of CPP (Table 1), as there is a difference in diapause incidence between the females and the males of this cross, even though these carry the same autosomal background (Supporting information Figure S4). Females are the heterogametic sex in Lepidoptera with a single copy of the Z and W chromosome, while males are homogametic with two copies of the Z chromosome. Thus, the female-specific response indicates a role of the Z chromosome. We then tested the association with the identified candidate loci by genotyping each individual in the F2 cross.
| Cross ♀ × ♂ | Diapause incidence (%) | Combined | Total (N) | |
|---|---|---|---|---|
| ♀ | ♂ | |||
| P: S × S | 2.1 | 0 | 1.6 | 93 |
| P: N × N | 97.8 | 100 | 98.9 | 91 |
| F1: N × S | 14.6 | 59.5 | 34.1 | 85 |
| F1: S × N | 45.4 | 50 | 48.1 | 77 |
| F2: SN × NS | 29 | 13.6 | 21.4 | 70 |
| BC: NS × S | 2 | 1 | 1.5 | 196 |
| BC: S × NS | 8.2 | 2.2 | 5.1 | 1,017 |
There was a significant association with the fixed SNP near the gene kinesin (p < 0.002), as well as the nonsynonymous substitution in the circadian clock gene timeless (p < 0.002), when analysing both sexes together (kinesin is on ms6084 and timeless is on ms6137; Supporting information Table S9). All individuals that developed directly were homozygous southern for both these two loci, while 80% of the females that were homozygous northern entered diapause, as well as 30% of the males that were homozygous northern, with the heterozygous group showing a diapause incidence of 10%–30% (Figure 1b, Supporting information Figure S7). Synteny analysis with B. mori suggests both these genes were located on chromosome 4 (Figure 1c). Investigating the sexes separately showed additional significant loci in females (Supporting information Table S10), but not males (Supporting information Table S11). In females, there was a marginally significant association with the nonsynonymous substitution in the Z-linked circadian clock gene period (p = 0.053), as well as a significant association with the gene carnitine O-acetyltransferase (CROT; p = 0.013; Supporting information Table S10, Figure 1b). Synteny analysis showed the gene CROT to also be located on chromosome 4 (Figure 1c).
3.4 Selection dynamics in candidate genes
We investigated nucleotide diversity, Tajima's D and number of polymorphisms in the significant candidate loci and surrounding regions to assess signatures of selection. The gene carnitine O-acetyltransferase (CROT) showed one amino acid polymorphism, while the genes period and timeless showed multiple amino acid variants (Supporting information Table S5). The genes kinesin, CROT and timeless, are in close proximity in D. plexippus and B. mori. This region, measured as the distance between the two most distant loci, kinesin and timeless, was 81 kbp in D. plexippus and 244 kbp in B. mori, and at least 130 kbp in our P. aegeria genome (Figure 1c). Within this region, there was a reduction in nucleotide diversity that is similar in both populations (South = 0.0023; North = 0.0019) significantly lower than the genomewide distribution of nucleotide diversity (P < 1E−4, Supporting information Figure S8), although nucleotide diversity appeared more variable at kinesin for the southern population. Average Tajima's D for this region (South = −2.34; North = −2.50) was also significantly lower than the genomewide distribution in both populations (P < 1E−4, Supporting information Figure S8), indicating an excess of rare alleles. Additionally, this region harboured 64 fixed SNPs between the N and S populations (31 intergenic, 29 intronic, and four exonic nonsynonymous substitutions: one in kinesin, one in CROT, two in timeless). At the period locus, the window of reduced nucleotide diversity was smaller at 50 kbp, with a mean (South = 0.0016; North = 0.0015) significantly lower than the genomewide distribution in both populations (P < 1E−4, Figure 1d, Supporting information Figure S8), and with a mean Tajima's D (South = −2.31; North = −2.41), again significantly lower than the genomewide distribution of Tajima's D of both populations, indicating an excess of rare alleles (P < 1E−4, Supporting information Figure S8). Within this region, there were five fixed SNPs between the N and S populations (one intergenic, two intronic and two exonic nonsynonymous substitutions). Thus, the size of the region of reduced diversity was nearly three times larger in kinesin/CROT/timeless region compared to that at period, and relative density of fixed SNPs between the N and S populations was higher in the kinesin/CROT/timeless region with five SNPs per 10 kb, compared to one SNP per 10 kb at period.
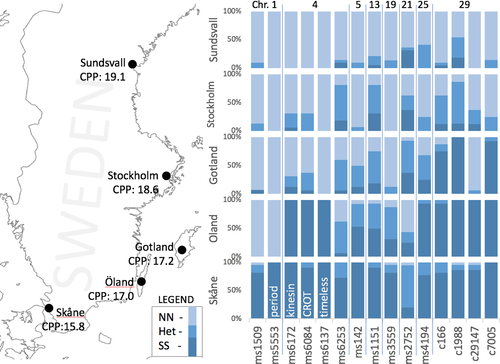
3.5 Latitudinal cline of candidate genes
We further investigated the shift in allele frequencies using the two previous populations studied above, and an additional three populations along a Swedish latitudinal cline that show variation in CPP. In the majority of the investigated genes, we found heterozygous individuals in several of the populations. In contrast, there was an abrupt shift in allele frequencies at timeless and period, from both being N homozygotes to both being S homozygotes, with an absence of heterozygous individuals in the cline (Figure 2). These shifts in allele frequencies were not completely matched between the two loci across the entire cline, as the Öland population was fixed for the southern allele at the timeless locus while being fixed for the northern allele at the period locus. Furthermore, the allele frequency shifts along the sampled cline do not fully match the gradient in CPP in the remaining populations. This is observed in the three northern populations, which exhibit northern genotypes for both loci but have clear differences in CPP as estimated in a previous study (Aalberg Haugen & Gotthard, 2015). This incongruence between the binary pattern of the timeless and period genotypes in the wild populations and the continuous cline in CPP suggests the action of additional loci in determining local CPP thresholds.
3.6 Effect of other loci
In order to investigate the presence of additional loci on CPP, we investigated genomic effects on CPP using an F1 cross of the two populations, wherein fixed variants between populations are in a heterozygous state. Thus, searching for genomic differences between diapausing and directly developing individuals in these F1 hybrids allows for the exploration of dominance or the effects of other loci not fixed between the populations. The F1s showed a sex-linked inheritance (Table 1). SNP genotyping confirmed this by revealing a highly significant association with Z-linked period (p = 0.008), reflecting that 90% of diapausing females carried only the N allele, compared to 44% of direct developing females (Supporting information Figure S9, Supporting information Table S12). Roughly 50% of males entered diapause (Table 1), and as they are heterozygotes at all loci fixed between populations, this indicated an absence of dominance effects at the previously tested loci. To identify whether any other loci exhibited associations with the induction decision, we scanned the genome for associations with diapause induction by generating two Pool-Seq data sets, one for the diapausing and one for direct developing group of males in the N × S F1 cross. The Pool-Seq analysis revealed low genomewide differentiation (FST = 0.031; Supporting information Figure S10), and an absence of even moderately differentiated outliers (highest FST = 0.18). FST values for the individual SNPs that were significant in the genotyping of previous crosses were <0.017 for period, <0.025 for kinesin, <0.012 for CROT, and <0.036 for timeless, reflecting that all males in this Pool-seq comparison were heterozygous for these loci.
Next, in order to investigate the possibility of additional population-specific loci, we generated an F2 backcross, wherein F1 hybrids of both sexes were crossed with a southern population individual. We expected loci with homozygous southern alleles to have a larger effect than loci with northern alleles in heterozygous states based upon our F1 cross results. Consistent with this, we found a low diapause incidence, with an effect of the predominantly southern background of the individuals in both crosses (Table 1). We genotyped the Z-linked locus period, and autosomal loci timeless, CROT, vasa, and trypsin in the SS × NS backcross, wherein females had either a S or a N Z chromosome. While there was a significant effect of sex, there were no significant associations with diapause at of any of the tested loci in this backcross (Supporting information Tables S13 and S14). This indicates that population-specific loci override the effects of a Northern Z chromosome.
4 DISCUSSION
Here, we assessed the genetic variation underlying clinal variation in CPP in wild populations of the butterfly P. aegeria, as well as the selection dynamics in candidate regions that associated with the diapause induction decision. We found genetic differentiation and evidence of local adaptation between populations that differ in their photoperiodic thresholds for diapause induction. We showed that the inheritance of CPP in crosses of these populations arises from both a sex-linked and autosomal component. Importantly, we were able to significantly associate several loci with this diapause induction decision on both the sex chromosome and autosomes, representing the sex-linked gene period and the autosomal kinesin/CROT/timeless region, likely at the gene timeless. These gene regions appeared to have a large effect size on the diapause incidence in F2 crosses, showing up to 80% difference in diapause incidence in individuals being northern versus southern homozygous for timeless. Still, the genotyping of additional populations along a latitudinal cline of CPP, as well as analyses of F1 and F2 backcrosses, strongly suggests the presence of additional genetic factors on diapause induction with effects that are smaller than we can detect in this study. In conclusion, we found evidence of both loci of large and small effect size underlying latitudinal variation in CPP, consistent with an exponential model of local adaptation (Kawecki & Ebert, 2004; Orr, 2000).
4.1 Genetic differentiation associating with diapause induction
The low level of differentiation found in this study between the southern and northern populations is in concordance with previous microsatellite work on these populations (Tison et al., 2014), reflecting their shared recent ancestry or ongoing gene flow (Kawecki & Ebert, 2004). In contrast to this, we also found regions exhibiting high levels of divergence. These could arise due to methodical artefacts (Schlötterer, Tobler, Kofler, & Nolte, 2014), as well as neutral or selective evolutionary events. Several of these regions of divergence showed significant associations with diapause induction, and represented both the Z chromosome and the autosomes, consistent with the finding of sex-linked and autosomal components affecting diapause induction in several previous studies (Chen et al., 2014; Fu et al., 2015; Hagen & Scriber, 1989; Ikten et al., 2011; Pruisscher et al., 2017; Rockey et al., 1987), including this species (Nylin et al., 1994).
4.2 Support for two regions
The narrow regions of divergence we observed in the FST analysis identify several candidate genes. The F2 hybrid cross allowed us to test the hypothesis that these outliers associated with diapause induction and identified two large-effect loci. In the case of the period locus, it is the only gene present inside the boundaries of the 50-kbp region of low nucleotide diversity and high FST, and it exhibits a lack of heterozygotes in the clinal samples. In the larger kinesin/CROT/timeless region, there are several genes present, making it challenging to distinguish between selection acting upon one or several of these loci. However, the samples from the latitudinal cline show that there is recombination between the tested loci, as the allele frequencies of the kinesin and CROT genes are not identical, or linked, within each population. Furthermore, there is a lack of heterozygous individuals for timeless. This suggests selection against heterozygotes at timeless, but with free recombination between northern and southern alleles in the kinesin/CROT region across the geographic cline. Consistent with this, we see higher nucleotide diversity at kinesin than CROT, which is in line with the former being more distant from timeless.
4.3 Selective sweep at two regions
The size and pattern of nucleotide diversity and negative Tajima's D values at the large-effect loci of period and kinesin/CROT/timeless are consistent with a signature of a selective sweep. A negative Tajima's D indicates that rare alleles are present at a higher than expected frequency. This could result from a recent selective sweep, linkage to a sweep, but also an expansion of the population after a recent bottleneck. As the entire genome is affected by demographic size changes, while regions under directional selection should be few in the genome, our finding of period and kinesin/CROT/timeless loci being significant outliers compared to the genomewide distribution is consistent with a selective sweep in these regions. Further empirical evidence or population genetics modelling is required to confirm the age and strength of these selective sweeps, ideally in combination with results from other systems. Finally, these insights should be interpreted with caution, given our shallow sampling of the site frequency spectrum in our Pool-Seq data sets. However, the pooling of our high-quality DNA should provide high-quality Illumina libraries and therefore proper sampling of our pools. Empirical support for this is seen in our FST results and permutation analyses that document low error in our estimation of allele frequencies within populations (since genomewide low FST arises only because of similar allele frequency estimations in the compared populations).
4.4 Evidence for additional loci in the latitudinal samples
While the F2 hybrid crosses identify large-effect loci, these loci neither fully control the diapause induction decision across populations, nor is their magnitude of effect consistent across populations. This is evident in several comparisons of the genotypes at these loci along our sampled cline for which CPP is well characterized (Aalberg Haugen & Gotthard, 2015). First, while the change in CPP among three northernmost populations is nearly two hours, all of these three populations have identical allele frequencies at period and timeless, and nearly identical frequencies at the neighbouring loci of timeless (kinesin, CROT) (Figure 2). Second, despite minimal difference in CPP between the population from Öland and Gotland, there is a complete switch between homozygous southern and homozygous northern timeless, no change at period, and a moderate shift in allele frequency at kinesin and CROT. Thus, neither variation at the period nor timeless locus alone fully controls local CPP, yet in distant population crosses they have large effects. Therefore, the response of CPP to selection over a latitudinal gradient appears to involve the loci investigated here at a relatively large geographic scale, but a significant part of the finer scale response must also be due to variation segregating at other loci. To what extent our other identified outlier loci are involved in these local decisions remains to be determined. The pattern found here is similar to findings in D. melanogaster, where different timeless variants in a latitudinal cline affected photoperiodically induced quiescence, which was modified by the genetic background (Tauber et al., 2007). The relationship between photoperiodically induced quiescence and diapause is intriguing given their similar clinal patterns (Pegoraro et al., 2017).
4.5 Evidence for additional loci in further hybrid crosses
The F1 hybrids and backcrosses further support the notion of additional loci acting on diapause induction. In the F1 hybrids, there is a strong sex-linked effect at the period locus, although in this cross period is a marker for an entire N or S Z chromosome. In contrast, our additional Pool-seq analysis of F1 hybrid males did not identify any particular genomic region that was strongly associated with the difference in diapause induction. All these males were heterozygous at the two major-effect gene regions, and we could not identify any dominance effects at these two loci. Consequently, the lack of high FST outliers between the diapause and direct development groups of F1 hybrid males suggests that any additional genetic factors influencing diapause induction have effects that are smaller than we can detect in this study.
The importance of other genetic factors is further corroborated by the results of the backcross, as the predominantly southern genetic background (~75% on average) overrides any Z-linked effect of a northern period, producing a predominately southern diapause response. Importantly, sample sizes here were larger than previous crosses, indicating that statistical power was sufficiently high. An alternative explanation could be that the photoperiod used is too far from the CPP of this backcross “population” and that an assay closer to the CPP of the southern population (≈16 hr daylength) would have been more informative. In combination, these findings suggest that the genomic architecture of local adaptation of CPP is variable and context dependent. While we discovered a number of loci with larger effect sizes, our results also suggest that there is considerable additional genetic variation present within and among populations that affect the CPP. Therefore, our study implies that the genetic architecture of CPP in P. aegeria should facilitate an evolutionary response to seasonal changes, such as predicted by climate change, should these changes be consistent for sufficiently long periods of time. Evolutionary responses of diapause induction to changing environmental conditions have been observed in other species (Bradshaw & Holzapfel, 2001).
4.6 Circadian clock genes and diapause induction
While there have been targeted candidate gene studies that investigated the connection between photoperiod and the circadian clock genes (Tauber et al., 2007), it is striking that performing a genomewide scan for genetic variation that influences CPP also revealed that allelic variation at period and timeless had the most significant effects. The products of these two genes directly interact with each other as part of the circadian clock by forming a heterodimer that together with mammalian-type cryptochrome affect the transcription of clock components (Goto, 2012; Yuan, Metterville, Briscoe, & Reppert, 2007). Studies have tested the association between photoperiodic induction of diapause and the circadian clock, predominantly due to the influence of the controversial Bünning hypothesis, which posits that photoperiodic timing is linked to the circadian timing mechanism (Bünning, 1936). However, the overall support for the hypothesis is inconclusive as several studies indicate an association between the clock genes and photoperiodism (Ikeno et al., 2010; Meuti et al., 2015; Pegoraro, Gesto, Kyriacou, & Tauber, 2014; Yamada & Yamamoto, 2011), while others have found these to be independent (Emerson et al., 2009).
It is possible that the amino acid changes found in the period and timeless genes affect the stability of their interaction with each other, which would affect the circadian clock by potentially slowing down or speeding up the circadian clock. Even if this were true, whether it is the circadian clock as a unit that is involved in photoperiodic induction in P. aegeria or the pleiotropic function of the clock genes remains an open question. Functional validation of the effects arising from the natural allelic variation observed here is challenging, as RNAi-knockdown and CRISPR/Cas knockouts of circadian clock genes often have deleterious or uninformative phenotypes for such questions. Here, by integrating whole-genome scans for local adaptation with phenotyping and targeted genotyping in population crosses, we demonstrate that loci underlying adaptive variation in seasonal life cycle regulation conform to an exponential model of effect sizes, and may be strongly influenced by allelic variation at few genes with known functions in the circadian clock. Gaining such insights into the genetic variation underlying clines of diapause induction from a larger number of species will significantly advance our understanding of the evolutionary history and dynamics of this complex phenotype.
ACKNOWLEDGEMENTS
The authors would like to acknowledge support from Science for Life Laboratory, the National Genomics Infrastructure, NGI and Uppmax for providing assistance in massive parallel sequencing and computational infrastructure. Funding was provided by the Swedish Research Council (VR grant numbers 2012-3715, 2010-5341, 621-2012-4001), Academy of Finland (grant number 131155), the Bolin Centre for Climate Research at Stockholm University and the Knut and Alice Wallenberg Foundation (grant number 2012.0058). We thank Philipp Lehmann for providing comments on earlier versions of this manuscript. We are very grateful for the hardwork of research assistant Beatrice Svensson in rearing and phenotyping the crosses. The authors declare there are no conflict of interests.
AUTHOR CONTRIBUTIONS
P.P., S.N., K.G. and C.W.W. designed research; P.P., K.G. and C.W.W. performed research; P.P. analysed data; and P.P., K.G. and C.W.W. wrote the manuscript.
DATA ACCESSIBILITY
All Illumina sequencing data, consisting of the two population samples and the F1 hybrid male diapause, and nondiapause groups are available in the NCBI BioSample database (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA484116). The genome assembly is available at the European Nucleotide Archive (ENA) at http://www.ebi.ac.uk/ena/data/view/PRJEB28004.




