Rapid evolution and the genomic consequences of selection against interspecific mating
Abstract
While few species introduced into a new environment become invasive, those that do provide critical information on ecological mechanisms that determine invasions success and the evolutionary responses that follow invasion. Aedes albopictus (the Asian tiger mosquito) was introduced into the naturalized range of Aedes aegypti (the yellow fever mosquito) in the United States in the mid-1980s, resulting in the displacement of A. aegypti in much of the south-eastern United States. The rapid displacement was likely due to the superior competitive ability of A. albopictus as larvae and asymmetric mating interference competition, in which male A. albopictus mate with and sterilize A. aegypti females, a process called “satyrization.” The goal of this study was to examine the genomic responses of a resident species to an invasive species in which the mechanism of character displacement is understood. We used double-digest restriction enzyme DNA sequencing (ddRADseq) to analyse outlier loci between selected and control lines of laboratory-reared A. aegypti females from two populations (Tucson, AZ and Key West, Florida, USA), and individual females classified as either “resisted” or “mated with” A. albopictus males via mating trials of wild-derived females from four populations in Florida. We found significant outlier loci in comparing selected and control lines and between mated and nonmated A. aegypti females in the laboratory and wild-derived populations, respectively. We found overlap in specific outlier loci between different source populations that support consistent genomic signatures of selection within A. aegypti. Our results point to regions of the A. aegypti genome and potential candidate genes that may be involved in mating behaviour, and specifically in avoiding interspecific mating choices.
1 INTRODUCTION
Biological invasions are a major threat to the functioning of ecosystems and can result in both direct and indirect effects on human health (Juliano & Lounibos, 2005; Vitousek, D'Antonio, Loope, Rejmanek, & Westbrooks, 1997). For example, invasive insect species may vector emerging pathogens that expose human populations to new diseases (e.g., dengue transmission by Aedes albopictus in Hawaii, Effler et al., 2005) or may threaten the food supply through crop damage (Mack et al., 2000). In addition to the impacts on humans, invasive species may compete with native or naturalized, resident species with similar ecology, although the exact arena of competition is not always clear (Sax, Stachowicz, & Gaines, 2005). In many cases, this can result in competitive displacement, wherein the superior competitor's population grows (often the invasive species), while the inferior competitor's population declines (Lounibos, 2007; Mooney & Clelland, 2001; Reitz & Trumble, 2002). If the competitive displacement is complete, the inferior competitor goes extinct in the new range of overlap between the two species. However, during an invasion, evolutionary theory predicts if the resident species is not extirpated, selection on the poorer competitor to avoid competition over generations can result in character displacement (Grant, 1972; Strauss, Lau, & Carroll, 2006). Character displacement can be seen by changes in behavioural and/or physical phenotypes that lessen the degree of overlap in niche between the invasive and resident species (Anderson & Lawler, 2016; Losos, Marks, & Schoener, 1993; Pfennig & Murphy, 2000). In the case of competition for resources, this may result in habitat partitioning, exemplified in the evolution of benthic and limnetic sticklebacks (Schluter & McPhail, 1992). For a reproductive character, like courtship displays, this should result in changes in reproductive behaviour, either pre- or postcopulation (Dobzhansky & Koller, 1938; Knowles & Markow, 2001). While a number of invasions have documented the phenomenon of character displacement, it has been rarely connected to selection in the genome (Stuart et al., 2014), let alone about the phenomenon of competition through mating interference. Linking phenotypic changes with changes in the genome can provide insight into the genetic pathway and physiological consequence of traits under selection and provides an opportunity to understand the connection between genes, selection, evolution and invasion ecology.
Thirty-four years ago, the Asian tiger mosquito (A. albopictus) invaded the United States which resulted in a well-documented decline in the resident yellow fever mosquito, Aedes aegypti, except for urban areas in south Florida, Louisiana, Texas and Arizona (Hahn et al., 2017; Hawley, Reiter, Copeland, Pumpuni, & Craig, 1987; Hopperstad & Reiskind, 2016; Moore, 1999). The rapid displacement of the resident mosquito was likely due to both the superior competitive ability of A. albopictus as larvae and asymmetric mating interference competition, in which male A. albopictus mate with and sterilize A. aegypti females (satyrization) (Ribeiro & Spielman, 1986). Juliano (2010) observed A. albopictus is a superior competitor for limited larval resources, providing evidence of asymmetric interspecific competition during mosquito development. In addition, there is growing support for a role of asymmetric interspecific sexual competition (Bargielowski, Lounibos, & Carrasquilla, 2013; Tripet et al., 2011). In this system, males of either A. aeygpti or A. albopictus will mate with females of the other species (interspecific mating), as documented in the laboratory and in field settings (Bargielowski et al., 2013; De Jesus & Reiskind, 2016; Tripet et al., 2011). However, when a male A. aegypti mates with a female A. albopictus, she can and will remate with a conspecific male and produce viable eggs. In an alternative manner, when a male A. albopictus mates with a female A. aegypti, she is rendered permanently sterile and will not produce viable eggs even when given the opportunity to remate with a male A. aegypti (Nasci, Hare, & Willis, 1989; Tripet et al., 2011). This asymmetrical sexual interaction, or satyrization effect, results in a competitive advantage for A. albopictus and can work in conjunction with exploitative competition to displace an inferior competitor (Ribeiro & Spielman, 1986). The combination of both of these effects, larval resource competition and satyrization, helps explain the rapid decline of A. aegypti in both the United States and Bermuda (Kaplan, Kendell, Robertson, Livdahl, & Khatchikian, 2010; Lounibos, Bargielowski, Carrasquilla, & Nishimura, 2016).
One line of evidence for the importance of the satyrization effect, given the difficulty of observing interspecific mating in the field, is an increase in the ability of female A. aegypti to resist mating with male A. albopictus and therefore regain the potential to contribute progeny to the next generation (Bargielowski et al., 2013). The phenotype of resisting satyrization is associated with the biogeography of A. aegypti populations with the invasive mosquito, with populations sympatric with A. albopictus resisting interspecific mating better than A. aegypti populations allopatric with A. albopictus (Bargielowski et al., 2013). Furthermore, the evolution of this resistance phenotype was confirmed in the laboratory through selection experiments (Bargielowski & Lounibos, 2014; Bargielowski et al., 2013). In addition to an increase in selected A. aegypti avoiding interspecific mating (from about 50% to 90%), A. aegypti females selected for resistance were smaller and less fecund, suggesting a high cost to satyrization resistance (Bargielowski & Lounibos, 2014).
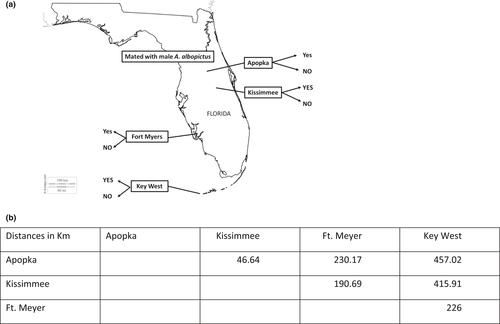
Here, we tested the hypothesis that the satyrization resistant phenotypes are detectable in changes in the genome of A. aegypti. We did this by comparing four populations of mosquitoes generated from a previous selection experiment (Bargielowski & Lounibos, 2014), and four wild-derived populations from Florida, USA, that show varying levels of satyrization resistance between each population (Figure 1, Lounibos et al., 2016). The selection experiments started with A. aegypti populations from Arizona and Key West, FL, that had likely never interacted with A. albopictus during the years of invasion of A. albopictus in the United States and then were selected over 5–6 generations (Bargielowski & Lounibos, 2014). They phenotyped individuals from each wild-derived population as resisting or succumbing to satyrization. We used a double-digest restriction enzyme DNA sequencing (ddRADseq) approach to generate single nucleotide polymorphisms (SNPs) in a reduced-representation genomic library in an effort to localize the signal of selection associated with the effect of satyrization by A. albopictus on A. aegypti. This study provides insight into the genomic regions and potential genes involved in and effects of selection on mate choice, with implications for the utilization of intraspecific, male-based control approaches for mosquito virus vectors (Alphey, 2014; Degner & Harrington, 2016; Wise de Valdez et al., 2011).
2 METHODS
To evaluate genomic signatures of the character displacement (A. aegypti female's mating resistance with interspecific males, in a broad sense), we used samples from a controlled laboratory setting where only exposure to interspecific males was different between control and selected lines (Bargielowski & Lounibos, 2014). We called these “the artificial selection experiment.” There were two female sources for this part, one from Tucson, AZ, and the other from Key West, Florida, populations with no known history of exposure to A. albopictus (Bargielowski & Lounibos, 2014). Given the artificial selection scenario in this study, we also wanted to compare genomic signatures from the laboratory selected populations to variation in female choice in wild mosquitoes from four populations in Florida. The wild-derived samples were individually phenotyped as “mated with” or “resisted” interspecific mating (YES or NO, respectively). We called these “the Florida wild-derived experiment.” For the Florida wild-derived experiment, only the Key West individuals were allopatric to the conspecific A. albopictus, with the other Florida wild-derived populations sympatric with A. albopictus since at least the mid-1990s (Moore, 1999).
2.1 Mosquito sources
2.1.1 Artificial selection experiment
The previous study by Bargielowski and Lounibos (2014) generated the material for the artificial selection experiment. We analysed 93 individuals from four separate lines of mosquitoes. These mosquitoes originated from populations allopatric with A. albopictus, collected from Key West, Florida (KW) or Tucson, Arizona (TUC). They were kept in a single insectary maintained at 27 (±0.62)°C, and 89 (±5.28) % rH with a 14L:10D light regime. For each population (KW or TUC), Bargielowski and Lounibos (2014) established a selected (G) and control line (F), by exposure to A. albopictus males (selected lines) for 3 weeks. They did not expose control lines to A. albopictus males. This yielded four groups from which we genetically sampled females: TUG (selected line originating from Tucson, n = 21), TUF (control line originating from Tucson, n = 24), KWG (selected line originating from Key West, n = 24) and KWF (control line originating from Key West, n = 24). They carried the Key West and Tucson selection group and the Tucson control group through six generations (G6, F6), but the control Key West group was in its 9th generation from the field (F9). The selected lines resisted interspecific mating in 85%–90% of the encounters, compared to 50%–60% in control lines.
2.1.2 Florida wild-derived sample trials
We collected four populations of female A. aegypti in Florida from Apopka, Kissimmee, Ft. Myers, and Key West and exposed 150 F2 females to 150 male A. albopictus for 3 weeks (see Lounibos et al., 2016 for details; Figure 1). From this experiment, we analysed 79 individual F2 A. aegypti females from the four populations (Apopka n = 19, Kissimmee n = 20, Ft. Myers n = 20, and Key West n = 20). These A. aegypti females showed different population levels of resistance to satyrization when exposed to interspecific males: Key West: 57%; Kissimmee: 70%; Apopka: 78%; Ft. Myers: 85%, estimated from three replicates of 150 females from each population. We considered Key West allopatric with A. aegypti, and it had low population levels of resistance, while Apopka and Ft. Myers had high levels of resistance to satyrization and Kissimmee was intermediate (Lounibos et al., 2016). Individual females either mated with A. albopictus males (YES) or they did not (NO), as assessed by filled versus empty spermathecae after 3 weeks.
2.2 DNA extraction
We extracted genomic DNA from the whole body of the mosquito. We used a Qiagen DNA Extraction Kit (Qiagen Inc., Valencia, CA) and quantified template DNA using a fluorometer (Qubit 2.0; Invitrogen, Carlsbad, CA) following both manufacturer's protocols, with the exception that we used 30 μl of proteinase K, digested the samples for 72 hrs and eluted in H2O to allow for subsequent concentration of DNA if needed.
2.3 Double-digest RAD sequencing (ddRADseq) library building
We built ddRADseq libraries using the enzyme pairs SphI and MluCI and following the protocol and method outlined in Burford Reiskind et al. (2016). We built two libraries, one of 93 (artificial selection experiment), and one of 79 individuals (wild-derived samples) using 200 ng of template DNA per individual (Table 1). We conducted paired-end sequencing of 100 bp fragments of the first library on the Illumina HiSeq 2000 at University of North Carolina, Chapel Hill, but given the low quality of second reads, we only used the single-end reads. For the second library, we conducted single-end sequencing of 100 bp fragments on the Illumina HiSeq 2500 at North Carolina State University Genomic Sequencing Laboratory. Specifications for sequencing were 10 nmol/L in 20 μl. For the wild-derived samples, we used single-end reads because it produced a greater number of high-quality polymorphic loci than the paired-end sequencing we abandoned with the selection experiment.
| Population | N | H E | H O | F IS |
|---|---|---|---|---|
| Tucson control | 21 | 0.174 | 0.144 | 0.175 |
| Tucson selected | 24 | 0.171 | 0.148 | 0.137 |
| Key West control | 24 | 0.172 | 0.143 | 0.165 |
| Key West selected | 24 | 0.174 | 0.148 | 0.147 |
| Apopka | 19 | 0.206 | 0.170 | 0.177 |
| Kissimmee | 20 | 0.187 | 0.170 | 0.087 |
| Fort Myers | 19 | 0.232 | 0.180 | 0.225 |
| Key West | 21 | 0.207 | 0.168 | 0.186 |
Note
- The selected line analysis uses 15,695 loci and the Florida wild-derived mating trial analysis uses 47,448 on loci.
2.4 Double-digest RAD sequencing library analysis
2.4.1 Initial quality control
The Illumina platform automatically demultiplexed the two indices into separate fastq files. We used fastqc (Babraham Bioinformatics; http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) to check the quality of the reads, using a high base score criterion (Phred > 33), prior to processing the barcodes as outlined in Burford Reiskind et al. (2016). We then ran the process_radtags script to filter and demultiplex our variable length barcodes in stacks v.1.24 (Catchen, Hohenlohe, Bassham, Amores, & Cresko, 2013). We trimmed the reads to 90 base pairs, to make all read lengths identical in length as required by the stacks platform.
2.4.2 Single nucleotide polymorphism detection
For SNP detection, we ran the denovo pipeline (denovo.pl) available in stacks. We ran all runs through the denovo pipeline with the following parameters: m = 3 (minimum stack depth), M = 2 (mismatches allowed between reads within an individual for creating loci) and n = 2 (mismatches allowed between loci when combining them in a catalog for all individuals) (Catchen et al., 2013). We then used population pipeline (populations) in stacks with parameters as follows: minimum number of stacks per individual at a locus (m = 5), number of populations loci present in (p = 2), proportion of individuals within a population that have these loci (r = 0.75) and appropriate output files for downstream analyses. While stacks pipeline provides the possibility to create data sets in various formats, we used the plink v1.19 format (Purcell et al., 2007) as it is versatile for large NextGen sequence data. We used the program pgdspider v.2.1.1.0 (Lischer & Excoffier, 2012; http://www.cmpg.unibe.ch/software/PGDSpider/) to transform the plink data set in various input file formats required by the following software: genepop v.4.2 (Rousset, 2008), lositan (Antao, Lopes, Lopes, & Beja-Pereira Luikart, 2008), structure 2.3.4 (Hubisz, Falush, Stephens, & Pritchard, 2009; Pritchard, Stephens, & Donnelly, 2000), geneland (Guillot, Renaud, Ledevin, Michaux, & Claude, 2012), bayescan (Foll & Gaggiotti, 2008) and discriminant analysis of principal components (dapc) implemented in adegenet (Jombart, 2008). For the artificial selection samples, we first filtered the data set for minimum allele frequencies (MAF) in plink (–maf 0.01), removing monomorphic loci which would interfere in some analyses such as geneland. For the wild-derived samples from Florida, we assessed genetic differentiation using two types of filtering method for each data set: (a) one containing loci filtered through the MAF plink filter (–maf 0.01); (b) the second “neutral loci” data set that filtered loci using the plink filters (–maf 0.01), missing genotypes (–geno 0.02) and Hardy–Weinberg filter (–hwe 0.05) for the analysis of geographic genetic structure.
2.4.3 Genetic characteristics
To characterize the genomic data and confirm whether or not there was genetic differentiation among samples, we measured genetic diversity (HE), inbreeding coefficient (FIS) and genetic differentiation (pairwise FST and pairwise exact test (MCMC parameters: 20,000 dememorization, 500 batches, 10,000 iterations per batch)) in genepop using the above-mentioned respective data sets. For the artificial selection samples, we analysed control and selected samples for both locations (Tucson and Key West, total of four sample groups) in genepop. For the wild-derived samples, we organized the samples in two different data sets: (a) by geographic location called “Geographic” (four distinct geographic locations) and (b) by mating phenotype called “MatedGeo” (two groups containing locations that had mated females “YES” and those that were not “NO”). For the wild-derived samples, we measured the genetic differentiation using the Bayesian assignment program structure for both the Geographic and Mated Geo using the neutral loci data set. We ran structure with 100,000 burnins, 100,000 MCMC replicates, a K ranging from 1 to 5, and with 10 iterations per K for the Geographic data set, using a random number seed. We used Structure Harvester (Earl & vonHoldt, 2012) to determine the likelihood of number of clusters and significance among sample locations. We also ran geneland for comparison to structure using the following parameters: 100,000 burnins, 250,000 MCMC replicates, a K ranging from 1 to 5, with 20 iterations per K for Geographic (four groups). At last, we compared the results from both structure and geneland to the clustering analysis in dapc after we ran a cross-validation analysis with 95 iterations in the adegenet package (Jombart, Devillard, & Balloux, 2010) using the full data set.
2.4.4 Outlier loci
The main goal of this study was to associate genomic loci under selection with respect to satyrization resistance. Therefore, we detected outlier loci between selected and control individuals in the artificial selection experiment and between YES and NO mating in the wild-derived samples using three different methods: bayescan 2.1, lositan and dapc. We generated outlier loci using the default parameters with the exception of 100,000 burnin and 10,000 prior odds in bayescan 2.1, 10 reps of 1,000,000 simulations in lositan and conducted a dapc in r using the package adegenet. To obtain the optimum number of principal components to retain in the dapc, we performed a cross-validation method using a 90% training set and 95 replicates, and conducted it with the chosen number of principal components using average linkage clustering method to set a threshold for outlier loci. We applied false discovery rate correction factor of the p-value of 0.05 based on reported high false positive loci rates in bayescan and lositan's main algorithm FDIST2 (Beaumont & Nichols, 1996). For outlier loci, we first checked all three programs for those loci that overlapped, compared the pairwise comparisons of the selection experiment (e.g., Tucson G vs. F with Key West G vs. F) to see whether similar genomic regions were under selection between the two origins and then evaluated a subset of outlier loci against the A. aegypti draft genome (From the A. aegypti Genome Working Group—Assembly AegL5.0-GCA_002204515.1 submitted to Vectorbase). We also checked for LD among outlier loci in genepop and aligned the outlier loci against the chromosome map in Vectorbase.
We ran the following comparisons to look for outlier loci. For the laboratory selected and control lines, we first compared all control to all selected lines and then compared selected and control lines within the Tucson lineage and then within the Key West lineage. For the outlier analysis of the wild-derived samples, we compared all YES to all NO combining all populations (two groups). This grouping allowed us to both control for any geographic signature across locations to avoid outlier loci related to geographic structure and to increase the power to detect differences between the two phenotypes.
For specific outlier loci shared between the two origins for the selection experiment, we also looked at the pattern of allele frequencies. Our goal here was to determine whether allele frequencies found in either the control or selected lines were significantly different from each other and whether this pattern held in the different source lines. For example, we asked if a locus was fixed for an A in the selected line from Tucson, was it also fixed for A in the selected line from Key West. To test for significant differences, we conducted a two-tailed Fisher's exact test comparison between the selected and control lines for each of the source populations.
For a subset of outlier loci that we found in both replicate selection lines (Tucson & Key West) and/or between the YES and NO wild-derived samples, we aligned outlier loci to the A. aegypti draft genome (AaegL5.0-GCA_002204515.1) to both identify locations of potential outlier loci and evaluate the degree of clustering of outlier loci throughout the genome. We aligned the sequences containing outlier loci to the draft genome using geneious 9.5 algorithm (http://www.geneious.com, Kearse et al., 2012). We calculated degree of clustering of aligned sequences and calculated distance to the closest protein-coding genes and evaluated the proposed functions of annotated genes. We recognized that using the least conservative outlier loci detection method would result in false-positives. However, the goal was to identify whether the loci were linked, clustered or spread throughout the genome and identify potential regions for further study. Therefore, we biased towards type 2 error over type 1 error to not exclude true outlier loci.
3 RESULTS
3.1 Library quality and SNP detection
For the artificial selection experiment, the stacks pipeline generated 6,022,063 loci, the population pipeline generated 18,466 polymorphic loci, and after filtering with plink, there were 15,695 polymorphic loci. For the wild-derived Florida samples, the stacks pipeline generated 3,563,841 loci, the population pipeline generated 49,249 polymorphic loci, and after filtering using plink, there were 47,488 polymorphic loci. For population genomic questions, we analysed the neutral data set of 5,612 loci from the Florida wild-derived sample with the structure and geneland analyses. The discrepancy in number of useable polymorphic loci between the artificial selection and the wild-derived experiments reflected the increase in number of distinct populations in the wild-derived sample to four geographically distinct populations and the change to single-end reads. For the selection experiment, the second pair of the paired-end reads produced a larger number of low-quality loci that were filtered out.
3.2 Genetic diversity
Within population, genetic diversity was not different among the populations whether from the selection experiment or from the wild-derived trial samples (Table 1). In the wild-derived female mating experiment, there was slightly higher genetic diversity in Ft. Myers, Florida. We also found higher genetic diversity in the wild samples as opposed to the artificial selection samples, but this was not significantly different (two-tailed, two-sample t test p = 0.065, data not shown). We found higher inbreeding coefficients in the wild-derived samples at Ft. Myers and to a smaller degree at Key West, and higher inbreeding coefficients for the control versus selected lines in the artificial selection experiment. The lowest inbreeding coefficient for any of the samples was at Kissimmee, Florida, in the wild-derived experiment. However, these were only small differences, which was supported by previous findings of little difference in inbreeding between wild-derived and laboratory-reared mosquitoes that might be found in other laboratory-reared mosquito systems (Armbruster, Hutchinson, & Linvell, 2000).
3.3 Genetic differentiation
For the artificial selection experiment, we found significant genetic differentiation between the two source populations Tucson and Key West in the FST analysis and the exact test in genepop (Table 2). We did not find significant genetic differentiation between the selected and control lines within either of the two source populations. Therefore, we did not find genomewide differentiation that would be expected with genetic drift (Table 2).
| Line 1 | Line 2 | F ST |
|---|---|---|
| Tucson_Selected | Key West_Control | 0.149 |
| Tucson_Selected | Key West_Selected | 0.158 |
| Key West_Control | Tucson_ Control | 0.123 |
| Key West_Selected | Tucson_Control | 0.132 |
| Key West_Control | Key West_Selected | 0.021 |
| Tucson_Selected | Tucson_Control | 0.038 |
Note
- Significant pairwise comparisons from the exact test are bolded based on an exact test of significance implemented in genepop.
We found similar results with the wild-derived mating trial of A. aegypti females, with genetic differentiation among geographic source locations but not between individuals that resisted satyrization versus those that mated with A. albopictus within a location (Table 3). Geographic genetic structure revealed a pattern of genetic differentiation that did not reflect an isolation-by-distance pattern (regression of direct line distance KM by FST/(1 − FST) p = 0.698; regression of driving distance KM by FST/(1 − FST) p = 0.771; data not included). All pairwise FST values among locations were significant (Table 3). One location, Kissimmee, had higher pairwise FST values than other pairwise comparisons (Table 3, Figure 1 and Supporting Information Figure S1). The results from the structure harvester analysis and geneland both supported a ΔK of 4 for the Geographic data set (four groups), which corroborated the findings from genepop (Supporting Information Figure S1; geneland data not shown). In addition, the dapc cluster analysis supported this pattern for the Geographic data set using 35 PCAs (Supporting Information Figure S2).
| Apopka | Kissimmee | Fort Myers | Key West | |
|---|---|---|---|---|
| Apopka | – | – | – | – |
| Kissimmee | 0.214 | – | – | – |
| Fort Myers | 0.134 | 0.189 | – | – |
| Key West | 0.192 | 0.239 | 0.158 | – |
Note
- The significant pairwise comparisons are from the exact test and are bolded.
3.4 Outlier loci
The results from bayescan, lositan, and dapc all showed several outlier loci in both the artificial selection experiment and wild-derived samples (Tables 4 and 5; Supporting Information Figures S3–S6). We found a pattern where lositan had a greater number of outlier loci than either bayescan or dapc for all combinations of data analyses (Tables 4 and 5). In general, the outlier loci identified by either bayescan or dapc overlapped with lositan. We found a greater number of outlier loci in the wild-derived samples (mated YES vs. NO) than in the artificial selection samples (selected vs. controlled), likely because we started with a greater number of loci in the Florida wild-derived experiment than in the artificial selection experiment.
| Nonoutlier loci | Outlier loci | ||
|---|---|---|---|
| Selected versus control | bayescan | 15,692 | 3 |
| lositan | 14,222 | 1,473 | |
| dapc | 15,647 | 25 | |
| Tucson selected versus control | bayescan | 10,691 | 0 |
| lositan | 9,674 | 1,017 | |
| dapc | 10,680 | 11 | |
| Key West selected versus control | bayescan | 11,824 | 0 |
| lositan | 11,160 | 664 | |
| dapc | 11,816 | 8 |
Note
- bayescan, lositan and dapc are the three programs implemented for outlier loci discovery.
| Nonoutlier Loci | Outlier Loci | ||
|---|---|---|---|
| Mated versus nonmated | bayescan | 47,406 | 41 |
| lositan a | 33,312 | 5,611 | |
| dapc | 47,442 | 6 |
Note
- a The nonoutlier loci do not include the loci that show balancing selection.
3.4.1 Selected lines
When we compared two groups, selected and control, regardless of population origin, we found 1,473 outlier loci in lositan, and only three and 25 outlier loci with bayescan and dapc, respectively (Table 4, Supporting Information Figure S3). When we compared selected versus control lines within each population, we found 1,114 outlier loci in the Tucson and 664 outlier loci in the Key West comparison, and only 49 specific loci that were the same between the two comparisons in lositan (Supporting Information Figure S4). These 49 loci were found in 32 reads (sequences), with some reads containing two to three SNPs. In analysing the specific base pair that generated the SNPs for each loci using Fisher's exact test, the identical 49 outlier loci had significant differences in allele frequencies between selected and control lines at eight and nine loci unique to Tucson or Key West, respectively (Table S1). We also found that 16 of the 49 outlier loci had significant differences in allele frequencies between the selected and control lines for both origins (Table S1). Of these 16 loci that were significant for both, 12 loci showed a significant difference in allele frequencies between selected and control lines in the same direction (e.g., both had a higher frequency of A in the control line and T in the selected line) and four loci had significant differences in allele frequencies in the opposite direction (e.g., a higher frequency of A in the control line of Tucson and higher frequency of T in the control line of Key West) (Table S1).
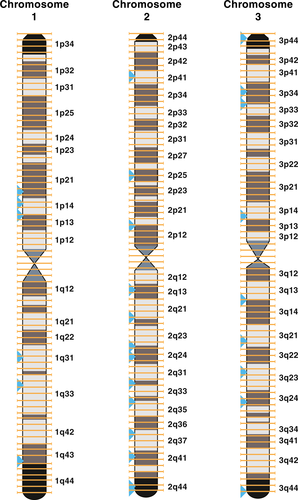
Aligning the 32 sequences containing the 49 outlier loci identified by lositan to the AaegL5 draft genome, we found that they were in different regions of the genome, with similar SNPs per base pair for each of the three chromosomes when we considered size of the chromosome, and all sequence regions more than 1 CentiMorgan (cM) apart (i.e., 1 million base differences in alignment position within a chromosome; Table in DRYAD Digital Repository). We did not find evidence of LD among loci except for those that were on the same sequence read and three pairwise comparisons two of which were between linked loci on the same sequence and another loci on a sequence nearby on chromosome 2 (Catalog # 179777 and 40963, respectively; Table in DRYAD Digital Repository 2, Figure 2), and the final one was linkage between two loci that mapped to different chromosomes, chromosomes 1 and 2 (Catalog # 126816 and 163493; Table in DRYAD Digital Repository, Figure 2). We cannot determine whether this linkage is an artefact of the assembly, the number of pairwise comparisons, or biologically important. We also found that many of these sequences were in or near protein-coding regions including proteins involved in immune function, metabolic proteins, nervous system development/insecticide resistance, odorant receptors, and several studies showed differential expression in females versus males at these genes (Table in DRYAD Digital Repository). Specific examples included ATPase subunit beta, which was found to be differentially expressed in females versus males, peflin a Ca2+ binding protein, cadherin gene exon 5 implicated in insect resistance (Jin et al., 2014), acetylcholinesterase (Ace1) gene implicated in disease and insect resistance (Weill et al., 2002); Clip Domain Serine Protease gene (CLIPD9) family implicated in immune function (Kanost & Jiang, 2015).
3.4.2 Florida wild-derived samples
For the Mated YES versus NO group, we found 5,611 outlier loci in lositan and 42 and six outlier loci in the bayescan and dapc, respectively (Table 5, Supporting Information Figures S4 and S6).
When we aligned the outlier loci found in lositan between YES and NO to AaegL5 draft genome, we found the 5,611 outlier loci on 4,023 reads (sequences), and most of these sequences aligned on chromosome 2 (Table S2). However, when we compared the size of each chromosome, we did not find a difference in the number of SNPs per base per chromosome (Table S2). In addition, we found a greater amount of clustering of the sequences compared to the artificial selection experiment with approximately 32% of the SNPs within 100,000 bps of each other, a pattern that held across all three chromosomes (Table S2). We also found 20% of the sequences identified in the selection experiment overlapped with the sequences that contained outlier loci in the wild-derived experiment and approximately 60% were within 250,000 bps of each other (Table S3).
4 DISCUSSION
We found outlier loci between selected lines of female A. aegypti exposed to interspecific (A. albopictus) males compared to control females, some of which were convergent between the selected lines from Tucson, Arizona, and Key West, Florida, suggesting these outlier loci were important in resistance to satyrization. We also found outlier loci between wild-derived females from Florida that either mated with or resisted interspecific males (YES or NO). While there were more outlier loci found in the wild-derived samples than in the artificial selection experiment, we did not have enough statistical power to determine if this was due to the short-term nature of the selection environment in the laboratory, a difference in genetic diversity, or an artefact. Indeed, we suspect it was likely due to the larger database of the wild-derived sample from the sequencer. In addition to evidence of outlier loci between the mating trials, we also found genetic divergences among geographic locations of the wild-derived samples in Florida. In a collective way, the results suggest that strong selection against satyrization led to rapid evolution at the genomic level. Aligning a subset of the outlier loci in the controlled experiment to the most recent draft genome, we found 32 separate locations that contained outlier loci shared by both comparisons of selected and control lines. This finding suggests that putative selection regions are spread across all three chromosomes, do not overlap and are close to a variety of protein-coding regions. We were encouraged that the outlier loci in the artificial selection experiment either overlapped or were in close proximity to the outlier loci in the wild-derived study. Despite the de novo analysis of the sequences generated in this study, the majority of the outlier loci were within or near genes for A. aegypti.
4.1 Laboratory selection experiment
Our findings supported a genomic signature of the changes in mating phenotype documented by Bargielowski et al. (2013) in the selected lines. The high cost of loss of reproductive potential of female A. aegypti due to interspecific mating suggests selection against interspecific mating would be strong enough to detect at the genomic level. Female choice in this system comes at a high cost and the females from the selected lines had lower fecundity and smaller body size (Bargielowski & Lounibos, 2014). This further supports the strong selection for female interspecific mating resistance, as the selection needed to be strong enough to overcome these costs. The cost of selection may reflect behavioural and physiological adaptations necessary to avoid satyrization. For example, it is possible that smaller (and therefore less fecund) A. aegypti females are better able to avoid male A. albopictus. While male body size has been shown to be important in mating (De Jesus & Reiskind, 2016; Helinski & Harrington, 2011), there is little work on female body size and mating behaviour. It is also possible that traits associated with body size and fecundity are pleiotropic with resistance to satyrization through a variety of pathways yet to be described.
The specific outlier loci shared between the selected and control lines in two different source comparisons suggested that the phenotypic variation in female choice had similar patterns of selection in the genomes. Considering these shared outlier loci located on 32 sequence reads, 30 of the 32 reads aligned with the A. aegypti draft genome within or near protein-coding regions. While several had no known function, most of the annotated genes that these outlier sequences aligned within or near do. For example, we found outlier loci within or near the cadherin gene exon5, an acetylcholinesterase gene associated with pesticide resistance, ATP synthase subunit beta, CLIPD9 family and hepatic arginase. Two of the genes, cadherin and acetylcholinesterase, are implicated in insecticide resistance in other insect species (Jin et al., 2014; Weill et al., 2002) and one of the genes CLIPD9 is important in immune function (Kanost & Jiang, 2015). We also found several of the aligned sequences near protein-coding regions associated with differential expression in males versus females or in females associated with forest versus domestic environments (Dissanayake et al., 2010; Hall et al., 2015; McBride et al., 2014). While these particular genes have not been implicated in female choice before this study, these genes may have pleiotropic or epistatic effects on mating behaviour. This could be through modification of detoxification enzymes or changes in insecticide target-site confirmations, which have been associated with changes in response to alarm cues in other insects (Foster et al., 2003). Male mosquito mating competitiveness is affected by target-site insecticide resistance in Anopheles coluzzi, a vector of malaria in Africa, although the impact on female mating behaviour has not been addressed (Platt et al., 2015). These results further support that these outlier loci are in regions of interest to pursue in either targeted sequencing or in a future RADseq QTL study that address mating resistance in female A. aegypti.
In contrast, there were several loci that were unique to one of the two selected lines. Therefore, while there may be similarity in some of the genomic regions, there are potential differences in genomic regions that may be due to the genetic background. If true, this suggests that the phenotypic trait of mating resistance under directional selection may differ between the two source populations in their overall genetic architecture. This genetic architecture may include differences in minor effect genes, which could depend in part on the genetic origin of the A. aegypti population.
As with any association study, a follow-up study to isolate the resistant phenotype would strengthen the connection between mating resistance and candidate genes. Other studies have detected outlier loci associated with rapid evolutionary response in several systems, although not in response to changes in mating behaviour (Moran & Alexander, 2014). In addition, some studies have shown rapid evolution at the phenotypic level, but have not analysed whether this was due to phenotypic plasticity or directional selection at the genome level (Angert & Schemske, 2005; Burford, Scarpa, Cook, & Hare, 2014; Reznick, Bryga, & Endler, 1990; Sultan, Horgan-Kobelski, Nichols, Riggs, & Waples, 2013). While Stuart et al. (2014) found evidence of the genetic component of character displacement between an invasive and resident anole species, confirming this in a common garden experiment, they did not attribute it to specific genomic regions or outlier loci. Previous studies conducting genomic scans for outlier loci associated with adaptation across a selection gradient provided promising results, but identified the limitations of conducting correlations with selection sources which lacked the control of multiple inputs or variables that we controlled in the artificial selection experiment (Bonin, Taberlet, Miaud, & Pompanon, 2006; Eckert et al., 2010; Nunes, Beaumont, Butlin, & Paulo, 2011). However, a novel approach to understanding environmental gradients in a marine system created common gardens and tested for gene expression differences and polymorphic outlier loci (Pespeni, Oliver, Manier, & Palumbi, 2010; Pespeni & Palumbi, 2013). Without the type of control we had in the selection experiment, it is unclear whether some of the outlier loci found in these previous studies were due to neutral events, evolutionary history or some other force that was not measured and not due to the specific type of selection measured. In an alternative manner, the control provided by a selection experiment that exists for many generations can also suffer from intrinsic issues due to small population dynamics (Gerke, Edwards, Guill, Ross-Ibarra, & McMullen, 2015). Yet, we found these results after only six generations of selection and without indication of reduced genetic diversity in the selected lines. The intention of the artificial selection experiment was to replicate what occurred when A. albopictus invaded an area with resident A. aegypti. However, the setting of our selection experiment is obviously artificial and may be too different to reflect natural selection in the wild. To confirm whether the patterns we observed in the selected lines from the laboratory, we looked for the same patterns in the wild-derived samples.
4.2 Wild-derived female mating trials
An important consideration is whether an artificial selection scenario, as exemplified by the selection scenario in this study, is something that might also contribute to strong directional selection or female choice in the wild. While we found significant population genetic structure in the Florida wild-derived samples from four different locations, suggesting limited gene flow among these populations, we did not find a signature of isolation by distance. Once we corrected for geographic genetic structure, by grouping all YES and all NO separately across all populations, we found the wild-caught females revealed evidence of directional selection. Given the association with either YES or NO in the wild-derived trial, these outlier loci may be related to female resistance to interspecific mating. We were also encouraged that those loci we found in the selection experiment were close to or overlapped with loci we found in the wild-derived females. While the number of outlier loci for this experiment was greater than that of the selection experiment, many of these outlier loci were likely tightly clustered or linked and not functioning independently. We did not find any indication that a particular chromosome showed a greater amount of clustering of outlier loci than or any other support for a chromosomal modification. Previous studies showed chromosomal rearrangements between the subspecies A. aegypti aegypti (Aaa) and A. aegypti formosus (Aaf) that may contribute to reproductive isolation (Dickson et al., 2016; McBride et al., 2014; Moore, 1979), but this was found within Africa and not in populations outside of Africa (Dickson et al., 2016). Here, we have only looked within Aaa and do not have evidence of chromosome rearrangement contributing to putative selection in these genomic regions.
Given the lower statistical power of only a few individuals (n = 9 or 10/population × phenotype) for these comparisons per population, we were encouraged that we found a similar pattern in outlier loci among all the comparisons. Furthermore, the additional analysis of the wild-derived female mating trial from multiple locations in Florida confirmed the importance of accounting for the geographic component of the genetic structure, which controls for background variation between populations or geographically based differential selection, when conducting outlier loci analyses in general. In future studies, increasing the replication of these mating trials with wild samples in sympatry and allopatry with the conspecific A. albopictus would help differentiated whether putative regions of selection differ between these two scenarios.
To understand the genetic background of A. aegypti in Florida and potential movement of mosquitoes in general, the high genetic differentiation of SNPs among samples in Florida that had close geographic associations was an important result from this study. While the higher degree of genetic divergence between Kissimmee and Key West followed an isolation by geographic distance pattern, the similar degree of genetic differentiation between neighbouring Apopka and Kissimmee did not. Apopka was genetically differentiated from Kissimmee and both were from other locations in Florida. To what degree this is a consistent pattern will require sampling throughout the Florida region at a finer scale and mapping this finer scale sampling to the landscape features and sympatry and allopatry to better understand the degree of genetic exchange or gene flow among locations. Previous studies using mitochondrial sequences suggested complex patterns of genetic divergence, with no support for isolation by distance (Damal, Murrell, Juliano, Conn, & Loew, 2013), as does a study comparing A. aegypti on Key West to populations on the mainland (Brown, Obas, Morley, & Powell, 2013). Despite the rapid generation time, and the ability to move among locations via human-mediated transport, A. aegypti populations have shown marked geographic genetic structure. In addition, Hopperstad and Reiskind (2016) showed evidence of local range expansions (or re-expansions, as A. aegypti was historically present in these locations) over short distances (3–5 km) in Palm Beach County and Lounibos et al. (2016) presented evidence for stable to slight increases in areas of A. aegypti since the invasion examining sites around Florida south of Orlando. This evidence of recent expansion combined with our findings suggests a landscape genomic approach would be important to understand gene flow in this species.
This study supports female choosiness or selection against aberrant mating with interspecific males at the genomic level and suggests that it is likely a complicated genotype involving multiple genomic regions. Previous studies have shown that female choosiness in animals is a complex behaviour and may be due to a variety of phenotypes that could possibly shift among geographic locations or over time (Chaine & Lyon, 2008; Lawniczak & Begun, 2004; MacKay et al., 2005). To add to the complexity of different genetic architecture, there may be different large effect or many small effect genes that may or may not interact epistatically or pleiotropically that contribute to these phenotypes (Chenoweth & Blows, 2006; MacKay et al., 2005). Our ability to only detect large effect genes or attribute outlier loci to adaptation is an issue that plagues both candidate gene approaches and reduced-representation sequencing (Bierne, Welch, Loire, Bonhomme, & David, 2011; Chenoweth & Blows, 2006). While previous studies may show the genetic architecture or candidate genes associated with postmating female choice (Lawniczak & Begun, 2004), the genetic basis for the interspecific mating avoidance of female A. aegypti is likely prior to copulation particularly as copulation even without sperm migration can cause female sterility (Carrasquilla & Lounibos, 2015). Therefore, this behaviour may involve other genes not currently identified. Moreover, as revealed by MacKay et al. (2005) increased female latency, which reduces female receptivity and leads to reproductive isolation, may be the behavioural phenotype associated with A. aegypti female avoidance of mating with interspecific males (Bargielowski & Lounibos, 2014). Variation in the timing of receptivity within the populations of A. aegypti could provide the pathway to rapid directional selection against making the wrong choice and may have a high fitness cost. Given the patterns of female choice highlighted by this study, we argue that it is important to consider geographic genetic structure, geographic origin and specific genomic regions when mapping the genes for such a complicated phenotype as female choice. Furthermore, we suggest that this complicated behaviour will have a genetic architecture that will likely involve epistatic and pleiotropic effects and potentially different sets of small effect genes depending on the geographic origin. Future directions will require a detailed analysis of these potential candidate genes found in this outlier analysis for evidence of selection.
In this study, we provide a genomic basis for understanding the impact of the invasion of A. albopictus on A. aegypti by finding signatures of selection in the A. aegypti genome to a specific type of interspecific interaction: mating. We found evidence of loci under selection within the genome by comparing artificially selected resistant phenotypes to controls and examining wild-derived populations of A. aegypti. Our work is important for understanding how organisms respond to biological invasion, the future of these two pathogen vectors, and has implications for the use of male-based control approaches for mosquitoes. The long-term patterns of these two species are difficult to predict, but the evidence for rapid evolution of character displacement in A. aegypti in response to A. albopictus may suggest a future in which these two species co-exist at fine and coarse scales and thus increase the risk of disease transmission to human populations. Comparing current to historical distributions of A. aegypti suggests at least a stable to slightly expanding range, both regionally and locally, but without a change in A. albopictus range (Hopperstad & Reiskind, 2016; Lounibos et al., 2016). Overall, a better understanding of how rapidly A. aegypti females can overcome strong selection, combined with increases in desiccation potential due to global climate change for A. albopictus (Juliano, Lounibos, & O'Meara, 2004; Lounibos et al., 2010; Reiskind & Lounibos, 2009), suggests a potential for an increase in the population sizes of A. aegypti. This is of great concern given that A. aegypti is a superior vector of established arboviruses like dengue virus, and recently emergent viruses like chikungunya and Zika.
ACKNOWLEDGEMENTS
We thank N.M. Haddad, R.B. Roberts and R.R. Dunn for comments on an early version of this manuscript and several anonymous reviewers for their comments that improved this manuscript. This research was funded by the Wynne Innovation Grant from the CALS Dean's Enrichment Grant programme at NCSU awarded to MO Burford Reiskind. We also thank both the Applied Ecology and Entomology Departments at NCSU for providing matching funds to further fund this collaborative research. Satyrization experiments and field work was supported by NIH Grant R21 AI095780 to LPL.
DATA ACCESSIBILITY
Supporting Information will be provided with the original submission for the online version of this manuscript. This will include the results of the GenBank blast search for both the artificial selection experiment and the wild-derived mating trial. We will also provide the results of the allele frequency analysis.
Data from this manuscript are available through Dr. Martha Burford Reiskind's DRYAD account associated with this publication (https://doi.org/10.5061/dryad.kj8kp94) in the DRYAD Digital Repository and at the following link: https://doi.org/10.5061/dryad.kj8kp94. This will include post-stack analysis plink input data files for pgdspider from which subsequent input data files can be generated. The list of outlier loci locations generated by geneious 9.5 that can be aligned to the draft genome on VectorBase. A list of aligned sequences from the outlier analysis to the draft genome generated by geneious 9.5 and includes the nearest annotated gene name and function. The results of the LD analysis from genepop. In addition, the raw sequence data generated in this study will be available upon request, as the data files far exceed the limits at DRYAD without addition payment.
AUTHOR CONTRIBUTION
M.O.B.R. conceived the study, generated and analysed the data, and wrote and edited the manuscript. P.L. and M.H.R. help generate and analyse the data and edited the manuscript. I.B. and L.P.L. generated the samples for both the selection and the field-based study and edited the manuscript.




