Does pollution influence small mammal diet in the field? A metabarcoding approach in a generalist consumer
Abstract
Mammals are mainly exposed to trace metals (TMs) via consuming contaminated food. Several studies have demonstrated relationships between metal concentrations in food and in animal tissues. However, potential effects of TMs on feeding behaviour of wildlife have been poorly documented under field conditions, despite experimental evidence showing that food selection is impacted by resource contamination. Here, we test the hypothesis that the diet of a generalist rodent, the wood mouse (Apodemus sylvaticus), is altered by soil TM contamination in the field. Wood mice were sampled in spring and in autumn along a gradient of soil contamination in the surroundings of a former smelter located in northern France. Available resources in the field were inventoried, and the diet of the animals was analysed using DNA “metabarcoding.” We demonstrated that (a) relationship between the resource richness in the diet and their richness in the field was altered by soil metal contamination. Wood mice specialized their diet along the gradient of soil metal contamination for both plant and invertebrate resources in spring. We also showed that (b) preference for Salicaceae, a plant family accumulating metals, decreased when soil contamination increased. These results suggest that environmental TM pollution could act as a force modulating trophic interactions in terrestrial food webs, thereby affecting wildlife exposure to contaminants by trophic route.
1 INTRODUCTION
Feeding behaviour is determined by life history traits and life stages of animals (Pyke, Pulliam, & Charnov, 1977; Schoener, 1971), abundance and distribution of food resources in nature (Crampton, Longland, Murphy, & Sedinger, 2011; White, 2008), and food quality (i.e., nutrient and toxin content; Butet, 1986a; Kerley & Erasmus, 1991; Lewis, Clark, & Derting, 2001; Schmidt, 2000). All these factors are driven by ecological determinants such as season, climate, community interactions or landscape features (Polis, Anderson, & Holt, 1997; Visser & Both, 2005; White, 2008). The optimal foraging theory assumes that animals consume resources providing high energy intake per unit time (Stephens & Krebs, 1986) and that the diet of animals tends to be specialized when food density is high (MacArthur & Pianka, 1966). However, animals require not only energy but also nutrients such as vitamins or essential metals. The nutrient requirements can thus hardly be satisfied from a single plant species, and thus, animals seek a variety of diet for nutrient balance (i.e., nutrient balance hypothesis; Westoby, 1978). Likewise, Freeland and Janzen (1974) proposed that generalist mammalian herbivores are unable to detoxify large amounts of similar plant secondary metabolites (i.e., those from a single plant species), and thus, a variety of plant sources allow them to avoid similar plant toxin load (i.e., detoxification limitation hypothesis). When the use of a resource is disproportional to its availability in the field, the feeding process by which an animal chooses the resource is called “selection” (Johnson, 1980; Manly, McDonald, Thomas, McDonald, & Erickson, 2002). The nature of selection (i.e., preference or avoidance) reflects the likelihood of a given resource for being chosen when this resource is offered with others (Johnson, 1980). The food selection hence manifests a relationship between individual requirements and resource constraints.
Trace metals (TMs) are environmental pollutants of concern for both human health and wildlife. They occur often as a legacy of past industrial activities. Even if the emissions of many of them have been reduced in the past decades thanks to environmental regulation and technical improvements at least in so-called developed countries, and the pollution still persists over vast surfaces due to nondegradability of metals and to their current emissions in many countries (EMEP, 2013; Kabata-Pendias, 2011). TM pollution may have effects on plants and invertebrates at several levels of organization, from subindividual physiological effects reducing survival or reproduction to the modification of community composition and structure (Walker, Hopkin, Sibly, & Peakall, 2012). For instance, a recent meta-analysis about the responses of terrestrial biota to TM pollution—dealing with 206 pollution point sources worldwide—showed a decrease in diversity for most of the taxa (producers, secondary consumers and decomposers; Kozlov & Zvereva, 2011). In addition, Dazy et al. (2009) demonstrated that plant species richness correlated negatively with TM concentrations in soils in eastern France. Metal pollution can also reduce species richness in earthworm communities (Nahmani, Lavelle, Lapied, & van Oort, 2003; Spurgeon & Hopkin, 1999) and impact the structure and composition of soil macrofauna communities (Nahmani & Lavelle, 2002). Trophic transfer, that is, the transfer of matter via food consumption, is generally considered to be the main route for TMs from the environment to animals of higher trophic levels (Shore & Rattner, 2001; Smith et al., 2007). For small mammals, several studies have demonstrated relationships between soil pollution by TMs, contamination of food and, subsequently, high concentrations of TMs in wild animals’ tissues (Hunter & Johnson, 1982; Hunter, Johnson, & Thompson, 1987a; Rogival, Scheirs, & Blust, 2007; Torres & Johnson, 2001; van den Brink, Lammertsma, Dimmers, Boerwinkel, & van der Hout, 2010). Avoidance of TM-contaminated food can be a mechanism that mitigates organisms’ exposure to contaminants (Gillet & Ponge, 2003). This has been reported for the wood mouse (Apodemus sylvaticus) under laboratory settings but not confirmed in natural conditions (Beernaert et al., 2008). To the best of our knowledge, no study has reported variations of richness in the diet and selection as a response to both the variation of resource availability and the contamination of the environment in natura in terrestrial ecosystems.
One of the main difficulties in assessing wildlife diet is the identification of items actually consumed. Identifying items with the highest taxonomic resolution is almost impossible with classical macro- or microscopy-based methods, which then makes it difficult to match diet with field records of resources. In recent times, DNA-based techniques have arisen as a powerful approach for the taxonomic identification. Although DNA-based identification is not novel, current technological developments such as DNA metabarcoding approach enable to simultaneously identify different taxa in environmental samples, such as soils or faeces, containing multiple specimens and potentially highly degraded DNA (reviewed in Pompanon et al., 2012; Taberlet, Coissac, Hajibabaei, & Rieseberg, 2012; Valentini, Pompanon, & Taberlet, 2009). The efficiency of this approach for analysing the diet has been demonstrated in various species including herbivorous mammals, birds and invertebrates (Soininen et al., 2009; Valentini, Miquel, et al., 2009), insectivorous bats (Bohmann et al., 2011) or omnivorous animals like the brown bear (De Barba et al., 2013).
In this study, we focused on the wood mouse (Apodemus sylvaticus), which is a generalist small mammal (Butet, 1990; Hansson, 1985; Watts, 1968), and therefore a model of choice when studying diet plasticity as a response to environmental variations. Indeed, provided its opportunistic feeding behaviour, the diet richness (i.e., total number of resources’ species in the diet) of the wood mouse is expected to be positively correlated with resource richness in the field. We hypothesized that: (a) Diet richness of the wood mouse would be negatively correlated with TM contamination of soils given the reported negative effects of pollution on plant and invertebrate richness in community, and (b) food selection by the wood mouse would be affected by TM contamination in soils possibly due to avoidance behaviour.
2 MATERIALS AND METHODS
2.1 Study sites
This study was carried out in the surroundings of the former lead (Pb) and zinc (Zn) smelter named “Metaleurop Nord” located in northern France (Noyelles Godault, Hauts-de-France, France). The surrounding soils were highly contaminated by TMs, mainly cadmium (Cd), Pb and Zn, over a large area of about 120 km2 because of metal-contaminated dust released for decades from this smelter and another one named “Umicore,” located about 4 km east of the first (Douay et al., 2008, 2009; Sterckeman, Douay, Proix, Fourrier, & Perdrix, 2002; Waterlot et al., 2016). A study area of 40 km2 was defined and divided into 160 sites of 25 ha (500 m × 500 m; Fritsch et al., 2010), from which seven are used in this study (see below). Total TM concentrations in the soil of woody patches ranged from 0.1 to 236.5 mg/kg of dry soil for Cd, from 16 to 7331 mg/kg of dry soil for Pb and from 44 to 7264 mg/kg of dry soil for Zn in an area of 40 km2 in the surroundings of the former smelter (Fritsch et al., 2010). These values largely exceed background TM concentrations in soils of the region Hauts-de-France (Sterckeman, Douay, Fourrier, & Proix, 2002). Those TM concentrations in woody patches were used in our modelling (cf. below). The seven sites are located along a soil pollution gradient and composed of three types of dominant habitats (woodland, urban features and arable lands) to provide a wide range of resource richness in the field (for details, see Supporting Information Data S1).
2.2 Rodent trapping
At each site, we trapped wood mice in spring (April) and in autumn (September and October 2012) according to Fritsch et al. (2011) and in accordance with current French legislation about ethics and use of animals in research. In brief, small break-back traps were used with peanut as rodent's bait. Ten lines, composed of 10 traps 3 m spaced each, were used per site and per season. All trap lines were set in woody habitats of the sites, and their location was georeferenced. The trap lines were checked in the morning for three consecutive days and reset and/or rebaited, if necessary. After being weighed in the field, animals were immediately frozen at −20°C for further analyses.
Two hundred and forty-six rodents (117 in spring and 129 in autumn; 116 females and 127 males) were used in the study. Age was estimated using body weight according to four classes (approximately, class I < 50 days < class II < 130 days < class III < 230 days < class IV), referring to Tête et al. (2014) and Vandorpe and Verhagen (1980). Age structure differed between seasons: No mouse of age class I was found in spring, and the proportions of animals in the other three classes were significantly different (χ2 = 31.04, p-value < 0.001) such that animals in spring were older than in autumn.
2.3 Stomach content samples
Consumed items were identified by biomolecular analysis of stomach contents (SCs). Wood mice were thawed at room temperature. Their SCs was extracted from the body with a spatula, which was thoroughly cleaned with disposable tissue, then washed with ultrapure water (18.2 MΩ/cm2) and wiped off with other tissue between each extraction. Remaining bait was removed from SCs. Each SCs was then homogenized and split into two aliquots: One (about 10 mg) was stored in 95% ethanol for metabarcoding analysis (see below), and the other was reserved for another study. For 13 mice among the 246 rodents, faeces taken from intestines were used as an alternative material for food identification because their stomach was empty. The 13 mice were equitably distributed according to the explanatory factors tested in this study.
2.4 Metabarcoding analysis
The DNA extraction, amplification and the PCR purification were performed at SPYGEN facilities (www.spygen.com). DNeasy Blood and Tissue Kit (Qiagen GmbH) was used for extracting total DNA, following the manufacturer's instruction. DNA amplification was performed with three sets of primers (Table 1): The P6 loop of the chloroplast trnL (UAA) intron g/h (Taberlet et al., 2007) was used for identifying general plant species; primer targeting a short fragment of mitochondrial 16S gene (16S mtDNA) was used for arthropods and molluscs DNA (16SMAV-F/16SMAV-R; De Barba et al., 2013); and primer targeting short region of 16S mtDNA for earthworms (ewD/ewE; Bienert et al., 2012). PCR amplifications were carried out on Applied Biosystems Veriti 96 Wells (Life Technologies). The amplification was realized in a final volume of 25 μl using 3 μl of DNA extract. Two PCR replicates were performed per each sample. For the amplification of arthropod and mollusc DNA, 2 μM of a blocking primer for mammal's DNA (MamMAVB1; De Barba et al., 2013) was added in the PCR mix. The amplification mixture contained 1 U of AmpliTaq Gold DNA Polymerase (Applied Biosystems), 10 mM of Tris-HCl, 50 mM of KCl, 2.5 mM of MgCl2, 0.2 mM of each dNTP, 0.2 μM of group-specific primers, 0.2 μg/μl of bovine serum albumin (Roche Diagnostic) and ultrapure water (18.2 MΩ/cm2) to bring each sample to the final volume. The mixture was denatured at 95°C for 10 min, followed by 45 cycles of 30 s at 95°C, 30 s at 50°C for trnL-g/h, at 55°C for 16SMAV-F/16SMAV-R and at 58°C for ewD/ewE and 1 min at 72°C, followed by a final elongation at 72°C for 7 min. Extraction and PCR negative controls were analysed in parallel to monitor potential contamination. After amplification, the samples were titrated using capillary electrophoresis (QIAxcel; Qiagen GmbH) and purified using a MinElute PCR Purification Kit (Qiagen GmbH). Before sequencing, purified DNA was titrated again using capillary electrophoresis. The purified PCR products were pooled in equal volumes, to achieve an expected sequencing depth of 10,000 reads per sample. Libraries were prepared using TruSeq Nano DNA genomic kit (Illumina), and a pair-end sequencing (2 × 100 bp) was carried out with an Illumina HiSeq sequencer (Illumina) using TruSeq SBS Kit v3 (Illumina) following the manufacturer's instructions. Library preparation and sequencing were performed at Fasteris facilities (www.fasteris.com).
| Taxonomic group | DNA type | DNA region | Primer name | Primer sequence 5′–3′ | Reference |
|---|---|---|---|---|---|
| Plants | Chloroplast | trnL (UAA) | g (forward) | GGGCAATCCTGAGCCAA | Taberlet et al. (2007) |
| h (reverse) | CCATTGAGTCTCTGCACCTATC | ||||
| Arthropods and molluscs | Mitochondrial | 16S mtDNA | 16SMAV-F | CCAACATCGAGGTCRYAA | De Barba et al. (2013) |
| 16SMAV-R | ARTTACYNTAGGGATAACAG | ||||
| Earthworms | Mitochondrial | 16S mtDNA | ewD (forward) | ATTCGGTTGGGGCGACC | Bienert et al. (2012) |
| ewE (reverse) | CTGTTATCCCTAAGGTAGCTT |
Reads were handled by the software mothur pipeline (Schloss et al., 2009). In brief, forward and reverse reads were assembled in contig sequences. Then, sequences were filtered based on length (20–90 bp for trnL-g/h; 36–38 bp for 16SMAV; 69–81 bp for ewD/ewE), homopolymer (<10 nucleotides) and no ambiguous nucleotides. After dereplication (occurrence count of each different sequence), only unique sequences with a minimum count of 10 (sum of all samples) were used for further analysis.
2.5 Field inventory of plant and invertebrate resources
Vegetation survey was realized once per site from June 4 to September 5, 2012. Three different strata were defined based on height: tree stratum (woody species >8 m high), shrub stratum (woody species < 8 m high) and herbaceous stratum. Taxa were identified at species level in the field. Nomenclature of species followed Lambinon, Delvosalle, and Duvigneaud (2004) and Dudman and Richards (1997). Cover-abundance of vascular plant species was visually estimated as the vertically projected area following Braun-Blanquet, Roussine, and Nègre (1952). Vegetation habitats were determined by plants’ composition of one or more strata and delineated as polygons with the aid of aerial pictures. The polygons were georeferenced and digitalized using the qgis software (http://www.qgis.org/). We listed 236 different plant taxa in the field (see Supporting Information Data S1 for the taxonomic richness in the field per site and Supporting Information Data S2 for the list of the taxa and their abundance per site).
Invertebrate fauna was sampled at the same time as the rodent trapping (i.e., in the two seasons). Ground-dwelling invertebrates were captured near rodent traps using three pitfall traps per line. Flying invertebrates were captured with yellow pan traps containing a mix of soap and water. Four yellow pan traps were set in each site. Yellow colour attracts especially flower-visiting insects. The traps were checked every morning for three consecutive days. All traps were georeferenced. Captured invertebrates were conserved in ethanol or in freezer at −20°C and identified to the highest possible taxonomic resolution by morphological characteristics (the main references are Coulon, 2003; Forel & Leplat, 2001; Jeannel, 1941; Trautner & Geigenmueller, 1987). Captured ground-dwelling and flying invertebrates consisted of 88 and 95 taxa, respectively (see Supporting Information Data S1 for the taxonomic richness in the field per site and Supporting Information Data S2 for the list of the taxa and their abundance per site). Because of the different taxonomic resolution, we used mainly family level (and higher levels) for invertebrate richness in the field (cf below): 24 and 95 families (or higher) in ground-dwelling and flying invertebrates, respectively.
We then estimated available resources for wood mice near their trapping location. Although notion of “availability” is distinct from “abundance” in selection studies (Manly et al., 2002), we considered them equivalent for wood mice given its generalist diet behaviour. Plant resource availability was estimated by the cover-abundance of each plant species found within a buffer of 10 m around trap lines (referred to as “buffer” thereafter). This buffer is equivalent to an area of about 1,000 m2, considered as the average size of wood mouse vital domain (Quéré & Le Louarn, 2011). We considered the data set collected between June and September to be representative of food availability in spring and autumn. As the traps used to capture invertebrates (pitfall trap and yellow pan trap) were inappropriate for catching earthworms and molluscs, both earthworm and mollusc richness in the field were unknown. Resource availability of ground-dwelling invertebrates in buffers both in spring and in autumn was estimated by the abundance of each taxa found per trap line (sum of the three traps per line), whereas availability of flying invertebrates was based on abundance in the yellow pan trap nearest to the trap line.
2.6 Diet diversity analyses
2.6.1 Sequence data for diet diversity analyses
Diet diversity analyses were performed by employing molecular operational taxonomic units (MOTUs): Groups of DNA sequences were clustered according to similarity, which are thus independent of any reference database (Sun et al., 2012). Sequences were clustered for each primer with average neighbour algorithm using Needlman–Wunsch distance. Distances cutting clusters were arbitrarily chosen as 0.032 for sequences obtained from primer trnL-g/h, as 0.042 for 16SMAV and as 0.034 for ewD/ewE (see Supporting Information Data S3 for sequence data of plant, arthropod and earthworm MOTUs). Deagle, Thomas, Shaffer, Trites, and Jarman (2013) showed that the number of sequences obtained from degraded DNA is likely not proportional to the biomass of each taxa really eaten. The quantitative sequence information data were therefore converted into the presence/absence of MOTUs using an occurrence threshold of 100 to remove background sequencing data.
2.6.2 Diet richness and modelling
Richness in the diet of each mouse was calculated as the total number of MOTUs per mouse by three types of food: plant, arthropod and mollusc (referred to as “arthropod” thereafter) or earthworm. We assumed that plants potentially consumed by the invertebrates would not artificially increase the number of plant MOTUs consumed by the rodent (i.e., there may be no secondary predation; for detail, see Harwood, Phillips, Sunderland, & Symondson, 2001) because the number of plant MOTUs was not significantly different between SCs containing or not containing invertebrate food (arthropod and/or earthworm; data not shown). The diet richness per mouse was compared among seasons, wood mouse sexes and age classes, and landscape features using the nonparametric Wilcoxon–Mann–Whitney (for comparison of two categories) or Kruskal–Wallis tests (for more than two categories), due to skewed distribution of MOTUs data. Frequency of occurrence was also compared by chi-square goodness-of-fit test.
Relationship between diet richness, richness in the field and TM concentrations in soil was assessed by statistical modelling. To estimate the richness in the diet of all mice in a buffer, the total number of MOTUs of all mice captured over a given buffer (referred to as “diet richness” thereafter) was calculated, per type of food or as total diet richness by summing plant, arthropod and earthworm MOTUs. The relationship was analysed using multimodel inference on the basis of the information-theoretic approaches (Burnham & Anderson, 2002). A set of candidate models was established by combinations of several explanatory variables. Second-order Akaike information criterion (AICc: corrected AIC for small sample size in relation to the number of model parameters) was used for ranking the proposed models. Probability of the “best” model over the set was estimated by Akaike weight (w). In situations where the best model was uncertain (i.e., w of the first ranked model < 0.9), we applied a model averaging approach for reducing model selection uncertainty. A confidence set of candidate models, whose ΔAICc < 6 (difference of AICc from the first ranked model < 6, which is equivalent to a likelihood ratio from the first ranked model >0.05), was reselected, and each parameter of those reproposed models was averaged by recalculated Akaike weight (Burnham & Anderson, 2002). When a given parameter was not included in a proposed model, the value of the parameter was considered as 0 in this model (Burnham & Anderson, 2002; Lukacs, Burnham, & Anderson, 2010).
All candidate models were established as generalized linear models (GLM) with logarithm link function for Poisson distribution. Models were built separately per season, given potential seasonal diet variation of the animal (Abt & Bock, 1998; Butet, 1986b), as well as per TM, considering their different effects on organisms and transfer pathways (Eisler, 2000). We used the diet richness in buffer as response variable and number of analysed mice per trap line as one of the explanatory variables. Soil TM concentrations of woody patch characterized by Fritsch et al. (2010) were applied to buffer set in or near a given woody patch. Mice of age class I were discarded from modelling because diet of juveniles could differ from adults (Montgomery & Montgomery, 1990; Watts, 1968). The modelling was carried out on 38 buffers in spring and 37 buffers in autumn. Number of mice used in the analysis was 181, and their number per line ranged from 1 to 6.
For plant richness in the diet, combinations of three variables and one interaction were considered as candidate models: number of mice, TM concentrations in soils of woody habitat (referred by the data of Fritsch et al., 2010; see “Study site” above) with a natural logarithm transformation and plant richness in the field, as well as an interaction between the TM concentrations and the plant richness in the field (i.e., 10 models including the intercept-only model). For arthropod richness in the diet, four variables and two interactions were used: mouse number, TM concentrations, both ground-dwelling and flying invertebrate richness in the field, as well as two interactions between the TM concentration and the richness in the field (i.e., 26 models). For earthworm richness in the diet, we used only two variables: mouse number and TM concentrations (i.e., four models). For total diet richness, we used three variables and one interaction: mouse number, TM concentrations and total richness in the field (sum of plant and both ground-dwelling and flying invertebrate richness at family level), as well as interaction between the TM concentrations and the total richness in the field (i.e., 10 models). Overdispersion (observed variance higher than theoretical one) was checked according to Cameron and Trivedi (1990). Explanatory value of our final models was estimated by deviance R2 (R2D: proportion of deviance explained by the given model; Zuur, 2009).
2.7 Food selection analyses
2.7.1 Sequence data for selection analysis and association with field survey data
Reference sequences of the species recorded in our study area were taken from the GenBank sequence database (www.ncbi.nlm.nih.gov/genbank). Each sequence extracted from SCs (i.e., query sequence) was compared to the reference sequences for checking their similarity, where we accepted difference of only one nucleotide. When query sequences could match with several reference sequences, they were gathered into one group composed of the corresponding species, called “sequence group (Grp)” (see Supporting Information Data S4 for details of sequence groups). Nonassigned query sequences were excluded. Query sequences with occurrence lower than 100 were not considered. Food Grp data were finally converted into the presence/absence. We represented Grps using both ID number and family name(s) corresponding to their component species. We applied such method only to plant food because almost all invertebrate resources in the field were identified to family level only.
2.7.2 Plant food selection and modelling
Frequency of occurrence of each plant food item (i.e., percentage of number of occurrences of a given item compared to the total number of occurrence of all items, referring to Klare, Kamler, & Macdonald, 2011) was calculated at family level using Grp. Food selection was assessed, for each plant food item, per season, comparing the frequency of occurrence and relative proportion of resources in the field (i.e., cover-abundance of a given plant food of all strata compared to the total cover-abundance of all plants of all strata). Plant resource taxa in the field were gathered into corresponding Grp and then into the same plant family. Resource availability was estimated based on the abundance of the plant families in each buffer, summed up per family for all mice in each season. Significance of food selection was evaluated per plant family using Bailey's confidence intervals at p-value < 0.05 (Bailey, 1980; Cherry, 1996): When relative availability of a given resource was located below or above the confidence interval of its frequency of occurrence, this resource is considered as preferred or avoided, respectively.
Preference change according to richness in the field and/or TM concentrations in soil was then assessed by multimodel inference approaches. First, food selection was estimated in each buffer as described above. Buffers where selection for a given resource was observed were considered 1 (i.e., selection for the resource is present), whereas the other buffers were considered 0 (i.e., selection for the resource is absent). We used GLM with logit link function for assessing change of proportion of buffers where preference was observed. There was only one plant family (Salicaceae) for which selection was significantly observed in more than five buffers, allowing statistical analyses. Salicaceae was preferred in spring in six among 38 buffers. Candidate models were established by combinations of three variables and one interaction: mice number in buffer, plant richness in the field and TM concentrations, as well as an interaction between the richness in the field and the TM concentrations. The modelling was performed for each TM. Model selection methods were the same as described above.
All statistical analyses were computed using the statistical software r (ver. 3.4.2; R Development Core Team). GLM was handled by “glm” function in “lme4” package and model averaging by “model.avg” function in “mumin” package. Overdispersion checking was carried out by “dispersiontest” function in “aer” package.
3 RESULTS
3.1 Resources richness in the field, trace metals and diet richness
Plant items occurred in the diet of 96.3% of wood mice, arthropod items in 65.9% and earthworm items in 29.3%. The occurrence of earthworm items was significantly lower in forest landscape and significantly higher in urban landscape (χ2 = 7.619, p-value = 0.022; for details see Supporting Information Data S5). The variations of the total richness in the diet and of the relative proportion of the three food item categories (plants, arthropods and earthworms) along the soil contamination gradient are shown in Supporting Information Data S6 and S7, respectively.
Number of plant MOTUs per mouse ranged from 0 to 7, and median was 2 MOTUs. It was significantly higher in autumn than in spring (W = 5394.5, p-value < 0.001). Number of arthropod MOTUs ranged from 0 to 9, and median was 1 MOTUs. In autumn, the number was significantly lower in age class I than in class II (Kruskal–Wallis χ2 = 7.972, p-value = 0.047; N.B. no mouse of class I was found in spring). Number of earthworm MOTUs ranged from 0 to 3, and median was 0 MOTUs. The number was significantly higher in urban area than in forest area in spring only (Kruskal–Wallis χ2 = 8.068, p-value = 0.018; see Supporting Information Data S8).
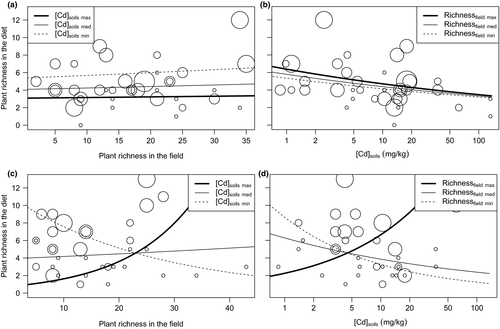
No single “best” model was evidenced, and thus, model averaging was applied for all sets of models. Complete results of modelling are shown in Supporting Information Data S9. The final model for plant richness in the diet in buffer included mouse number, soil TM concentrations, richness in the field and the interaction between the last two for the three TMs in the two seasons. Figure 1a,b shows plant richness in the diet according to plant richness in the field and Cd concentration in soils in spring, respectively. The pattern was similar for the other metals. Influence of richness in the field on the diet richness was tiny in spring (Figure 1a), whereas diet richness decreased according to TMs in soils regardless of the richness in the field (e.g., from 6 to 4 plant MOTUs along the range of TM concentration; Figure 1b). R2D was 0.301, 0.313 and 0.307 for Cd, Pb and Zn, respectively. Such tendency changed in autumn (Figure 1c,d for Cd, similar for other TMs). Diet richness was negatively correlated with plant richness when soil contamination was low, while this correlation disappeared, or even reversed, when TM concentration increased (Figure 1c). A similar tendency was predicted for the relationship between diet richness and TM contamination in soils (Figure 1d): Diet richness was negatively correlated with TM concentration when plant richness was low and positively correlated when the plant richness was high. R2D was 0.624, 0.492 and 0.601 for Cd, Pb and Zn, respectively.
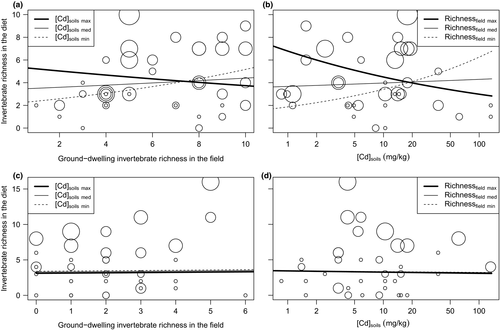
The model for invertebrate richness in the diet included all proposed explanatory variables in the two seasons, except the interaction between TMs in soils and richness of ground-dwelling invertebrates for the models for Cd and Zn in autumn. In spring, arthropod richness in the diet increased according to richness of both ground-dwelling and flying invertebrates when soil contamination was low. This correlation disappeared and then reversed, when TM concentration in soils increased. For example, our model for Cd predicted that arthropod MOTUs increased from 2 to 5 along the gradient of ground-dwelling invertebrate richness in the field when Cd concentration in soils was low, whereas arthropod MOTUs decreased from 5 to 3 when Cd concentration was high (Figure 2a; the pattern was similar for flying invertebrates). The relationship between arthropod richness in the diet and TM concentration in soils followed the same tendency (Figure 2b). In autumn, arthropod richness in the diet was explained almost only by mouse number. Neither TM concentration in soils nor richness of both types of invertebrates had sensible influence on diet richness (Figure 2c,d). R2D for Cd, Pb and Zn was, respectively, 0.502, 0.472 and 0.495 in spring and 0.495, 0.504 and 0.497 in autumn.
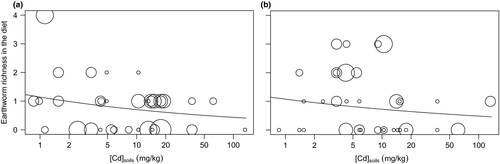
The averaging model for earthworm richness in the diet included mouse number and TM concentrations in soils in both seasons. Earthworm richness in the diet slightly decreased along TM gradient (e.g., difference of about 0.5 MOTUs; Figure 3a,b), and mouse number on trap line had a larger influence in autumn than in spring. R2D for Cd, Pb and Zn was, respectively, 0.101, 0.056 and 0.075 in spring and 0.169, 0.158, 0.192 in autumn.
3.2 Plant food selection
We found 48 Grps in the food of the 246 mice. Sapindaceae, Salicaceae, Cornaceae, Adoxaceae, Asteraceae and Rosaceae were plant taxa frequently eaten in our area (see Supporting Information Data S10 for details). Selection for some plant families was observed at the scale of all study sites together and at the two seasons: preference for Fagaceae and avoidance of Urticaceae. Furthermore, selected plant resources changed between the two seasons, for example preference for Salicaceae, Sapindaceae and Rosaceae in spring and preference for Poaceae, Adoxaceae, Asteraceae and Cornaceae in autumn (see Supporting Information Data S11 for details).
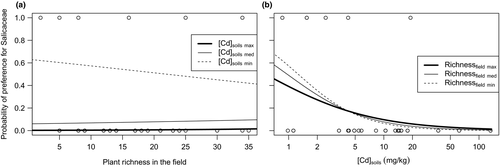
Model averaging was used for the final model for Salicaceae preference in spring (see Supporting Information Data S12 for complete results). The model included all variables: mouse number, plant richness in the field, TM concentration in soils and the interaction. Preference for Salicaceae was negatively correlated with plant richness in the field when soil contamination was low (e.g., from 0.6 to 0.4; Figure 4a). On the other hand, preference for Salicaceae disappeared along the gradient of TM concentration in soils, whatever the plant richness in the field was (e.g., preference probability for Salicaceae decreased from 0.6 to 0; Figure 4b). R2D of the final model was 0.280, 0.165 and 0.271 for Cd, Pb and Zn, respectively.
4 DISCUSSION
We first hypothesized that soil TM contamination would reduce diet richness of wood mouse due to adverse effect of TMs on resources’ richness in the field. Our study revealed negative correlations between diet richness and TMs in soils. This was true for both earthworms and plants in spring and in autumn (although only at low and medium levels of plant richness in autumn), and for arthropods in spring when richness in the field was high. On the contrary, positive correlations were observed, for plants in autumn, when plant richness in the field was high, and for arthropods in spring when arthropod richness in the field was low. A negative relationship between diet richness and pollution by TMs is expected if resource richness in the field is negatively affected by TM concentrations. Literature about the effects of pollution on various taxonomic groups suggests a global negative effect of pollution on the richness of organisms and an absence or a positive correlation for a few taxa groups or situations. Although decreasing richness of earthworms with increasing TM concentrations gradients in soils has been widely reported (Pérès et al., 2011; Spurgeon & Hopkin, 1996, 1999), several studies have reported that relationships between species richness of soil macrofauna and TM contamination are not straightforward (Gillet & Ponge, 2003; Migliorini, Pigino, Bianchi, Bernini, & Leonzio, 2004; Nahmani & Lavelle, 2002). Moreover, several studies showed that community composition was affected by soil TM concentrations. Tolerance to, and/or accumulation capacity of, metals differ according to taxa, organs and metals (Broadley, White, Hammond, Zelko, & Lux, 2007; Das, Samantaray, & Rout, 1997; Pourrut, Shahid, Dumat, Winterton, & Pinelli, 2011). Lower resistance to toxic effects can make metal-sensitive plants disappear in contaminated areas, which may favour metal-tolerant ones, sometimes without modification of taxonomic richness. Such modification of community composition has been reported by several authors in plants (Banásová, Horak, Čiamporová, Nadubinská, & Lichtscheidl, 2006; Dazy et al., 2009; Strandberg, Axelsen, Pedersen, Jensen, & Attrill, 2006) and in invertebrates (Babin-Fenske & Anand, 2011; Migliorini et al., 2004; Nahmani & Lavelle, 2002). In our study area, we found no correlation or even positive trends between TM concentration in soils and both plant and invertebrate richness in the field (S. Ozaki, C. Fritsch, F. Mora, T. Cornier, R. Scheifler, & F. Raoul, unpublished data). However, there were confounding factors in our site like the anthropogenic increase in habitat diversity (see Douay et al., 2009) and possibly of plant richness along the gradient of contamination on the Metaleurop Nord site. Community composition also differed according to soil contamination levels or other soil properties affecting metal bioavailability (S. Ozaki, C. Fritsch, F. Mora, T. Cornier, R. Scheifler, & F. Raoul, unpublished data). Difference in resource composition could alter resource availability and thus modify feeding behaviour and diet composition. Few studies documented the effects of pollution on the diet of terrestrial vertebrates, most of them dealing with diet composition rather than richness or diversity. Clare et al. (2014), describing the diet of insectivorous bats Myotis lucifugus in different locations, showed a trend of lower diet species richness in area of lower habitat quality (acidification or organic pollution). Eeva, Ryömä, and Riihimäki (2005) showed a significant difference in diet composition of nestlings of two insectivorous birds, Parus major and Ficedula hypoleuca, between unpolluted and TM polluted sites, reflecting differences in resources composition, phenology and feeding ecology among the two bird species: In polluted areas where caterpillars were less abundant, both species ate more beetles and different larvae of flying insects but less moth. The study of Heroldová (2002) demonstrated that the diet of field vole Microtus agrestis in forest clearings caused by air pollution was dominated by grasses, reflecting higher abundance of grasses and a poorer diversity of food supply in clearings. Thus, it is suggested that diet richness (as demonstrated in the present study) and/or composition can be affected by pollution-induced changes in trophic resources.
We also hypothesized that TM contamination would affect food selection by wood mouse. Our model predicts that preference probability for Salicaceae in spring in the highest contaminated sites decreases to become almost null. Avoidance of TM-contaminated soil or food has been shown in invertebrates but has rarely been investigated in vertebrates and is particularly hard to prove under field conditions in comparison with controlled experiments. This behaviour has been observed under laboratory conditions for some invertebrates like isopods or small moths (Odendaal & Reinecke, 1999; Scheirs, Vandevyvere, Wollaert, Blust, & De Bruyn, 2006; Zidar, Drobne, Štrus, Van Gestel, & Donker, 2004), and for the wood mouse (Beernaert et al., 2008), but mechanisms of such aversion are not yet elucidated. In Beernaert et al. (2008), wood mice chose acorns (Quercus robur, Fagaceae) sampled from unpolluted sites rather than ones from metal-polluted sites (mainly by Cd, copper, Pb and Zn), but such avoidance behaviour was no longer detected under field conditions, perhaps due to ecological cofactors such as food shortage, and/or the presence of competitors and predators. Avoidance of contaminated food, leading to shifts in diet, can be one of the mechanisms allowing animals to mitigate their exposure in highly polluted environments (Gillet & Ponge, 2003). In fact, Salicaceae family is considered as TM accumulator and resistant to highly TM-contaminated soils (Pulford & Watson, 2003). Migeon, Richaud, Guinet, Chalot, and Blaudez (2009) showed that Salicaceae family in the surroundings of Metaleurop Nord, composed of two genera Salix and Populus, accumulated larger amount of Cd and Zn in their shoot parts than the other families like Fagaceae or Sapindaceae. These two last are also preferred plant families in our study and known as common food of wood mice (i.e., Watts, 1968). However, mechanisms of changes in preference remain unclear. The nutritional quality of metal-contaminated plant organs has been shown to be lowered, with for instance a decrease in sugar or protein concentrations (Beernaert et al., 2008; Scheirs et al., 2006), features which play an important role for food choice in rodents (Butet, 1990; Kerley & Erasmus, 1991). It is also possible that animals might identify metal-contaminated plants due to metal-induced metabolic compounds (Poschenrieder, Tolrà, & Barceló, 2006), largely known as compounds limiting feeding (Foley & Moore, 2005). Dearing, Mangione, and Karasov (2000) reported that plant secondary compounds might play more important role than nutrient level in the diet of the generalist woodrat, Neotoma albigula. It is therefore supposed that mice could directly or indirectly detect noxious substances (plant toxins or TMs) and avoid ingesting such items. Also, Behmer et al. (2005) demonstrated from their experiments using nutritionally balanced synthetic food that high Zn concentration per se altered the feeding behaviour of the desert locust Schistocerca gregaria. Whatever the mechanism, the hyperaccumulation of metals in plant tissues contributes to defensive effects of plants against natural enemies, including invertebrate herbivory (Boyd, 2007; Poschenrieder et al., 2006). Such defensive effect has not been reported against vertebrates so far, but the disappearance of the preference for Salicaceae family along TM contamination in soils could be associated with its high metal accumulation capacity. Furthermore, plant richness in the diet in autumn increased along the soil TM concentration gradient when richness in the field was high. Apart from changes of resource composition, this pattern could be explained, at least partly, by the nutrient balance hypothesis or the detoxification limitation hypothesis: If nutritional quality of all plant resources was altered (i.e., if nutritional quality was not clearly contrasted among resources), diet richness of animals could increase for completing nutrient and/or for avoiding loads of similar secondary compounds.
Contrary to plants, selection for invertebrate food could not be analysed in part because of the primer used for arthropods and molluscs: Its short size is more reliable for the analysis of degraded DNA in the case of diet (Taberlet et al., 2012), but the precision in taxonomic assignment was low. We also did not sample earthworms in the field. Moreover, it is difficult to determine selection for invertebrate resources because of their mobility. Foraging strategy of predators can be modified according to mobility of preys (Sih & Christensen, 2001), suggesting that relative quantity in the field is not the only factor to consider when analysing mobile prey selection. Even though we supposed that decreased earthworm diet richness might be linked to their richness in the field, invertebrate food selection in wood mice remains as a further challenge.
An important feature of our results is the strong seasonal difference of patterns, for both food selection and correlations with TM contamination. In temperate ecosystems, seasons have a key influence on the dynamics of ecosystems and on the various aspects of biology and ecology of living organisms. Seasonal difference of patterns might result from the adaptation of wood mice feeding behaviour to phenology of resources. Indeed, wood mice consume mainly seeds and other reproductive organs (Butet, 1986a; Watts, 1968), seeking high-energy or protein-rich tissues (Butet, 1990). Although parts of plant resources actually consumed could not be distinguished using DNA metabarcoding, we supposed that parts of plants preferably consumed have changed between spring and autumn. Moreover, the nutritional composition and quality of plants and invertebrates are likely to vary according to seasons. Also, resources functionally important for wood mice might have a strong phenology, such as mushrooms in autumn. However, the molecular primers we used did not target mushrooms, which prevents further conclusion. At last, TM accumulation in organisms is also subject to seasonality at contaminated site (i.e., Hunter, Johnson, & Thompson, 1987b; Migeon et al., 2009). Seasonal variation of TM accumulation pattern can be related to growth season or physiological changes of each taxa (Hussein, Obuid-Allah, Mohammad, Scott-Fordsmand, & Abd El-Wakeil, 2006; Kabata-Pendias, 2011). Disentangling the combined effects of these factors on the patterns of interest in this study is beyond our present scope, but it is clear that seasonality is a key feature of the interplay between pollution, resource dynamics and consumer feeding behaviour.
Our study sheds light on the effects of soil TM contamination on patterns of feeding behaviour of a generalist consumer, by applying DNA metabarcoding for diet identification. We underline potential altering effects of TMs on the diet richness, which possibly partly results from modifying food selection, at least for plant resources. Such modification of diet related to habitat contamination might lead to a different pattern of contaminant exposure in the animals. We hence suggest that ecosystem pollution should now be considered as a driver of changes in food web structure and dynamics in time and space.
ACKNOWLEDGEMENTS
This study was financially supported by the project BIOTROPH, cofunded by the Agence De l'Environnement et de la Maîtrise de l'Energie (ADEME; contract No. 1172C0030) and the Conseil Régional du Nord-Pas de Calais (CRNPC; orders No. 12000921 and 14001044; joint call with the Fondation pour la Recherche sur la Biodiversité). The first author was also financially supported by a grant from the Conseil Régional de Franche-Comté (contract No. 2015C-06107). The authors gratefully thank Cécile Grand from ADEME for fruitful scientific discussions. We also thank Eva Bellemain and Alice Valentini from SPYGEN Company for their help in DNA analyses and interpretation. We finally thank François Gillet, Nadia Crini, Dominique Rieffel and Anne-Sophie Prudent for their precious assistance.
DATA ACCESSIBILITY
DNA sequence data, soil TM concentration in buffers, resources availability in buffers and wood mouse data are available from the metadata portal “dat@osu”; https://doi.org/10.25666/dataosu-2018-07-17.
AUTHOR CONTRIBUTIONS
F.R. and R.S. conceived the study; S.O., R.S., C.F. and F.R. designed the study; R.S., C.F., F.M., T.C. and F.R. performed field samplings and laboratory treatments; B.V. performed bioinformatics treatments; S.O. performed the statistical analyses; S.O., F.R., R.S. and C.F. interpreted the results. S.O. wrote the manuscript under the supervision of F.R, R.S. and C.F.




