Response of host–bacterial colonization in shrimp to developmental stage, environment and disease
Abstract
The host-associated microbiota is increasingly recognized to facilitate host fitness, but the understanding of the underlying ecological processes that govern the host–bacterial colonization over development and, particularly, under disease remains scarce. Here, we tracked the gut microbiota of shrimp over developmental stages and in response to disease. The stage-specific gut microbiotas contributed parallel changes to the predicted functions, while shrimp disease decoupled this intimate association. After ruling out the age-discriminatory taxa, we identified key features indicative of shrimp health status. Structural equation modelling revealed that variations in rearing water led to significant changes in bacterioplankton communities, which subsequently affected the shrimp gut microbiota. However, shrimp gut microbiotas are not directly mirrored by the changes in rearing bacterioplankton communities. A neutral model analysis showed that the stochastic processes that govern gut microbiota tended to become more important as healthy shrimp aged, with 37.5% stochasticity in larvae linearly increasing to 60.4% in adults. However, this defined trend was skewed when disease occurred. This departure was attributed to the uncontrolled growth of two candidate pathogens (over-represented taxa). The co-occurrence patterns provided novel clues on how the gut commensals interact with candidate pathogens in sustaining shrimp health. Collectively, these findings offer updated insight into the ecological processes that govern the host–bacterial colonization in shrimp and provide a pathological understanding of polymicrobial infections.
1 INTRODUCTION
Aquatic animals directly ingest microbes from the waters of rearing facilities from the opening of their mouths onward. Thus, intuitively, the gut microbiota of aquatic animals is directly governed by the microbial community of the rearing water (Schryver & Vadstein, 2014). Consistent with this assertion, it has been shown that the dynamics of the gut microbiota are closely associated with those in rearing bacterioplankton communities (Giatsis et al., 2015; Yan et al., 2016). However, there is increasing evidence of strong host filters on the symbiotic microbiota, where hosts of the same species living in different aquatic habitats share similar gut bacterial communities (Rungrassamee et al., 2014; Schmidt, Smith, Melvin, & Amaralzettler, 2015; Tzeng et al., 2015). Recently, it became clear that host diseases could attribute to dysbiosis (shifts in the microbial community that have a negative impact on the host [Petersen & Round, 2014]) in the gut microbiota (Dai, Yu, Xuan, Zhen, & Xiong, 2018a; Nie, Zhou, Qiao, & Chen, 2017; Xiong et al., 2015a; Yu, Cao, Dai, Qiu, & Xiong, 2018). In this regard, a better understanding of the route of transmission or acquisition of gut symbionts is indispensable, because it aids in designing better disease management strategies.
Given the functional importance of gut microbiota in preventing pathogen colonization (Inohara, 2013; McFall-Ngai et al., 2013; Xiong, 2018), digestion and energy acquisition (Dai et al., 2018b; Pereira & Berry, 2017; Xiong et al., 2017a), and other conditions, extensive studies have shown that the gut microbiota compositions of aquatic animals are affected by factors such as developmental stage (Xiong et al., 2017b; Yan et al., 2016), health status (Nie et al., 2017; Yu et al., 2018; Zhu et al., 2016), rearing water quality and planktonic microbes (Giatsis et al., 2015). From an ecological perspective, the gut can be considered an “island” for colonizing bacteria within a “sea” of surrounding (e.g., rearing water) environments (Delong, 2014; Yan et al., 2016). Within this framework, a successful colonization of external taxa into the gut is determined by complex ecological processes, such as dispersal, host selection and competition with indigenous taxa (Mallon, Elsas, & Salles, 2015; Xiong, Dai, & Li, 2016). The neutral theory assumes that members in a community are randomly lost and are rescued by random dispersal from the surroundings and ecological drift. Thus, species that deviate from the neutral expectations are the candidates that are imposed upon host selection, for example selected against or adapted to host filters (Loudon et al., 2016; Sloan et al., 2006). Such models offer a powerful tool for untangling the relative importance of the aforementioned processes that determine bacterial community maintenance between the host gut and surrounding source (Burns et al., 2016; Loudon et al., 2016; Zhu et al., 2016). While considerable progress has been made to investigate the underlying ecological processes that govern the assembly of gut microbiota, no consensus has emerged on the importance of deterministic processes over host ontogenesis. For example, it was reported that host selection strength increased with the maturity of healthy individuals (Burns et al., 2016), while other studies showed the opposite trend (Loudon et al., 2016; Xiong et al., 2017b). In addition, it appears that disease and environmental stress can decouple host genetics and its associated bacterial communities (Wegner, Volkenborn, Peter, & Eiler, 2013; Xiong et al., 2015a). The gut microbiotas vary concurrently with rearing conditions, bacterioplankton, host life stage and health, such that these variables are confounded (Burns et al., 2016; Xiong et al., 2017b). Consequently, little is known about how or to what extent the host–bacterial colonization is affected by host ontogenesis and disease, although this information is essential from both scientific and commercial perspectives.
The “Like will to like” hypothesis proposes that the presence of phylogenetically related species facilitates the colonization of external species into the gut (Stecher et al., 2010). However, according to Darwin-based niche theory, closely related taxa may compete for niche habitats and growth-limiting resources, thereby conferring colonization resistance (Mallon et al., 2015). This discrepancy could be due to the high density and diversity of gut commensals that interact with each other to form symbiotic and/or antagonistic relationships, rather than a simple sum of the traits of individual species (Dai et al., 2017; Faust & Raes, 2012). Evidence, albeit limited, shows that the synergistic interactions among gut taxa are fundamental in sustaining proper functions, while disruption of these cooperative activities would increase the susceptibility of hosts to microbial invasion (Korgaonkar, Trivedi, Rumbaugh, & Whiteley, 2013; Zhu et al., 2016). The paradigm shift towards a consortium of commensals provides a protective effect during health disease (Vonaesch, Anderson, & Sansonetti, 2018). Under this premise, exploring the commensals that facilitate or restrict the establishment of pathogens would provide implications for both prevention and treatment.
Shrimp are an ideal model for studying the interactions between hosts and their associated microbiota. This is in large part due to the feasibility of cohabitating a large number of congeneric individuals, thereby ruling out the effects of host genetics, environment condition and source pool on the variations in gut microbiota. In addition, the frequent occurrences of shrimp disease enable us to evaluate the response of gut microbiota to disease, which is an ultimate goal for such microbial ecology studies (Schryver & Vadstein, 2014; Xiong et al., 2016).
We applied ecological approaches to analyse the host–bacterial colonization pattern in shrimp with the following questions:
-
To what extent is the host–bacterial colonization pattern affected by shrimp ontogeny, disease and environmental conditions? It has long been recognized that the function of gut physiology increases as hosts mature (Lovat, 1996). Thus, we hypothesized that the ability of host selection on external taxa increased with host maturity, while disease could skew this trend. To determine this, we adopt a natural model in which we consider the gut bacterial communities of shrimp to be local communities that are a part of a broader metacommunity, that is, the rearing bacterioplankton communities (Burns et al., 2016; Loudon et al., 2016).
-
Are there gut signatures that indicate shrimp disease, irrespective of shrimp ontogeny? Gut bacterial community structures vary over shrimp life stages and between health status (Huang, Li, Wang, & Shao, 2016; Zhu et al., 2016). In this case, we ruled out the normal variation within gut microbiota over healthy shrimp development, thereby identifying disease-associated signatures that reflect deviations therefrom.
-
How does shrimp disease alter the co-occurrences of gut commensals and candidate pathogens? Given that commensals impose colonization resistance to pathogens (Inohara, 2013), we speculated that the antagonistic interactions between pathogens and resident taxa would shift into synergistic interactions when disease occurred. In doing so, we focused on the gut taxa that directly interact with the candidate pathogens.
2 METHODS
2.1 Experimental design and sample collection
The shrimp (Litopenaeus vannamei) ponds investigated here are located in Ningbo, China (29°32′N, 121°31′E). The standard glasshouses (rectangular, 60 m × 30 m, with a depth of 1.2 m) were identically managed, including rearing sea water, 5% daily water exchange, stocking density (1,500 larvae per m3), feeding schedule and a stable temperature (28 ± 0.5°C). The rearing sea water was disinfected with sodium hypochlorite and alum for removal of microorganisms and particles, and then was aerated in open reservoirs for 3 weeks. Bottom aeration was applied to sustain a sufficient level of dissolved oxygen.
Congeneric larval shrimp were inoculated into each pond on April 8, 2016. Water and shrimp samples were collected from six selected ponds on the April 22 (2 weeks after inoculation to enable the adaptation of the shrimp to new rearing conditions) and April 29, on the May 27 and June 3, and on the July 4 and July 10, representing shrimp larval, juvenile and adult life stages, respectively (Supporting Information Table S1). A disease occurred in three of our regularly monitored ponds on the July 4, 87 days after inoculation. The diseased shrimp exhibited typical symptoms of white faecal syndrome (WFS), such as inactivity, lack of appetite, red hepatopancreas, white guts and white faecal strings (Sriurairatana et al., 2014), and died within a few days (Supporting Information Figure S1). The cultivation was forcefully terminated on the July 10, due to massive mortalities. Healthy larvae and juveniles were collected from the six monitored ponds, resulting in six biological replicates for each sampling (Supporting Information Table S1). Given the small size of larvae, 10 individuals were collected from each fishing net during sampling. In addition, there were only three ponds of healthy or diseased adults when disease occurred; thus, we applied three fishing nets (technical replicate for improving statistical power) to collect shrimp in each pond. This design resulted in nine replicates (three technical replicates × three ponds) for healthy or diseased adults on the July 4 and July 10, respectively. In the same way, duplicate water samples were collected from each pond at the adult stage (Supporting Information Table S1). Shrimp were aerated in tanks with water from the corresponding pond during sampling and transportation. Water samples were stored in an icebox.
Water parameters were measured as described elsewhere (Xiong et al., 2015b; Yang et al., 2018). In brief, water temperature, pH, salinity and dissolved oxygen (DO) were determined in situ using corresponding probes (Oxi 340i; WTW, Weilheim, Germany). The levels of chemical oxygen demand (COD), total nitrogen (TN), total phosphorus (TP), NH4+, NO3−, NO2−, PO43+ and SiO43+ were analysed following standard methods (AQSIQ 2007). Dissolved inorganic nitrogen (DIN) was the sum of NH4+, NO3− and NO2−.
2.2 Measurement of immune enzyme activities
Using a 1-ml sterile syringe, haemolymphs were collected from the ventral sinuses of every three shrimp (captured from every fishing net across six ponds, corresponding intestines were pooled for DNA extraction) and then were centrifuged at 6,000 g for 5 min to isolate haemocytes. Superoxide dismutase (SOD) defenses against toxic oxygen derivative production during bacteria and other foreign body invasion, whereas phenoloxidase (PO) enables a rapid response of shrimp to pathogen invasion (Amparyup, Sutthangkul, Charoensapsri, & Tassanakajon, 2012). In addition, acid phosphatase (ACP) is an index in humoral immunity to reflect the phagocyte ability to remove the foreign bodies (Liao, Ren, He, Jiang, & Han, 2014). Given the uncertain causal agent of shrimp WFS, the three immune activities that commonly respond to external invasion were measured using corresponding kits (Jiancheng Bioengineering Institute, Nanjing, China).
2.3 DNA extraction, bacterial 16S rRNA amplification and Illumina sequencing
On the sampling day, 0.5 L of water was prefiltered through nylon mesh (100 μm pore size) and subsequently filtered onto a 0.22-μm membrane (Millipore, Boston, MA, USA) to collect microbial cells. Given that an inadequate amount of DNA could be obtained from the intestine of a single larval shrimp in the initial trial runs, every three shrimp intestines across the six monitored ponds, three fishing nets (three technical replicates for each pond at the adult stage) and six sampling dates were dissected on ice and pooled to compose a single sample. In total, we collected 60 and 48 samples to analyse the bacterial communities of the shrimp gut and rearing water, respectively (Supporting Information Table S1). The filters and shrimp intestines were placed into a sterile tube and were stored at −80°C prior to DNA extraction. Genomic DNA (gDNA) was extracted using a FastDNA Spin kit (MP Biomedicals, Carlsbad, CA, USA).
We amplified the V3-V4 regions of bacterial 16S rRNA gene with the primer sets 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′). For each sample, triplicate 50-ml PCRs were performed, each containing 50 ng of purified DNA as a template with cycling conditions: 25 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 45 s, with a condition of 72°C for 10 min for the final elongation step. Triplicate amplicons for each sample were pooled and purified using a PCR fragment purification kit. Equimolar amounts of amplicons from each sample were pooled and then sequenced on a single run using the Illumina MiSeq 2 × 300 bp platform (Illumina, San Diego, USA).
Paired-end sequences were merged using flash (Magoč & Salzberg, 2011). The merged sequences were processed following the quantitative insights into microbial ecology pipeline (qiime version 1.9.0) (Caporaso, Kuczynski, & Stombaugh, 2010). In brief, the sequences with ambiguous bases or truncated at any site of more than three consecutive bases receiving a Phred quality score (Q) <20 were deleted. Chimeric sequences were deleted using the UChime algorithm (Edgar, Haas, Clemente, Quince, & Knight, 2011). Sequences with a distance-based similarity of 97% or greater were grouped into operational taxonomic units (OTUs) using uclust (Edgar, 2010). The most abundant sequence from each OTU was selected as representative and then was taxonomically assigned a closed reference using script “pick_closed_reference_otus.py” (Greengenes Database, release 13.8) (DeSantis et al., 2006), which enables each identified OTU has a close relative. Singletons were excluded from the data set. To correct for uneven sequencing efforts, the OTU table was 10× randomly rarefied subset of 19,400 sequences per sample for calculating distances between each pair of samples.
It is reasoned that the genes present in microbial genomes are much more similar among related bacteria. Under this premise, the 16S gene copy of a given OTU without an available genome sequence can be estimated using their sequenced relatives as a reference (Kembel, Wu, Eisen, & Green, 2012). In particular, the OTU table was normalized by dividing the abundance of each OTU by its predicted 16S copy number. The metagenome prediction of each sample and functional categorization based on the KEGG Orthology were performed with the phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) on the Galaxy platform (Langille et al., 2013).
2.4 Statistical analysis
The following analyses were performed in r 3.3.2, unless otherwise stated (R Core Team 2015). Alpha diversity of bacterial community was compared to reveal changes over shrimp development stages and between health status using a one-way analysis of variance (ANOVA) followed by the Tukey post hoc test. Beta diversity was calculated with weighted UniFrac metrics, and an analysis of similarity (ANOSIM) was performed to determine whether there were significant differences between each pair of groups. A nonmetric multidimensional scaling (NMDS) analysis was used to visualize the distances of bacterial communities among samples. A parametric permutational multivariate analysis of variance (PERMANOVA) was conducted to quantitatively evaluate the effects of developmental stage, health status and habitat on the variations in the bacterial community using the adonis function (Anderson, 2001). The Pearson correlation analysis was employed to test the association between bacterial compositional and predicted functional profiles. A forward selection was used to identify the most important variables shaping the bacterial communities in a distance-based multivariate linear model (DistLM), which sequentially added one variable that improves the selection criterion (R2) the most at each step, until no improvement can be reached in R2 (McArdle & Anderson, 2001). The same subset of environment variables were implemented in a structural equation model (SEM) to evaluate the interrelationships among rearing conditions, bacterioplankton and gut communities in amos 18.0 (IBM, Chicago, IL, USA) (Byrne, 2001). The a priori and theoretical assumptions made to establish the SEM were as follows: (a) Rearing conditions can directly influence the bacterioplankton and gut communities, and (b) the bacterioplankton community can lead to changes in gut microbiota. The IndVal (indicator values) method combined with a linear model was used to identify species that were linearly correlated with healthy shrimp developmental stages (Dufrêne & Legendre, 1997). After ruling out these shrimp age-discriminatory taxa, a response ratio analysis was employed to identify gut signatures that consistently changed between the health statuses of the two samplings (Zhu et al., 2016).
The Sloan neutral community model was independently applied to evaluate the host–bacterial colonization pattern over shrimp development and between health status. This model predicts that the probability of detecting an OTU in shrimp gut due to random dispersal is directly proportional to its abundance in the corresponding water community (Sloan et al., 2006). OTUs were sorted into three categories depending on whether they occurred more frequently (over-represented), less frequently (under-represented) or within (neutrally distributed) the 95% confidence interval of the neutral model predictions. The cumulative relative abundance of sequences in these three categories of OTUs in shrimp gut served as a proxy for evaluating the relative importance of stochasticity (∑ Relative abundance of sequences within neutrally distributed OTUs) and determinism (∑ Relative abundance of sequences in over-represented and under-represented OTUs) in determining the gut bacterial assembly (Loudon et al., 2016).
The interspecies interaction of gut microbiota for each developmental stage and diseased shrimp was constructed by the regular random matrix theory-based network inference approach (Deng et al., 2012). To ensure that network topologies were comparable, all interspecies interactions of different groups were generated with a uniform threshold. In addition, to identify bacteria that displayed a strong inhibition against or that facilitated colonization of the candidate pathogens, we only focused on the co-occurrence pattern of candidate pathogens and their interacted taxa. The networks were visualized in cytoscape 3.3.0 (Shannon et al., 2003).
3 RESULTS
3.1 Shrimp immune response to disease
The three measured immune enzyme activities, SOD, ACP and PO, consistently increased (p < 0.05 in the three cases) in diseased shrimp compared to the healthy subjects (Supporting Information Figure S2). Therefore, disease markedly induces the shrimp inflammatory response.
3.2 Composition and dynamics of shrimp gut and bacterioplankton community
Due to low sequencing depth, eight samples from the gut bacterial communities of healthy larvae (one sample), adults (one sample), the water bacterial communities of healthy (one sample) and diseased (five samples) adults were excluded in the data set (Figure 1a). The remaining 100 samples contained 2,811,540 high-quality sequences, with 19,435–36,635 sequences per sample (mean ± SD, 28,115 ± 4,747). After rarefaction, the averaged OTUs in shrimp gut were 899 ± 295, which was significantly lower (p < 0.001, paired t test) than those in water (1,854 ± 516). The dominant phyla in the gut microbiotas were Gammaproteobacteria, followed by Alphaproteobacteria, Bacteroides and Actinobacteria, whereas the phyla Alphaproteobacteria and Bacteroides were predominant in the bacterioplankton communities (Supporting Information Figure S3). In particular, the relative abundances of these dominant phyla significantly (p < 0.05 for each phylum) changed between the healthy and diseased shrimp (Supporting Information Figure S3). The temporal dynamics of the dominant phyla were parallel with shrimp development; for instance, the relative abundances of gut Gammaproteobacteria and Actinobacteria linearly increased as the shrimp aged, whereas the opposite trend was observed in the bacterioplankton communities (Supporting Information Figure S3).
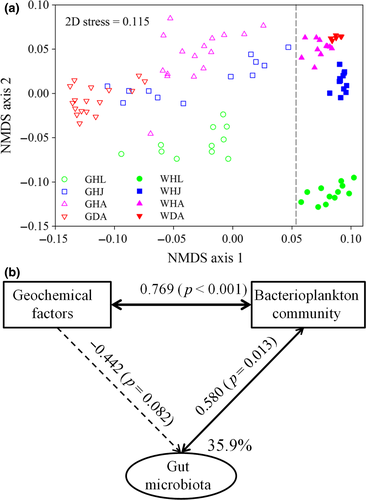
The α-diversity of the healthy shrimp gut microbiotas was relatively stable, although the corresponding bacterioplankton diversity linearly increased over the same period, a finding that was supported by both the Shannon and phylogenetic diversity indices (Supporting Information Figure S4). However, the α-diversity of gut microbiota significantly decreased when disease occurred, whereas that of the bacterioplankton community exhibited the opposite pattern (Supporting Information Figure S4). The NMDS ordination biplot depicted that bacterial communities varied markedly over the developmental stages, between health statuses and habitats (Figure 1a). In addition, a sequential assembly of gut microbiota over healthy shrimp life stages was evidenced by linearly increased distances along axis 2, which is also the case for bacterioplankton communities (Figure 1a). This pattern was further supported by PERMANOVA, revealing that habitat, shrimp developmental stage and healthy status contributed variations in the bacterial communities of 17.8%, 15.2% and 8.5%, respectively (p < 0.001 in the three cases) (Supporting Information Table S2). However, the compositions of the bacterial community significantly differed (p < 0.05) between any two of the compared groups, with an exception of the bacterioplankton communities between healthy and diseased adults (Supporting Information Table S3). Thus, it is likely that the gut microbiota is more sensitive for indicating host health status compared to the rearing bacterioplankton community.
3.3 Interplay among rearing environment, bacterioplankton and gut microbiota
To minimize the autocorrelation between the measured geochemical factors, a forward selection procedure was employed to reduce the number of explanatory variables, which retains only the most important variables (McArdle & Anderson, 2001). As a result, DO, NO2−, pH, PO43−, NO3−, TN and temperature were selected, which cumulatively constrained 56% of the variations in the bacterioplankton community (Supporting Information Table S3). For this reason, the SEM was applied to untangle the interplay among geochemical variables, bacterioplankton and the gut communities (Figure 1b). The SEM explained 35.9% of the variance in the shrimp gut microbiota. Bacterioplankton communities were positively (λ = 0.769, p < 0.001) affected by geochemical factors, which subsequently altered (λ = 0.580, p = 0.013) the shrimp gut microbiota. In contrast, the direct effects of geochemical factors on the gut microbiota were negative and marginal (λ = −0.422, p = 0.082; Figure 1b). In this regard, geochemical variables in the ponds led to changes in the water bacterial community. These changes in the bacterioplankton communities are then also reflected in the gut microbiotas to a certain extent.
3.4 Linkage between the gut bacterial phylogenetic and functional structures
The mean nearest sequenced taxon index (NSTI, the sum of phylogenetic distances for each organism in the community to its nearest relative with a sequenced reference genome) was 0.182 ± 0.023 across the samples. A comparable mean NSTI has been detected for the human gut microbiota, wherein PICRUSt produced accurate metagenome predictions (Langille et al., 2013). In this regard, the functional traits predicted in this study could be reliable to a certain extent. The predicted functional compositions of gut microbiota significantly changed over the shrimp developmental stages and between healthy and diseased adults (Supporting Information Figure S5A). To further evaluate the effects of disease on the interplay between the phylogenetic and functional structures, we separately tested the interplay for the healthy and diseased cohorts. There was a significant (Pearson correlation, r = 0.339, p < 0.001) association between the phylogenetic and functional structures of the healthy cohorts, while this was not the case for the diseased shrimp (r = 0.166, p = 0.080; Supporting Information Figure S5B). Thus, shrimp disease decoupled the association between the phylogenetic and functional structures of gut microbiota.
3.5 Gut bacterial taxa indicating shrimp developmental stages and health status
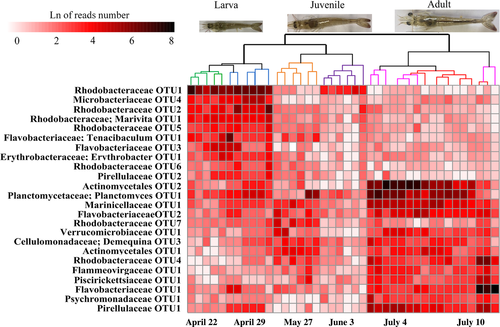
To identify the OTUs most responsible for the temporal variability of the gut bacterial communities, strict IndVal values (r > 0.6 and p < 0.01) were employed to identify taxa that were closely associated with shrimp developmental stages. Furthermore, only the taxa whose abundances were significantly correlated with shrimp age were considered. As a result, 24 taxa were identified whose abundances cumulatively accounted for 24.8 ± 3.9% sequences of the communities. The profiles of these age-discriminatory taxa contributed a clear and sequential separation of the gut microbiota as healthy shrimp aged (Figure 2). In contrast, the 24 age-discriminatory taxa that were detected in bacterioplankton communities did not generate a parallel clustering (Supporting Information Figure S6). In this regard, shrimp age-discriminatory taxa are not directly mirrored in the temporal changes in the corresponding bacterioplankton communities.
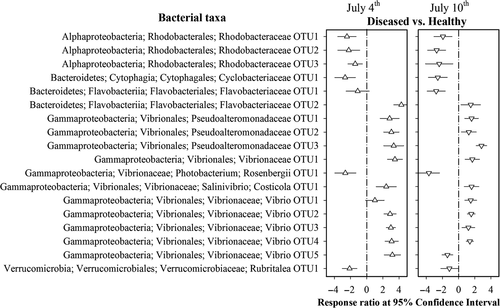
The 24 taxa were removed from the data set to rule out host age effects on the identification of indicators for health status. After this optimization, 18 taxa exhibited consistent changes in their abundances between health statuses on the two sampling days (Figure 3). There were significantly greater abundances of Vibrionaceae and Pseudoalteromonadaceae members as a response to shrimp disease. In contrast, the abundances of the Rhodobacteraceae species decreased in the diseased shrimp compared to the healthy ones (Figure 3). In particular, the occurrences of these 18 taxa contributed a clear discrimination between health statuses with a low error rate (8.6%, five of 58 cases) (Supporting Information Figure S7). Thus, the profiles of these 18 taxa are indicative of shrimp health status.
3.6 Neutral processes over host development and under disease
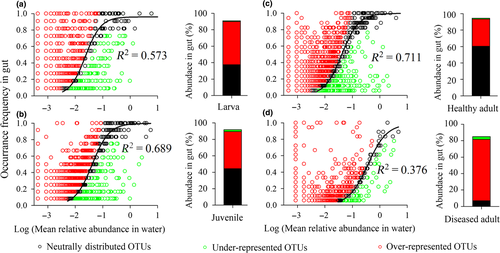
We used a neutral model (Figure 4) to analyse the OTUs that were shared between the shrimp gut and rearing water for each developmental stage and under disease. The fit of the model tends to be decrease over shrimp development stage (increasing R2 values, Figure 4). The sequences sorted into the OTUs consistent with neutral distribution (black points), which accounted for 37.5% in larvae and linearly increased to 60.4% in healthy adults. In contrast, the OTUs categorized as over-represented (red points) linearly decreased from 52.5% in larvae to 32.9% in healthy adults. However, there was no clear trend for the OTUs that were categorized as under-represented (green points) (Table 1; Figure 4). Cumulatively, the deterministic processes that governed gut microbiota tended to be less important as healthy shrimp aged. However, this stage-dependent trend was skewed when disease occurred; that is, the relative importance of determinism markedly increased, from 33.9% in the healthy to 77.8% in the diseased adults (Table 1). We noted that this departure was attributed to the over-representation of OTU27681 and OTU14490 in the diseased shrimp. Searching public databases using their representative sequences as queries, we found that they shared 98.3% and 99.1% similarities with the 16S rDNA genes of Vibrio tubiashii and V. alginolyticus, respectively, which are two notorious pathogens in shrimp aquaculture. In addition, the two OTUs enriched in the gut microbiota of diseased subjects, but they were extremely low in the rearing water (Supporting Information Figure S8). The pure cultures of the two strains were isolated from the gut of diseased shrimp, with accession numbers of MH182062 and MH182063 for the 16S gene sequences in GenBank. In addition, Vibrio spp. OTU27681 and OTU14490 were inferred as the candidate pathogens, due to their high discriminative accuracy between shrimp health status, enriched abundances of coded virulence genes and gatekeeper roles in the gut microbiota of diseased shrimp (unpublished data).
| Life stage | Neutrally distributed/stochasticity | Over-represented | Under-represented | Determinism |
|---|---|---|---|---|
| Larvae | 37.5 | 52.5 | 0.98 | 53.5 |
| Juvenile | 44.3 | 45.2 | 2.22 | 47.4 |
| Healthy adult | 60.4 | 32.9 | 1.02 | 33.9 |
| Diseased adult | 4.8 | 74.5 | 3.35 | 77.8 |
- The relative importance of determinism was the sum of over-represented and under-represented OTUs.
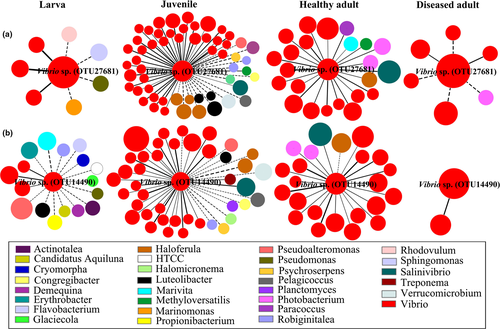
3.7 Co-occurrence pattern of the candidate pathogens
To allow for direct comparisons, an identical threshold (St = 0.700) was chosen for the four networks (Supporting Information Table S5). The harmonic geodesic distance and modularity values were significantly higher than those of the corresponding random networks, suggesting that small-world and modular properties were prevalent. The average clustering coefficients ranged from 0.173 to 0.486, but all were higher than their random values, revealing that the nodes in these networks were more connected than the corresponding random networks (Supporting Information Table S5). To explore the taxa that facilitate or constrain the outgrowth of pathogens, we focused on the modules of the candidate pathogens (Figure 5). Topologies of the two candidate pathogens in the networks changed markedly over healthy shrimp development. Vibrio sp. OTU27681 harboured a few neighbours at the larval stage, but quickly reached a maximum of 55 neighbours at the juvenile stage and decreased to 22 connections in healthy adults (Figure 5a). In contrast, its connections sharply decreased in the diseased adult subjects. In particular, Vibrio sp. OTU27681 cooperated with the neighbours that were affiliated with Vibrio species but were antagonized by non-Vibrio species at the larval and juvenile stages. However, all neighbours positively interacted with Vibrio sp. OTU27681 at the adult stage. A similar co-occurrence pattern was detected for Vibrio sp. OTU14490 (Figure 5b).
4 DISCUSSION
Despite recent progress, the ecological properties of host gut microbiota and its rearing source remain underinvestigated. Shrimp have aquatic embryos that hatch into an aquatic larval stage. It is thus apparent that rearing water offers a major, if not the sole, microbial pool to the gut of shrimp. However, it is unclear to what extent the shrimp gut microbiota is affected by the rearing water bacterial communities, especially how this relationship is modulated when disease occurs. Here, we demonstrate that the association between the bacterioplankton community and shrimp gut microbiota is strongly affected by the shrimp developmental stage and health status. In addition, we track the co-occurrence dynamics of candidate pathogens, which yields novel insights into the pathogenesis from an ecological perspective.
4.1 Shrimp ontogeny and disease govern the gut microbiota
The α-diversity of healthy shrimp gut microbiota was relatively stable, whereas the α-diversity of those in the pond of origin linearly increased (Supporting Information Figure S4). Thus, larval shrimp can rapidly acquire sufficient and diverse bacterial species, although only a few proportions of bacterioplankton successfully colonize the gut. Similar results have been observed in previous studies (Huang et al., 2016; Xiong et al., 2017b). However, this pattern is contradicted in the gut microbiota of zebrafish (Stephens et al., 2015) and aquaculture fishes (Yan et al., 2016), where the α-diversity decreases over fish life stages. Considering the lack of adaptive immunity in shrimp, a stable α-diversity may confer its colonization resistance against pathogens. Consistent with this assertion, both experimental and mathematical studies have proposed that a more diverse community exhibits a greater probability of having a member with antagonistic traits towards pathogens (Mallon et al., 2015; Schubert, Sinani, & Schloss, 2015). Some evidence of this is provided here, as the gut α-diversity significantly decreased in the diseased shrimp compared to the healthy ones, although there was an increased diversity in its species pool (Supporting Information Figure S4).
The shrimp gut microbiotas and bacterioplankton communities both exhibited sequential changes along axis 2 (Figure 1a). However, it should be stressed that the parallel changes do not mean passive colonization, because habitat (e.g., gut or rearing water) was the predominant factor (17.8% vs. 15.2% by developmental stage) in governing the variations in bacterial community (Supporting Information Table S2). In addition, the shrimp gut microbiota was significantly distinct from its corresponding bacterioplankton community (Figure 1a and Supporting Information Table S1). This is also the truth at the coarser phylum level (Supporting Information Figure S3). Environmental variables, such as temperature, could directly alter the gut microbiota of aquatic ectotherms (Giatsis et al., 2015; Kohl & Yahn, 2016; Li et al., 2017). However, only 35.9% of the variation in shrimp gut microbiota was explained by external environmental factors and bacterioplankton community (Figure 1b). It has been proposed that ontogenetic factors are more important than environmental factors in determining the bacterial colonization (Burns et al., 2016; Mortzfeld et al., 2016). These findings give rise to the hypothesis that the gut bacterial communities are experiencing a winnowing process over shrimp development. A possible explanation is that host physiological and immunological developments impose strong filters on the symbiotic taxa, thereby changing the abundances of associated bacteria or the acquisition of new species (Burns et al., 2016; Mortzfeld et al., 2016), as also shown here (Figure 1 and Supporting Information Figure S2). However, the occurrence of shrimp disease markedly disrupted the gut microbiota (Figure 1). Disease-induced gut inflammation (Supporting Information Figure S2) commonly facilitates an outgrowth of anaerobic bacteria, such as Gammaproteobacteria (Supporting Information Figure S3), which has been supported by a recent finding (Winter & Bäumler, 2014). Thus, both shrimp life stage and health status are key forces in determining the host–bacterial colonization.
4.2 Disease decouples phylogenetic and functional association in gut microbiota
The clear shifts in gut microbiota give rise to the question of whether such shifts are coupled with functional adjustments. It appears that the gut microbial function can be sustained without taxonomic coherence, that is functional redundancy (Moya & Ferrer, 2016). However, we found that the predicted functional compositions were intimately associated with the corresponding compositional structures of the gut microbiota among healthy individuals (Supporting Information Figure S5).
We propose the following explanations for this discrepancy. First, the shrimp diet is developmental stage-specific. Thus, to increase the host's fitness, such as by degrading the recalcitrant substrate, the host could recruit functionally active specialists (Mortzfeld et al., 2016), thereby intensifying the link between bacterial functional and phylogenetic compositions. Alternatively, available niches for external taxa are temporally dynamic as the host matures; thus, a successful colonizer means it has the ability to explore new ecological niches (Burns et al., 2016; Mallon et al., 2015). In the same way, a metagenomic study illustrates that the functional qualities of the gut microbiome significantly differ from healthy child to adult (Hollister et al., 2015). In addition, it has been shown that two shrimp species of the same age share congruent functional potentials, although the gut bacterial communities are distinct (Tzeng et al., 2015). These findings support the idea that the host developmental stage is a robust predictor for bacterial composition and function (Greenhalgh, Meyer, Aagaard, & Wilmes, 2016). In particular, this interplay was disrupted when shrimp disease occurred (Supporting Information Figure S5B). Disease-induced changes in the gut functional composition have been extensively detected in shrimp (Dai et al., 2018a; Xiong et al., 2015a), fish (Li et al., 2017) and humans (Hollister et al., 2015). A possible explanation is that disturbance, for example disease, skews the association between host genetics and its associated microbiota, with consequent dysbiosis in functions (Moya & Ferrer, 2016; Wegner et al., 2013). In addition, our recent works show that shrimp disease results in a stochastic colonization of alien species (Xiong et al., 2017b; Zhu et al., 2016). In other words, the loss or acquisition of a given strain is not determined by its functional traits, thereby decoupling the association between phylogenetic and functional compositions. However, given the “novelty” of the shrimp gut system and its inherent variability from the human gut, a metagenomic study on the shrimp gut microbiome is required to validate the pattern observed here.
4.3 Gut signatures of shrimp disease
Given the marked changes in gut microbiota in response to disease (Figure 1), it is of great interest to identify the taxa that are indicative of shrimp disease. However, the stage-specific gut microbiota impedes the identification of features characteristic of a “healthy” microbiome (Greenhalgh et al., 2016; Yu et al., 2018). As such, we ruled out the 24 taxa that were closely associated with shrimp developmental stages (Figure 2). There were 18 taxa with relative abundances that were consistently different on the two sampling days (Figure 3). It is worthwhile to note that the pattern of enrichment or decrease for a given taxon is in accordance with its known biological functions. For example, Vibrionaceae and Pseudoalteromonadaceae species have been long recognized to be opportunistic pathogens in shrimp aquaculture (Thitamadee et al., 2016; Xiong et al., 2015a), and these bloomed in the diseased shrimp (Figure 3). In contrast, the abundances of Rhodobacteraceae species significantly decreased in the diseased subjects (Figure 3). Members of Rhodobacteraceae are symbiotic with aquatic hosts, which have important roles in carbon biogeochemical cycling (Pujalte, Lucena, Ruvira, Arahal, & Macián, 2014). Therefore, the symbiosis of these taxa may aid in host digestion. Indeed, Rhodobacteraceae species have been applied as probiotics in L. vannamei, with marked improvements in growth and survival rate (Chumpol, Kantachote, Nitoda, & Kanzaki, 2017). In addition, based on the occurrences of these taxa, a clear stratification between shrimp health status was observed (Supporting Information Figure S7). Thus, it seems reasonable that the profiles of gut microbiota hold the potential to indicate shrimp health status.
4.4 Ontogeny and disease effects on the host–bacterial colonization
Based on recent progresses, it is apparent that the gut microbiota of aquatic animals is not a simple mirror of the microbes in their rearing pools, but that selection for specific bacterioplankton may be occurring (Burns et al., 2016; Mortzfeld et al., 2016; Zhu et al., 2016). There are important questions provoked by this observation. What is the association between the gut and water community structure? How is this association altered by host age and/or disease? To address these concerns, we compared the assembly of gut microbiota with shrimp life stages and health statuses.
There were a number of bacterial taxa with distributions that deviated from the neutral predictions. In particular, up to 52.5% of the sequences recovered from the larvae fell into OTUs that were over-represented in the gut, whereas these linearly decreased to 32.9% in healthy adults (Table 1; Figure 4). These OTUs are more widespread than expected, indicating that they have competitive advantages in surviving the biotic counterpressure imposed by resident microbes and host filters (Burns et al., 2016). In this regard, it is reasonable that the over-represented OTUs are candidates for probiotics, due to a higher chance of successful establishment in the shrimp gut (Mallon et al., 2015; Xiong et al., 2016). In contrast, portions of the OTUs that were neutrally distributed exhibited an opposite trend, ranging from 37.5% to 60.4% (Table 1; Figure 4). The presences of these OTUs in the gut are largely dependent on their abundances in the rearing source pool. Thus, the gut microbiota is affected by changes in the water community to a certain extent, with neutral processes becoming relatively more important over the shrimp developmental stages. Similar results were observed in the assembly of gut bacterial communities as fish and shrimp aged (Xiong et al., 2017b; Yan et al., 2016).
According to the co-evolution hypothesis (McFall-Ngai et al., 2013), it is mandatory for larva to recruit suitable taxa that expand the range of diet digestion due to their incomplete digestive systems. Thus, to improve the host's fitness, the colonization of the gut microbiota is filtered from the rearing species pool as a result of deterministic processes. However, when the gut histology and availability niches change as the host ages, the initial “winners” will be reassembled, thereby resulting in a host stage-specific gut microbiota (Greenhalgh et al., 2016; Stephens et al., 2015; Xiong et al., 2018; Yan et al., 2016). Instead, water quality tends to be deteriorative and eutrophic over shrimp cultivation (Xiong et al., 2014; Yang et al., 2018), which stresses shrimp and increases the virulence of pathogens (Penttinen, Kinnula, Lipponen, Bamford, & Sundberg, 2016). In turn, shrimp relocate energy in response to pathogen invasion, thereby attenuating their filtering abilities. The importance of determinism governing the gut microbiota markedly increased in diseased adults compared with healthy ones, which was unexpected (Figure 4). This pattern could not be attributed to an increased host-filtering effect. This assertion comes from the finding that the two candidate pathogens (over-represented OTUs) were prone to uncontrolled growth in the diseased shrimp (Supporting Information Figure S8). Indeed, it has been proposed that stochastic processes add to the establishment and the success of external taxa not only symbionts but also pathogens (Mallon et al., 2015). Collectively, our findings illustrate that the mechanisms that govern gut microbiota assembly are parallel with host ontogeny and highlight the integration of host health status for interpreting the assembly of gut microbiota.
4.5 Identification of commensal barrier against pathogen
It is now recognized that colonization resistance against pathogens is mediated by multiple microbial taxa interacting in a context-dependent manner (Schubert et al., 2015). The two candidate pathogens cooperated with resident Vibrio strains at the larval and juvenile stages (Figure 5). The “Like will to like” hypothesis claims that selective pressures impose similar forces upon closely phylogenetic relatives, whereas cooperative associations confer a fitness advantage (Stecher et al., 2010; Winter & Bäumler, 2014). To allow coexistence, phylogenetically related taxa could co-occur by adjusting to each other and segregating their realized niches in a process called niche differentiation (Pereira & Berry, 2017). In contrast, the diverse unrelated non-Vibrio taxa occupy different niches, thereby constraining the expansion of pathogens (Mallon et al., 2015). In addition, a gut taxon can only persist if it is capable of acquiring a particular limiting nutrient better than its competitors, as postulated by Freter's nutrient-niche theory (Freter, Brickner, Botney, Cleven, & Aranki, 1983). To sustain host health, it is thus conceivable that the candidate pathogens were antagonized by commensal species (Figure 5). However, this does not mean that a desirable interspecies interaction can be maintained over the host's life.
There is evidence that the protective commensal bacteria, such as Bacteroides and Propionibacterium, tend to decrease as the host aged (a trend also observed here, Supporting Information Figure S3), leading to a decrease in life quality (Greenhalgh et al., 2016; Mariat et al., 2009). Consistently, the keystone species affiliated with Robiginitalea (a genus of Bacteroides) and Propionibacterium disappeared in healthy adults, resulting in overall synergistic interactions with the potential pathogens (Figure 5). Thus, substantial alterations in the interspecies interaction may offer a “red flag” for disease risk. In particular, the interspecies interaction was collapsed when disease occurred, with a dramatic reduction in connectors (Figure 5). Consistently, it was reported that the complexity and cooperative activity among gut commensals were disrupted by a shrimp disease (Zhu et al., 2016). Enteropathogen-induced gut inflammation could create ecological niches that facilitate its expansion and its advantage to outcompete commensals (Mallon et al., 2015; Thiennimitr et al., 2011). As a result, disease outbreak generally contributes to a significant decrease in α-diversity, although the abundances of pathogens was enriched, as shown in this study and in previous reports (Li et al., 2017; Xiong et al., 2015a). Then, the co-occurrence equilibrium in the gut microbiota provides an index for evaluating the risk of disease.
5 CONCLUSIONS
Shrimp do not passively select their gut microbiomes. Rather, the internal gut conditions result in a microbiota that differs from that of the surrounding rearing waters. Host selection pressures tend to be weak, as neutral processes become more important as shrimp age. However, disease outbreak skews this trend due to the uncontrolled growth of two candidate pathogens. Further, the co-occurrence patterns afford clues on how commensals interact with pathogens in sustaining host health. Host stage-dependent gut microbiota leads to parallel changes in predicted functional composition, while this interplay is decoupled by disease. After ruling out the normal variation in bacterial communities over developmental stages, we identify gut signatures that are indicative of shrimp health status. Our findings provide updated insights into the ecological processes that govern the host–bacterial colonization of shrimp gut microbiota and improve pathological understanding. Studies such as this one are timely, as shrimp aquaculture is being threatened by polymicrobial infections.
ACKNOWLEDGEMENTS
We are grateful to comments from three anonymous reviewers as well as editorial comments from R. Biek, which together substantially improved this manuscript. This work was supported by the Public Welfare Technology Application Research Project of Zhejiang Province (2016C32063), the Project of Science and Technology Department of Ningbo (2017C10044) and the K.C. Wong Magna Fund in Ningbo University.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DATA ACCESSIBILITY
The MiSeq 16S rRNA sequence data used for the analyses were deposited in the Sequence Read Archive at DDBJ under an accession number DRA005782.
AUTHOR CONTRIBUTION
J.X. designed research. D.W., Q.Q., Z.J., Y.W. and C.L. performed genetic and field researches. J.X. analysed data and wrote the article.




