Differential biodiversity responses between kingdoms (plants, fungi, bacteria and metazoa) along an Alpine succession gradient
Abstract
Biological diversities of multiple kingdoms potentially respond in similar ways to environmental changes. However, studies either compare details of microbial diversity across general vegetation or land use classes or relate details of plant community diversity with the extent of microbially governed soil processes, via physiological profiling. Here, we test the hypothesis of shared responses of plant and rhizosphere bacterial, fungal and metazoan biodiversities (especially across-habitat β-diversity patterns) along a disturbance gradient encompassing grazed to abandoned Alpine pasture, on acid soil in the European Central Alps. Rhizosphere biological diversity was inferred from eDNA fractions specific to bacteria, fungi and metazoans from contrasting plant habitats indicative of different disturbance levels. We found that soil β-diversity patterns were weakly correlated with plant diversity measures and similarly ordinated along an evident edaphic (pH, C:N, assimilable P) and disturbance gradient but, contrary to our hypothesis, did not demonstrate the same diversity patterns. While plant communities were well separated along the disturbance gradient, correlating with fungal diversity, the majority of bacterial taxa were shared between disturbance levels (75% of bacteria were ubiquitous, cf. 29% plant species). Metazoa exhibited an intermediate response, with communities at the lowest levels of disturbance partially overlapping. Thus, plant and soil biological diversities were only loosely dependent and did not exhibit strictly linked environmental responses. This probably reflects the different spatial scales of organisms (and their habitats) and capacity to invest resources in persistent multicellular tissues, suggesting that vegetation responses to environmental change are unreliable indicators of below-ground biodiversity responses.
1 INTRODUCTION
The search for general processes structuring biodiversity typically focuses on single ecosystem compartments, with terrestrial plant communities traditionally receiving extensive attention due to the tendency of plants to “stand still and wait to be counted” (Harper, 1977). Despite the importance of plants as primary producers of organic compounds, the extent to which the diversity of rhizosphere communities is integrated with plant communities has received little attention, probably due to the difficulty of interdisciplinary studies involving expertise and methodologies tailored to specific taxa. Recent advances in DNA sequencing technologies promise to bridge the molecular taxonomy of rhizosphere communities with plant community structure allowing investigation of the extent to which processes shaping plant communities also impact rhizosphere biological diversity and the degree of interaction between these components of the ecosystem. Indeed, recent studies have investigated links between particular groups of organisms, and general relationships between diversity and processes have been found. However, studies either detail microbial diversity across general vegetation, land use or habitat classes (Chen et al., 2015; Flores-Rentería, Rincón, Valladares, & Yuste, 2016; Kaiser et al., 2016; Paula et al., 2014), correlate the general processes governed by microbes (such as soil respiration, microbial biomass or microbial enzyme activities) with detailed surveys of plant communities (Purahong et al., 2016; Strecker, González Macé, Scheu, & Eisenhauer, 2016) or infer microbial diversity indirectly from the physiological profiling of soil samples (“functional diversity”), rather than determining taxonomic diversity directly via genetic analysis (Araya, Bartelheimer, Valle, Crujeiras, & García-Baquero, 2017; Klimek, Chodak, Jaźwa, & Niklińska, 2016; Klimek et al., 2015; Markowicz, Woźniak, Borymski, Piotrowska-Seget, & Chmura, 2015; Mureva & Ward, 2017). The responses of microbial functional and taxonomic diversities to changes in plant productivity are not directly comparable (Zhang, Johnston, Barberán, Ren, & Lü, 2017) and likely reflect the operation of different processes. While these studies are of enormous value for understanding the role of microbial diversity in ecosystem processes, a direct comparison of plant, bacterial, fungal and metazoan taxonomic richness and community structure is necessary to understand whether diversity responses to environmental gradients follow shared, general principles across different kingdoms. A corollary of this is that, should different kingdoms exhibit similar responses, then processes that are relatively easy to study, such as those of plant communities, could provide general indicators for other groups.
In the search for generality in ecology, a prominent theory aiming to explain changes in biodiversity and community structure in response to environmental gradients (i.e., encompassing different habitats and thus general patterns of β-diversity; sensu Whittaker, 1960) is that of the humped-back model of species richness/productivity (Al-Mufti, Sydes, Furness, Grime, & Band, 1977; Grime, 1973). This assumes that a general productivity gradient arises from underlying gradients of stress (limitations to metabolism) or disturbance (biomass destruction), with diversity constricted where environmental extremes select for specialist life forms, resulting in a peak for both species and functional diversity at intermediate levels of environmental pressures. This theory can be interpreted as incorporating the intermediate disturbance hypothesis (IDH; Connell, 1977), and although some controversy exists (Adler et al., 2011; Fridley et al., 2012; Houston, 2014; Pierce, 2014), extensive supporting has emerged from worldwide studies of vegetation (Fraser et al., 2015) and no comprehensive alternative hypothesis has been proposed. Humped-back curves are evident for a range of organisms (reviewed by Grime & Pierce, 2012), including bacteria (Bell et al., 2010) and insects (Montagna, Lozzia, Giorgi, & Baumgärtner, 2012;). Crucially, the emergence of humped-back curves for communities of primary producers in terrestrial and aquatic ecosystems has been empirically confirmed to result from adaptive specialization and restriction of viable functional trait values in habitats of extremely high or extremely low productivity or disturbance (Cerabolini et al., 2016; Kelemen, Török, Valkó, Miglécz, & Tóthmérész, 2013; Török et al., 2016; Vallina et al., 2014). As plants with contrasting life-history strategies exhibit different capacities to produce both biomass (in terms of both amount and quality) and carbon-rich root exudates, plant strategies at different degrees of disturbance or productivity are capable of facilitating different soil communities (Grime & Pierce, 2012). Indeed, the character of root exudates is species-specific and allows plants to target and attract specific rhizosphere microorganisms (reviewed by Huang et al., 2014). While mucilage and proteins may form the bulk of root exudate mass, differences in primary metabolites such as amino acids, carbohydrates and organic acids account for root exudate diversity, and the degree of exudation and concomitant effects on rhizosphere processes such as denitrification depends on whether the plant species are slow-growing stress-tolerators or fast-growing competitor species, sensu Grime's (2001) CSR plant strategy theory (Guyonnet et al., 2017). Indeed, soil bacterial and fungal communities are known to change in association with plant traits that reflect plant productivity, such as foliar C:N and leaf dry matter content (Sayer et al., 2017). Links between plant CSR strategies, root exudates and the rhizosphere microbiome are also suggested by models (Krause et al., 2014; Schmidt, Gravuer, Bossange, Mitchell, & Scow, 2018; Thijs, Sillen, Rineau, Weyens, & Vangronsveld, 2016). Thus, changes in plant strategies along the productivity/disturbance gradients underpinning the humped-back curve should impose effects on rhizosphere biological diversity.
The occurrence of humped-back curves throughout different groups of organisms and the potential controlling effects of primary producers led Grime and Pierce (2012) to hypothesize that patterns of soil biological diversity should reflect those of plants. Equally, the soil biota plays a key role in conditioning the edaphic environment for plants, especially by governing processes such as N fixation and mineral nutrient turnover rates, and links between plant and bacterial diversity have been interpreted as an edaphic filter regulated in part by microorganisms (Araya et al., 2017). This strongly suggests that contrasting biotic compartments of ecosystems, such as bacteria, fungi, plants and metazoa, should be interdependent and thus exhibit similar diversity responses along environmental gradients.
In this study, through an integrated multidisciplinary approach using state-of-the-art massive data generation technologies and traditional systematics, the primary producer and rhizosphere communities of selected Alpine pastures were characterized along an environmental gradient ranging from undisturbed to heavily disturbed plant habitats. We addressed the following hypotheses: (i) Diversities (richness and evenness) of plant and rhizosphere communities show similar responses along a disturbance gradient, and this response is consistently unimodal; (ii) community structures of different kingdoms are correlated; and (iii) the amount of variation in plant and rhizosphere taxa composition is correlated with abiotic (soil physicochemical properties) and biotic (grazing disturbance level and plant functional type composition) variables.
2 MATERIALS AND METHODS
2.1 Experimental design, sampling and DNA extraction
This study evaluated the diversity of plant and rhizosphere soil biological (bacterial, fungal and metazoan) communities associated with Alpine pastures in relation to different levels of disturbance. To this end, we selected model study sites within the Valtellina valley (Lombardy, Italy; Figure 1), selected based on conformity with all of the following criteria: (a) traditional exploitation for summer cattle grazing; (b) location above the tree line (similar site geomorphological and microclimatic conditions: elevation, exposure, slope, bedrock); and (c) similar management histories and presence of livestock units. Six sites where identified: two located in the municipality of Chiesa in Valmalenco (Sondrio) and four in that of Albaredo per San Marco (Sondrio; Figure 1). All sites are characterized by acid soils (Umbrisols; code A7 of Costantini et al., 2012) in the soil region of “Western and Central Alps on igneous and metamorphic rocks” (code 37.1; Costantini et al., 2014). Local soil classification at each survey point was also checked by reference to the Regional Topographic database (www.geoportale.regione.lombardia.it).
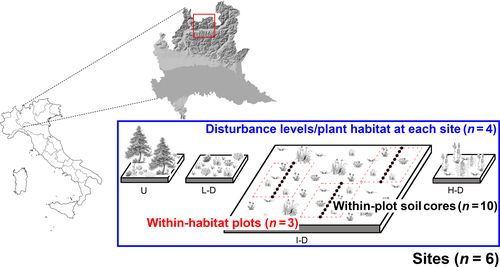
At each site, four different plant habitats were identified according to the intensity of livestock disturbance: (a) undisturbed habitats (U) characterized by climax or semiclimax vegetation dominated by Larix decidua, Rhododendron ferrugineum and Vaccinium myrtillus (European Community Habitat's Directive (92/43/EEC) habitat code 9420 Alpine Larix decidua and/or Pinus cembra forests, 9410 Acidophilous Picea forests of the montane to alpine levels (Vaccinio-Piceetea)); (b) abandoned pasture (low disturbance; L-D) not continuously grazed for at least 20 years, consisting of grassland colonized by small shrubs, for example, R. ferrugineum (habitat code 92/43/EEC: 4060 Alpine and Boreal heaths); (c) habitats exploited every summer by livestock (intermediate disturbance; I-D) consisting of meadows dominated by Nardus stricta (habitat code 92/43/EEC: 6230 Species-rich Nardus grasslands, on silicious substrates in mountain areas, 6150 Siliceous alpine and boreal grasslands); and (d) cattle resting areas (high disturbance; H-D) with the highest levels of both disturbance and cattle excreta (Pierce, Luzzaro, Caccianiga, Ceriani, & Cerabolini, 2007), dominated by nitrophilous species including Rumex alpinus.
At each disturbance level (plant habitat), three replicate plots of 100 m2, at least 10 m apart, were sampled (Figure 1). Within each plot, ten soil cores were collected every metre along the 10 m median transect of the plot, consisting of a volume of ~560 cm3 (cylindrical sampler with a diameter of 6 cm collecting to a depth of 20 cm through the O and A soil horizons and encompassing multiple root systems representing the above-ground plant community). The ten soil cores from each plot therefore represent subreplicates that together form a single rhizospheric soil sample (Figure 1). The plant community was characterized in terms of species relative abundance every 20 cm along the transect following Daget and Poissonet (1969). To avoid altering the biotic components of interest (bacteria, fungi and metazoa), soil cores were collected without removing the vegetation. Thus, a total of 72 rhizospheric soil samples (6 sites × 4 plant habitats within each site × 3 plots per habitat) were collected. Total genomic DNA was extracted using Nucleo Spin Soil kit Macherey-Nagel (Düren, Germany) from 1 g of each plot soil sample replicate, obtained after pooling and homogenizing the ten subreplicate soil cores (i.e., at each site, within each habitat, three plot-level samples of soil DNA were extracted). A schematic representation of the experimental design is reported in Figure 1; geographic coordinates of transects and the associated metadata are reported in Supporting information Table S1. All samples were collected within the first 2 weeks of July 2014.
2.2 Amplicon library preparation and sequencing
The extracted DNA was quantified using Bioanalyzer 2100 (Agilent Technologies, CA, USA). For each of the six replicate sites, the DNAs from the three within-habitat plots were pooled at equimolar concentration and used as a template for PCRs with primers targeting loci specific for the taxonomic groups studied: (a) 18S rRNA for metazoans with forward M620F 5′-GCAGCCGCGGTAATTCC-3′ and reverse M1260R 5′-TCRGCTTTGCAACTATACTTCC-3′ primers (Capra et al., 2016); (b) the internal transcribed spacers 1 and 2 (ITS1-ITS2) for fungi with forward ITS1 5′-CTTGGTCATTTAGAGGAAGTAA-3′ and reverse ITS4 5′-TCCTCCGCTTATTGATATGC-3′ (White, Bruns, Lee, & Taylor, 1990) and (c) the V3-V4 region of the ribosomal 16S rRNA for bacteria with forward Bakt_341F-mod 5′-CCTACGGGNGGCWGCAG-3′ and reverse R806 5′-GGACTACHVGGGTWTCTAAT-3′ (Caporaso et al., 2011; Herlemann et al., 2011). Thus, the entire study involved a degree of subreplication and, where necessary, sample pooling but also included true replication represented by the six sites.
PCRs were performed in a volume of 50 μl using Phusion High-Fidelity PCR Master Mix (Thermo Fisher Scientific, MA, USA), 0.2 mM of each dNTP, 0.5 pmol of each primer and 20 ng of genomic DNA. PCR conditions employed 30 s at 98°C, followed by 25 cycles of 1 min of denaturation at 98°C, 30 s of annealing at 58°C and 1 min of extension at 72°C, with a final single extra extension step of 7 min at 72°C. Finally, amplicons were cleaned up with Agencourt AMPure XP (Beckman, CA, USA) and the sizes were checked with a Bioanalyzer 2100 (Agilent Technologies, CA-USA). Libraries were prepared with a second-stage PCR using Nextera XT Index 1 Primers (N7XX) and Nextera XT Index 2 Primers (S5XX) from the Nextera-XT Index kit (FC-131-1001 or FC-131-1002), following 16S Metagenomic Sequencing Library Preparation protocol (www.illumina.com/content/dam/illuminasupport/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf). The libraries obtained were quantified by Real-Time PCR with KAPA Library Quantification Kits (Kapa Biosystems, MA-USA) pooled in equimolar proportions and sequenced by MiSeq sequencer (Illumina, CA-USA) using reagent kit v3 with paired-end reads of 250 bp.
The reads obtained by MiSeq assays were deposited in the European Nucleotide Archive (Accession PRJNA412149; sample IDs SAMN07701727-SAMN07701798).
2.3 Bioinformatics
Raw sequencing data were treated with mothur v1.33 (Schloss et al., 2009). After making contigs using the make.contigs command with default settings, bacterial 16S rRNA raw sequences were filtered based on the following specifications: Phred score >30, minimum fragment length of 250 bp, absence of ambiguous nucleotides (which are sometimes generated during contig formation when the delta between quality scores of a mismatched base is lower than 6), maximum 10-bp-long homopolymers and maximum primer mismatch of 1 bp. Sequences were clustered based on 100% similarity, and singletons were removed from the data set. Potential chimeric sequences were identified de novo and removed using the UCHIME algorithm (Edgar, Haas, Clemente, Quince, & Knight, 2011). Sequences were then clustered de novo into Operational Taxonomic Units (OTUs) at 97% similarity using the open-source vsearch tool (Rognes, Flouri, Nichols, Quince, & Mahé, 2016). Although the 16S rRNA does not evolve uniformly along its length, the 3% dissimilarity cut-off is often chosen to distinguish bacterial species (Schloss, 2010). Pruning of OTUs with low numbers of sequences (<10) was carried out on a per-sample basis, as an OTU that is common in one sample may occur as a low-abundance contaminant in others due to cross-contamination (Lindahl et al., 2013). The most abundant sequence of each OTU was selected as representative. Taxonomy was assigned through a search for similar sequences conducted with blast v2.2.29 (Zhang, Schwartz, Wagner, & Miller, 2000) against the 13_5 release of the Greengenes online database (DeSantis et al., 2006). Nontarget taxa including archaea, plants and metazoans were removed from the data set. At this point, rarefaction curves were computed to evaluate the sequencing efforts provided. As a normalization step to reduce bias associated with different sequencing depths, all samples were subsampled down to the size of the smallest sample. OTU counts were then corrected according to the 16S rRNA copy number using the bioinformatics software package picrust (Langille et al., 2013) and re-subsampled at ~90% of the minimum corrected sample size. New OTUs were clustered according to their Greengenes ID for further statistical analyses.
Contigs were not made for metazoan 18S rRNA and fungal ITS1-ITS2 raw sequences because the expected amplicon size of some target taxa exceeded the capability of the Illumina kit used. Thus, forward and reverse runs were processed separately. After trimming to the first 200 nucleotides, 18S rRNA and ITS1-ITS2 raw sequences obtained from both forward and reverse Illumina runs were filtered based on the following specifications: minimum fragment length of 200 bp, absence of ambiguous nucleotides and maximum 10-bp-long homopolymers. Sequences were then clustered based on 100% similarity, and singletons were removed from the data set. Chimera check was carried out in the manner as for 16S sequences. Taxonomy was assigned to sequences using the RDP classifier (Wang, Garrity, Tiedje, & Cole, 2007) and an 80% confidence threshold. The UNITE database (Abarenkov et al., 2010) was used as a reference for ITS1-ITS2 sequences, while the Silva database (Yilmaz et al., 2013) was used for 18S rRNA sequences. Clusters at 100% sequence similarity were then grouped into phylotypes (henceforth referred to as OTUs) based on their taxonomic affiliation down to the species level. OTUs with low numbers of reads (<10) were discarded on a per-sample basis. Nontarget taxa (bacteria, archaea and protists) were removed from both data sets. Rarefaction curves were then computed. All samples were subsampled down to ~90% of the size of the smallest sample, and OTU tables originated from forward and reverse runs were merged into a unique OTU table according to taxonomy.
All OTU tables are available in Supporting information Tables S2–S5.
2.4 Physical and chemical parameters of soil samples
Soil physical and chemical analyses were performed on the pool of the three true replicate samples of soil collected within each habitat type (i.e., 24 soil samples in total). Soil samples were analysed according to the official methods following Italian Regulation (1999) for (a) texture (g/Kg s.s.), determined by measuring the volumetric mass of the water–soil suspension and particle distribution by wet sieving and hydrometer; (b) reaction (pH unit), determined in CaCl2; (c) total CaCO3 (g/kg s.s.) determined by gas-volumetric determination of CO2 after HCl treatment; (d) soil organic matter (g/kg s.s.), organic C (g/kg s.s.), total N (g/kg s.s.) and C:N (relative units) determined by elemental analysis using the Dumas method from a measure of organic C; (e) cation exchange capacity [meq/100 g s.s.], determined by extraction with a solution of barium-triethylamine chloride adjusted to pH 8.2; (f) exchangeable Ca, Mg, K and Na (meq/100 g s.s.), Ca:Mg and Mg:K (relative units), degree of base saturation (%); exchangeable cations were determined by extraction with a solution of barium-triethylamine chloride adjusted to pH 8.2, by digestion-flame atomic absorption spectroscopy; (g) assimilable P, determined by the ascorbic acid method with spectrophotometry, sample treatment by H2SO4, H2O2 and HF; and (h) electrical conductivity and salinity Ece (dS/m), determined in water extracts (sample:distilled water ratio of 1:5, w/v) using a glass electrode and an EC meter (Hanna Instruments) (Panero, 1987). Soil physical and chemical parameters are reported in Supporting information Table S6.
2.5 Diversity and statistical analyses
Bacterial, fungal, metazoan and plant communities were used as input for the diversity analyses carried out with different r packages (R Project; http://cran.r-project.org/), in particular the r libraries vegan (Dixon, 2003; Oksanen et al., 2017) and packfor. The α-diversity was calculated using the Shannon index (Shannon, 1948), Pielou's evenness (Pielou, 1975), Chao-1 bias correction of total species richness (Chao, 1984; Chao & Lee, 1992; Smith & Van Belle, 1984) and the observed species richness as indicators. A Kruskal–Wallis one-way analysis of variance was performed on these indices to determine the effect of disturbance level/habitat type.
For each kingdom, the number of taxa shared among the different habitats was visualized by Venn diagrams. Venn diagrams were produced with the r package gplots on the presence–absence community data adopting the following filtering criterion: The presence of a taxon in a given habitat is counted when it occurred within that habitat for at least three of six sites.
The overall β-diversity for the four communities was estimated using the Sørensen-based multiple-site dissimilarity (βSOR; Baselga, 2010), implemented in R package betapart (Baselga & Orme, 2012). In addition, shifts in community structure among the habitats, the turnover and nestedness components of β-diversity, were calculated using Simpson-based multiple-site dissimilarity (βSIM; Baselga, 2010) and nestedness-resultant multiple-site dissimilarity (βNES; Baselga, 2010). β-Diversity analyses were performed on the filtered presence–absence matrices used to infer Venn diagrams.
For each community, a Nonmetric Multi-Dimensional Scaling (NMDS; Kruskal, 1964) biplot, based on the Bray–Curtis dissimilarity, was constructed to graphically ordinate samples and assess the differences among the four levels of disturbance. NMDS analysis was performed on the abundance OTU tables using the Bray–Curtis dissimilarity index (Bray & Curtis, 1957) and on the presence/absence OTU tables using both Bray–Curtis dissimilarity index on binary data (Bray & Curtis, 1957) and the Jaccard index (Jaccard, 1908). NMDS analyses were performed using the metaMDS function of the vegan package. The correlation between the four communities and the soil physicochemical parameters was investigated by fitting the previous NMDS ordination scores with the envfit function. The permutation of the community composition-based dissimilarity matrix allowed assessment of the significance of the fitted vectors, and a squared correlation coefficient (R2) was calculated. The differences in the structure and composition of autotroph and heterotroph communities of areas subjected to different levels of disturbance were estimated by a nonparametric one-way analysis of similarity (ANOSIM; Clarke, 1993) implemented in the vegan library based on the Bray–Curtis dissimilarity on binary data (99 permutations). Correlations between among-sites plant dissimilarities and dissimilarities of each heterotroph community was estimated by Mantel's (Mantel, 1967) test and visually represented by biplots. To quantify the fractions of bacterial, fungal and metazoan community variance explained by soil physicochemical variables and grazing-related disturbance level, the partition of variation was performed through the varpar function in vegan, including only forward-selected variables that presented no co-linearity within the abovementioned subsets (Borcard, Gillet, & Legendre, 2011). In addition, plant species adaptive strategies were included in the variance-partitioning analysis. Specifically, Grime's CSR plant strategies were determined for species recorded within three or more replicate plots for each habitat type (Grime, 2001). Leaf functional trait data (leaf area, specific leaf area and leaf dry matter content) were obtained from the “FIFTH” database (Cerabolini, 2010) and used to calculate the C-, S- and R- trade-off for each species using the method and spreadsheet tool of Pierce et al. (2017). The community-weighted mean (CWM) CSR value for each habitat type was also calculated based on species relative abundance data, following Cerabolini et al. (2016). For plants, the Nemenyi post hoc test was used to investigate differential abundance in the different levels of grazing.
3 RESULTS
3.1 α- and β-diversity of plant and rhizosphere communities and their response along a disturbance gradient
The metabarcoding libraries yielded to a total of 52,478,929 paired reads (13,076,606 18S rRNA reads, 13,594,742 ITS1-ITS2 reads, 25,807,581 16S rRNA reads). After the removal of low quality bases, chimeras, contaminants and rare sequence types, and following the normalization steps described in the materials and methods (set at 15,000 units/sample for bacteria, 47,000 units/sample for fungi, and 100,000 units/sample for metazoa), a total of 3,029 OTUs (1,484 for bacteria, 1,197 for fungi and 348 for metazoa; Supporting information Table S7) were obtained. Most rarefaction curves were able to reach the asymptote at a smaller number of sequences than the subsampling size, suggesting that the sequencing effort and the subsampling size were appropriate (Supporting information Figures S1, S2).
The taxa richness, as well as the values of the Shannon's diversity index, of the plant and rhizosphere communities did not exhibit a common pattern in response to the different levels of disturbance (Figures 2a; Supporting information Figure S3; Table S7). Species richness of plant communities differed across the four habitat types/levels of disturbance (Kruskal–WallisRichness, χ2 = 16.39, p < 0.001; Figure 2a; Supporting information Table S7), with the highest number of species observed in the communities of the low-disturbance habitat (Smedian = 35.5 ± 8.9) and the lowest in the high-disturbance habitat represented by cattle resting zones (Smedian = 10.5 ± 2.3). Significant differences in species richness were detected between low-disturbance and high-disturbance habitats (Nemenyi post hoc test, p < 0.001) but not between high-disturbance and undisturbed habitats (Nemenyi, p = 0.061) nor between high- and intermediate-disturbance habitats (Nemenyi, p = 0.088). Similar results were achieved also using Shannon's diversity index (Kruskal–WallisShannon, χ2 = 17.57, p < 0.001; Supporting information Figure S3; Supporting information Table S7), for which significant differences were observed between low- and high-disturbance habitats (Nemenyi, p < 0.001). No differences, in terms of both taxa richness and Shannon's diversity index, were observed among the bacterial communities across differentially disturbed habitats (Kruskal–WallisRichness, χ2 = 1.83, p = 0.608; Kruskal–WallisShannon, χ2 = 3.65, p = 0.302; Supporting information Table S7). A similar result was also recorded for fungal communities (Kruskal–WallisRichness, χ2 = 4.28, p = 0.232; Kruskal–WallisShannon, χ2 = 4.57, p = 0.206; Supporting information Table S7). However, in the case of metazoans, differences in species richness were evident among the four habitats/disturbance levels (Kruskal–WallisRichness, χ2 = 8.3, p = 0.04; Supporting information Table S7) but pairwise comparisons between particular habitats were at the limit of significance (e.g., high-disturbance versus undisturbed areas p = 0.068; intermediate-disturbance versus undisturbed areas p = 0.072). For this kingdom, no differences were observed among the inferred values of the Shannon's diversity index (Kruskal–WallisShannon, χ2 = 3.813, p = 0.282; Supporting information Table S7).
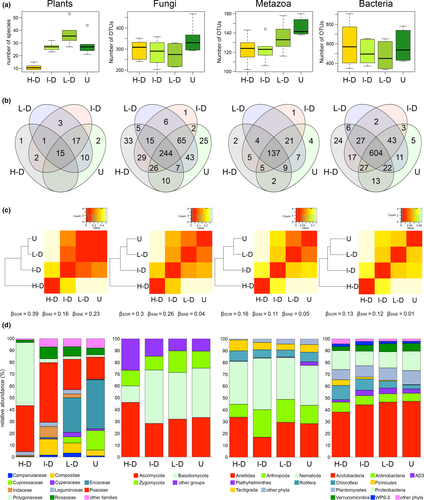
Except for plant communities (Kruskal–WallisPielou, χ2 = 11.89, p = 0.008), no differences were recovered in the Pielou's evenness of heterotroph communities across habitats (Supporting information Table S7). The comparison between Pielou's evenness of intermediately disturbed vs high-disturbance communities was significant (Nemenyi, p = 0.004; data not shown).
Venn diagrams of plant and rhizosphere communities, performed on the presence/absence OTU table, revealed the presence of a core of species/OTUs common to all habitats (Figure 2b). The abundance of the core biota relative to the whole community ranged from 29.4%, in the case of plant communities, to 75.1% in the case of bacterial communities (Figure 2b). Fungal communities exhibited intermediate results (47.7%), while metazoan communities demonstrated values comparable to those of bacterial communities (69.5%) (Figure 2b). In all communities, the environments harbouring the majority of the unique species/OTUs were those with extremes of disturbance, namely undisturbed and high-disturbance habitats.
The extent of change in the composition of plant and rhizosphere communities throughout the habitats subjected to different levels of disturbance, estimated by the Sørensen-based multiple-site dissimilarity index, ranged between 0.13 and 0.39 in the case of bacterial to plant communities, respectively (Figure 2c). The value of the Sørensen-based multiple-site dissimilarity index among the fungal communities was of a similarly high value to plant communities (βSOR Fungi = 0.3), while metazoan communities behaved more like bacterial communities, with little dissimilarity among sites (βSOR Metazoa = 0.16). In all communities, the biota associated with high-disturbance habitats was found to be the most dissimilar from other communities, in terms of Sørensen pairwise dissimilarity (Figure 2c), followed in order by communities characterized by decreasing disturbance levels, indicating a transition from high-disturbance to undisturbed areas.
The estimation of the two β-diversity components, viz. the nestedness and the spatial turnover of the OTU/species assemblages, revealed similar results for rhizosphere communities (Figure 2c). The turnover component accounted for the majority of the overall β-diversity, ranging from 89%, in the case of bacterial communities, to 69% in the case of metazoans (βSIM Bacteria = 0.12; βSIM Fungi = 0.26; βSIM Metazoa = 0.11; Figure 2c). The nestedness component contributed only marginally to the overall β-diversity, ranging from 11% (bacteria) to 31% (metazoans) (βSNE Bacteria = 0.01; βSNE Fungi = 0.04; βSNE Metazoa = 0.05; Figure 2c). The results indicate that in heterotroph communities, the replacement of OTUs among environments at different disturbance levels is relatively constant. Plant communities represent an exception to this trend as the nestedness was the major component of the overall β-diversity (βSOR Plants = 0.39, βSNE Plants = 0.23, βSIM Plants = 0.16; Figure 2c), indicating an ordered extinction or colonization along the disturbance gradient.
Plant and rhizosphere communities associated with habitats subjected to different disturbance levels had similar taxa compositions at least for the higher taxonomic levels (Figure 2d). However, some differences were recorded, especially in the case of plant and fungal communities. Plant communities associated with undisturbed and low-disturbance habitats were dominated by species of the Ericaceae (41% and 29%), while intermediate- and high-disturbance habitats were dominated by Poaceae (50.3%) and by nitrophilous taxa belonging to Polygonaceae (53.1%; Figure 2d), respectively. Basidiomycota was the dominant fungal group, with the exception of high-disturbance habitats, where the communities were dominated by Ascomycota (Figure 2d). Among metazoans, the nematodes represented the dominant group across the four habitats, with an abundance ranging from 33.7% (undisturbed habitat) to 43.6% (intermediate disturbance). Annelids were the second most abundant taxon, with the exception of the intermediate-disturbance habitat where the group was substituted by arthropods (Figure 2d). With regard to bacteria, Acidobacteria dominated all habitats, with abundance increasing slightly from high-disturbance to undisturbed habitat (i.e., from 38% to 47%; Figure 2d). All the remaining bacterial phyla showed comparable abundances across the four environments; Firmicutes represented an exception in that they decreased from high-disturbance (4.7% on average) to undisturbed habitat (0.2% on average), while Verrucomicrobia exhibited an opposite trend (Figure 2d).
3.2 Correlation among communities
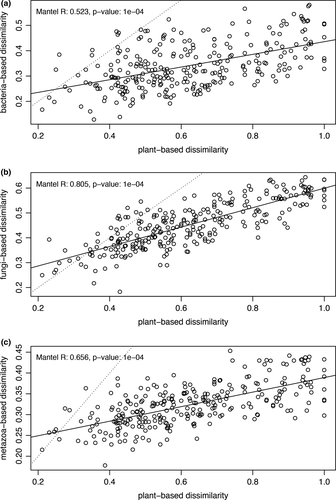
With regard to the hypothesis that plant communities represent reliable proxies of animal, fungal and bacterial communities, the Pearson product–moment correlation of the Mantel tests (p<0.01) was highest in the case of fungi versus plant communities (r = 0.8), indicating similar community structures. However, in the case of bacteria versus plant communities, the Pearson product–moment correlation was only 0.52. The correlation between the dissimilarity matrices based on metazoan and plant communities had intermediate values (r2 = 0.66). The linear regression on the biplot reporting the among-samples dissimilarities estimated on the basis of pairwise comparisons between communities (e.g., plant vs. bacteria) confirmed the different magnitude of correlation among plant and rhizosphere communities obtained by Mantel's tests (Figure 3).
3.3 Factors correlating with plant and rhizosphere communities and amount of variation in taxa composition correlated with abiotic and biotic variables
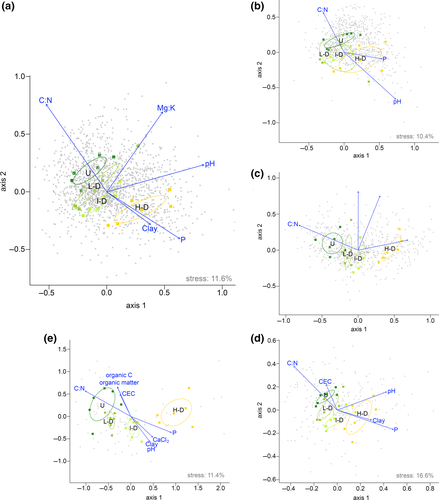
NMDS analyses performed on plant and rhizosphere communities (presence/absence OTU tables) achieved stable solutions in two dimensions (stresses ranged from 10.4% to 16.6%; Shepard plots Supporting information Figures S4, S5), fitted with soil physical and chemical properties and disturbance level (Table 1, Figure 4; NMDS adopting Jaccard index in Supporting information Figure S6 and Supporting information Table S8). Four of the 13 measured soil physical and chemical properties were significantly correlated with the ordinations of both plant and rhizosphere communities, namely pH, C:N and assimilable P (Table 1). Other soil physical and chemical properties were significantly correlated with the structure and composition of specific communities: Variation in sand and silt content was associated with variation in fungal and metazoan communities, while organic carbon and soil organic matter were associated with fungal and plant community diversity (Table 1). In addition to soil properties, the degree of disturbance significantly correlated with all communities (plant and rhizosphere organisms), with different values of squared correlation coefficients ranging from 0.29 to 0.8, in the case of bacterial and plant communities, respectively (Table 1). These results were further confirmed by the analysis of the merged OTU/species table, where disturbance level (r2 = 0.53, p = 0.01) and soil physical and chemical properties represented by pH in CaCl2 (r2 = 0.62, p = 0.01), assimilable P (r2 = 0.46, p = 0.01), Mg:K (r2 = 0.58, p = 0.01) and C:N (r2 = 0.70, p = 0.001) were the variables most highly associated with variability in the biotic communities (Table 1; Figure 4). NMDS axis 1 detailed a transition from undisturbed habitat (negative values) characterized by climax vegetation, to the high-disturbance habitats (positive values), detectable for both autotroph and heterotroph communities both when analysed separately and merged (Figure 4). These patterns are highlighted by the nonoverlapping standard error ellipses representing the 95% confidence area around the habitat mean; the only exception was evident for bacterial communities (Figure 4b).
| Tested variables/factors | Plant + Rhizosphere | Bacteria | Fungi | Plants | Metazoans | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r 2 | p | r 2 | p | r 2 | p | r 2 | p | r 2 | p | |
| Solid skeleton | 0.11 | 0.53 | 0.08 | 0.85 | 0.05 | 0.62 | 0.06 | 0.46 | 0.03 | 0.78 |
| Sand | 0.26 | 0.57 | 0.23 | 0.41 | 0.31 | 0.72 | 0.04 | 0.53 | 0.29 | 0.55 |
| Silt | 0.27 | 0.57 | 0.21 | 0.52 | 0.36 | 0.41 | 0.05 | 0.53 | 0.29 | 0.55 |
| Clay | 0.18 | 0.04 | 0.07 | 0.37 | 0.19 | 0.07 | 0.26 | 0.02 | 0.21 | 0.03 |
| pH in CaCl2 | 0.62 | 0.01 | 0.82 | 0.01 | 0.61 | 0.01 | 0.24 | 0.03 | 0.46 | 0.03 |
| Organic matter | 0.28 | 0.76 | 0.28 | 0.67 | 0.18 | 0.81 | 0.27 | 0.01 | 0.30 | 0.38 |
| Organic C | 0.28 | 0.76 | 0.28 | 0.67 | 0.18 | 0.81 | 0.28 | 0.01 | 0.30 | 0.38 |
| Total N | 0.31 | 0.84 | 0.15 | 1.00 | 0.28 | 0.70 | 0.12 | 0.09 | 0.18 | 0.99 |
| C/N | 0.70 | 0.01 | 0.34 | 0.02 | 0.73 | 0.01 | 0.72 | 0.01 | 0.63 | 0.01 |
| Cation exchange capacity | 0.05 | 0.27 | 0.04 | 0.39 | 0.70 | 0.20 | 0.16 | 0.05 | 0.13 | 0.04 |
| Ca/Mg | 0.02 | 0.89 | 0.05 | 0.57 | 0.03 | 0.92 | 0.08 | 0.35 | 0.02 | 0.99 |
| Mg/K | 0.58 | 0.01 | 0.47 | 0.12 | 0.61 | 0.02 | 0.02 | 0.74 | 0.48 | 0.28 |
| Assimilable P | 0.46 | 0.01 | 0.25 | 0.02 | 0.46 | 0.01 | 0.50 | 0.01 | 0.59 | 0.01 |
| Level of disturbance | 0.53 | 0.01 | 0.29 | 0.01 | 0.72 | 0.01 | 0.80 | 0.01 | 0.52 | 0.01 |
Note
- In bold are reported p < 0.05.
The analysis of similarity (ANOSIM) demonstrated that different kingdoms exhibited different degrees of separation between communities across disturbance levels, ranging from widely separated in the case of plants and fungi (R statistics = 0.64 and 0.67, respectively; Supporting information Table S9) through partially overlapping communities in the case of metazoans (R statistics = 0.48; Supporting information Table S9) to no discernible separation for bacteria (R statistics = 0.21; Supporting information Table S9).
The amount of variation in plant and rhizosphere taxa composition correlated with abiotic and biotic variables (i.e., the soil physical and chemical parameters, the grazing-related disturbance levels and plant community-weighted mean CSR values) as shown by variance-partitioning analyses (Figure 5). Forward selection analysis selected different sets of soil physical and chemical variables (p < 0.05), depending on the communities, while for plant CSR strategies, only the extent of C-selection was identified as important (Supporting information Table S10). An amount of variation ranging from ~64%, in the case of bacterial communities, to only ~24%, in the case of metazoan communities, was explained by soil variables (Figure 5).
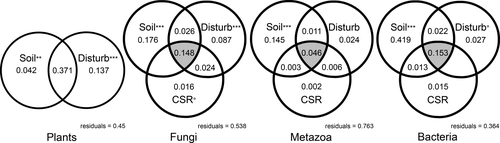
With regard to bacterial communities, soil variables explained a total of 60.7% of the variation in species composition: 41.9% was uniquely explained by these variables, and 2.2% and 1.3% were explained jointly by soil variables in combination with disturbance and mean CSR strategy, respectively (Figure 4). CSR strategies, disturbance and soil variables jointly explained 15.3%. Variation explained solely by disturbance level amounted to 2.7%, while 1.5% of bacterial variation was solely explained by the plant community CSR strategy (Figure 5).
In fungal communities, 17.6% of the variation was explained solely by soil variables; disturbance level and plant CSR strategy accounted for 8.7% and 1.6%, respectively. The remaining 19.8% of the variation was explained by the interaction among the selected variables, with the majority (14.8%) explained by the interaction of all variables (Figure 5). Variation within soil metazoan communities explained by the measured variables was limited (23.7%), with soil variables alone accounting for 14.5% of variation (Figure 5). With regard to plant communities, the selected variables accounted for 55% of variation, 13.7% solely explained by disturbance level, 4.2% by soil variables and 37.1% jointly by the two.
4 DISCUSSION
The hypothesis that rhizosphere community structure directly reflects that of the plant community was not supported here in the context of an Alpine environmental gradient. Thus, a strict link between primary producer and rhizosphere community structure is unlikely to be a general phenomenon. The emerging picture is that of loosely connected communities exhibiting common responses to environmental factors but which are not irrevocably tied to biodiversity patterns of primary producers. For example, the extent of community structuring across sites was similar for fungal and plant communities (i.e., was heterogeneous to a similar extent; Figures 3b, 4), whereas the bacterial community was essentially homogeneous and less structured (Figures 3a, 4). Beta-diversity patterns also suggest that the process of diversity change is fundamentally different for each community type, with plants exhibiting nested diversity loss (filtering of the larger species pool and substitution of the community), whereas the bacterial community changed via species turnover. The hypothesis that “everything is everywhere, but the environment selects” (Baas Becking, 1934) was not supported for our study system: For bacteria, it would perhaps be more precise to state that “nearly everything is nearly everywhere, but the environment can also select.” Plant communities were well separated taxonomically (with nitrophilous herbs dominating the heavily disturbed environment grading from grasses in the pasture to woody “climax” plants), reflecting classic patterns of succession that have been well characterized functionally (e.g., Caccianiga, Luzzaro, Pierce, Ceriani, & Cerabolini, 2006), and are apparently very different to the patterns in rhizosphere communities. While bacterial and plant communities represent extremes of β-diversity patterns, the fungal community exhibited similarities to the plant community and the metazoan community demonstrated an intermediate pattern most similar to that of bacteria. The higher metazoan diversity in the undisturbed habitat possibly reflects greater variability in the stature of plant species, which encompass small herbs, shrubs and small trees with concomitant heterogeneity in rooting depths between species, possibly providing a greater range of rhizosphere microhabitats. Additionally, in undisturbed habitat, soil is less compacted by cattle activity, and a relatively porous soil structure is likely to provide a greater range of microhabitats and facilitate organism movement (e.g., Collembola; Larsen, Schjonning, & Axelsen, 2004). Indeed, at most sites in the present study, soil organic matter contents and C:N were highest in undisturbed habitat (Supporting information Table S6), which suggests that more organic detritus could be available for exploitation by metazoans. Environmental stability and site history are key factors in the emergence of biodiversity (Gaston, 2000), and the greater stability of the undisturbed habitat is probably an additional factor encouraging metazoan diversity.
Why are patterns of diversity different for different biotic communities? A definitive response is difficult to provide because measured diversity values and patterns reflect overall local species pools rather than examining the fine-scale distribution of organisms within soil volumes. General determinants of the character of communities were edaphic, but notably of the 13 physical and chemical parameters measured, only four (pH, C:N, Mg:K, assimilable P) were associated with changes across primary producer and rhizosphere communities: essentially a gradient of soil fertility concomitant with the disturbance sequence, in agreement with the concept of concerted changes in multiple factors inherent to the humped-back model (Grime & Pierce, 2012). That plant communities change in agreement with the humped-back model (with relatively strict spatial zonation), whereas other communities do not, suggests that additional factors are at play. This is demonstrated by the extensive residuals (Figure 5) and the fact that only two factors (soil and disturbance) can explain most of the variance in the plant community. Which additional factors could be operating? The organisms under study exhibit dispersal mechanisms or motility with different degrees of effectiveness, especially for dispersal relying on dispersules versus growth. However, the plant community also exhibits a fundamental difference of scale, in that individuals are relatively long-lived and comparatively large, multicellular organisms. Plants occupy both soil and atmosphere—and must cope with extreme seasonal excursions in temperature, humidity and insolation—and thus, the investment of resources in tissues, and in the individual, is crucial to survival (Grime & Pierce, 2012). At the other extreme, bacteria are individually short lived and extensively dispersed, which appears to be evident from the number of ubiquitous OTUs (Figure 2b). Alternatively, the wide dispersal of bacteria could be due to the restricted and acid soil pH range, which may have selected for acid tolerance and thus for a subset of species that are generally adapted to this major soil characteristic. Also, while alpine plant communities show little change in species composition during a single growth season, changes in bacterial communities are potentially more rapid. Indeed, various studies demonstrate that seasonal changes, particularly in soil chemical characteristics, have extensive impacts on rhizosphere bacterial communities (Francioli, Schulz, Buscot, & Reitz, 2018; Lauber, Ramirez, Aanderud, Lennon, & Fierer, 2013; Regan et al., 2014). While the present study represents a “snapshot” of the communities at one point during the season (the first 2 weeks of July), we would argue that the alpine bioclimatic zone includes an inherently short growth season and that sampling in summer, the most active time of the year including the flowering peak of the plant community, is likely to provide a useful comparison of diversity across kingdoms. Thus, while it is possible that seasonal changes in rhizosphere communities may occur and were not detectable with our study system, we can nonetheless address hypotheses of common diversity patterns across kingdoms along a disturbance gradient. The differential reaction and structuring of the various communities studied here suggest that each one may exhibit an idiosyncratic response to land use change or environmental forcing, such as climatic change. Specifically, the relatively homogeneous structuring of the bacterial community, at one extreme, suggests that impacts are likely to be relatively limited in extent. In contrast, the way that the plant community changes, mainly by filtering or species loss, suggests that environmental changes are more likely to detract from the local species pool.
5 CONCLUSION
Plant and rhizosphere communities in an alpine environment characterized by changing disturbance levels and soil fertility were found to be only weakly interdependent and structured in idiosyncratic ways, mainly responding to the underlying environmental parameters rather than to each other. This can be explained by the complexity of relationships between natural and anthropogenic factors characterizing seminatural agroecosystems. Here, the plant community can only be assumed as a general proxy for the soil fungal component, but less so for other below-ground biotic components.
ACKNOWLEDGEMENTS
The authors are grateful to Dr. Giampaolo Della Marianna, Fondazione Fojanini di Studi Superiori—Sondrio (Italy) and to Dr. Emanule Capra, Istituto di Biologia e Biotecnologia Agraria, Consiglio Nazionale delle Ricerche—Lodi (Italy).
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests. The research was funded by the Agreement Regione Lombardia - National Research Council (CNR), Project FilAgro “Strategie Innovative e sostenibili per la filiera agroalimentare” assigned to F.P. and by the Research Supporting Plan 2015-17 (Line 2A) funded by the Dipartimento di Scienze Agrarie e Ambientali, Università degli Studi di Milano “MoBioS – Molecular Index of Biological Soil Quality” assigned to M.M.
AUTHOR CONTRIBUTIONS
M.M., G.G. and F.P. conceived the study. M.M., G.G., F.P., P.C. and F.T. performed fieldwork activities. F.G. characterized the plant communities and S.P. calculated the plant strategies. A.B., V.B., E.L. and R.G. performed the bioinformatics analyses. M.M. and A.B. performed the statistical analyses. M.M., A.B. and S.P. wrote the first draft of the manuscript, and all authors contributed substantially to revisions.
DATA ACCESSIBILITY STATEMENT
All the reads generated by MiSeq assays are deposited in the public repository European Nucleotide Archive (Accession No. PRJNA412149; sample IDs SAMN07701727-SAMN07701798). All the results of the soil analyses as well as all the OTU tables to reproduce the reported analyses are provided in the present study as supplementary materials.




