Long-term experimental warming alters community composition of ascomycetes in Alaskan moist and dry arctic tundra
Abstract
Arctic tundra regions have been responding to global warming with visible changes in plant community composition, including expansion of shrubs and declines in lichens and bryophytes. Even though it is well known that the majority of arctic plants are associated with their symbiotic fungi, how fungal community composition will be different with climate warming remains largely unknown. In this study, we addressed the effects of long-term (18 years) experimental warming on the community composition and taxonomic richness of soil ascomycetes in dry and moist tundra types. Using deep Ion Torrent sequencing, we quantified how OTU assemblage and richness of different orders of Ascomycota changed in response to summer warming. Experimental warming significantly altered ascomycete communities with stronger responses observed in the moist tundra compared with dry tundra. The proportion of several lichenized and moss-associated fungi decreased with warming, while the proportion of several plant and insect pathogens and saprotrophic species was higher in the warming treatment. The observed alterations in both taxonomic and ecological groups of ascomycetes are discussed in relation to previously reported warming-induced shifts in arctic plant communities, including decline in lichens and bryophytes and increase in coverage and biomass of shrubs.
Introduction
The greatest rates of climate warming have been observed in the Arctic, where the mean rate of temperature increase is nearly double of that in lower latitudes, and approaches 0.1 °C per year over the last three decades (Anisimov et al. 2007; IPCC 2007; Kaufman et al. 2009). This warming is leading to a suite of changes including (i) altering the extent and thickness of the arctic sea ice, (ii) shifts in plant community composition visible as tree line advancement into tundra and increase in shrub density and (iii) permafrost thawing and alterations in the net exchange of CO2 and CH4 with the atmosphere (Welker et al. 2000, 2004; IPCC 2013; Oechel et al. 2014). Warming-induced changes in the arctic ecosystems are of serious concern because most of them are leading to large positive feedback effects, promoting even greater warming of the climate. For example, rising concentrations of the greenhouse gases are followed by temperature increase and thawing of the permafrost – process that in turn, leads to increased efflux of CO2 and CH4 due to enhanced rates of decomposition in the warmer soils (Zona et al. 2009). Because northern circumpolar regions store approximately 50% of the Earth's soil carbon in seasonally or permanently frozen organic matter (Tarnocai et al. 2009), warming in the Arctic likely has tremendous consequences for atmospheric greenhouse gas concentrations that continue to rise (now at 400 ppm) (Cox et al. 2000). Another important feedback loop that is developing on tundra landscapes is the warming-induced expansion of shrubs (Hollister et al. 2005; Wahren et al. 2005), which trap snow during the winter and significantly increase winter soil temperature and, thus, soil microbial activity, promoting further the expansion of shrubs (Sturm et al. 2001).
Climate-induced changes in the arctic plant communities are among the most evident ones on our planet and have been the focus of intensive research (e.g. Welker et al. 1997; Arft et al. 1999; Wahren et al. 2005; Walker et al. 2006). In addition to long-term field monitoring, the responses of arctic vegetation to elevated temperatures have been estimated in experimental manipulations that revealed rapid shifts in arctic plant communities, including an increase in the biomass of graminoids and deciduous shrubs, higher plant canopy, accumulation of leaf litter and decrease in relative cover of shade-intolerant lichens and bryophytes (Hollister et al. 2005; Walker et al. 2006). The extent of these changes has been dependent on the duration of experiments and initial composition of the plant community (Hollister et al. 2005), with greatest responses exhibited by low arctic ecosystems, compared with high arctic and alpine areas (Arft et al. 1999). Within the low arctic, in both natural and experimental conditions, stronger responses to warming were exhibited by plant communities of the so-called moist tussock tundra. This vegetation type is clearly distinguished from another tundra types, for example, dry heath tundra characterized by Dryas octopetala, by visible differences in plant community composition that is dominated by the tussock-forming sedge Eriophorum vaginatum, deciduous and evergreen shrubs (Grime 2001; Walker et al. 2006; Mercado-Diaz 2011). In addition, there is approximately eightfold difference in the aboveground vascular plant biomass in the two tundra types (approximately 800 g/m2 in the moist tundra and approximately 100 g/m2 in the dry tundra) (Shaver & Jonasson 1999). Dry and moist tundra types are described in detail in Walker & Maier (2008; http://www.arcticatlas.org/maps/themes/tl5k/tl5kvg) including their surficial and glacial geology, soil carbon contents, elevations, relative percentage of the area, pH levels and plant community compositions.
Although the responses of arctic plant communities to warming have been well studied, our understanding of how warming in the Arctic will affect soil fungi remains rudimentary (Schaeffer et al. 2013). Fungi regulate nutrient-cycling processes and influence the plant fitness by forming various types of plant–fungus associations (Ludley & Robinson 2008; Hobbie et al. 2009; Newsham et al. 2009; Dahlberg & Bültmann 2013). These fungal associations can be especially important as they enhance plant acquisition of scarce nutrients, especially N and P, that generally limit plant growth in cold-dominated ecosystems (Nadelhoffer et al. 1996; Kytöviita & Ruotsalainen 2007; Hobbie et al. 2009). Previous research carried out on the responses of arctic fungi to rising temperatures focused on ectomycorrhizal basidiomycetes associated with the arctic shrub Betula nana and showed warming-induced increase in fungal diversity and biomass (Clemmensen et al. 2006; Deslippe et al. 2011). A more recent study by Morgado et al. (2014) reported a sharp decrease in richness of ectomycorrhizal basidiomycetes due to warming in the moist tundra of arctic Alaska, with the shifts in the hyphal exploration types that likely indicate increased potential for mineralization of recalcitrant nutrient pools in the soil. Neither basidiomycete richness nor community composition was altered by warming in the dry tundra type (Morgado et al. 2014). On the other hand, phylum Ascomycota is the most diverse group of fungi in the Arctic, (Geml et al. 2012; Timling et al. 2014) and their community composition and possible responses to increased temperatures are almost entirely unknown. Along with the other groups of fungi and bacteria, ascomycetes contribute to the functioning of tundra ecosystems through a broad spectrum of their ecological roles, such as: (i) decomposers of organic substances as saprotrophs, (ii) symbionts of photosynthetic algae and N-fixing cyanobacteria in the forms of lichens, (iii) ericoid, arbutoid and ectomycorrhizal partners of shrubs, (iv) mutualist or commensalist endophytes and (v) pathogens of plants, fungi and animals. Therefore, changes in the community compositions of ascomycetes due to warming are likely linked to changes in key ecological processes in tundra ecosystems.
We investigated the responses of ascomycetes to experimentally increased summer air and soil temperatures in dry heath and moist tussock tundra in northern Alaska. We hypothesized that (i) community composition and richness of taxonomic groups of ascomycetes will change under warmed conditions, (ii) the responses of ascomycete communities will be stronger in the moist tussock tundra type compared with dry heath tundra in agreement with trends observed for plant communities and (iii) warming will favour the growth of shrub-associated and saprotrophic fungi but will suppress lichenized ascomycetes. To test these hypotheses, we used deep Ion Torrent DNA sequencing of the ITS2 rDNA region, to compare ascomycete community compositions in ambient and elevated temperatures in the two tundra types that have been exposed to approximately 18 years of experimental warming as part of the International Tundra Experiment (Welker et al. 1997, 2005; Pattison & Welker 2014).
Materials and methods
Study site and sampling
Our research area was near the Toolik Lake Research Station, situated on the northern foothills of the Brooks Range, Alaska (68°38′N, 149°34′W). Two main vegetation types, dry heath tundra and moist tussock tundra are found throughout the region; dry heath tundra is dominated by Dryas octopetala, Salix polaris, Vaccinium species and fruticose lichens, while the moist tussock tundra is dominated by Betula nana, Salix pulchra and the sedge Eriophorum vaginatum (Walker et al. 1999). Experimental plots of warming treatments were established in 1994 as part of the International Tundra Experiment. Warming was accomplished using open-top chambers (OTCs) (Jones et al. 1998; Walker et al. 1999; Welker et al. 2000). An OTC is a hexagonal device of approximately 1 m2 constructed of translucent fibreglass that passively increases daytime air temperature by 1–5 °C during the snow-free period (Marion et al. 1997; Welker et al. 1999). OTCs are placed over experimental plots in every spring as soon as 50% of the plot becomes snow free and are removed at the end of the growing season in late August or early September (Walker et al. 1999).
The sampled plots had previously been used for vegetation studies that reported significant shifts in the plant community composition inside the OTCs, especially in the moist tundra type (Welker et al. 1999). In each tundra type, we sampled soil at five OTCs (warming treatment) and five control plots, resulting in twenty plots used for the whole analysis. Five soil cores, 2 cm in diameter and approximately 20 cm deep, were taken randomly to provide a composite sample for each plot that included all the organic and parts of the mineral soil horizon. Coarse litter and aboveground vegetation parts were removed from the sample, although some fine roots were present in the samples. Composite samples were kept frozen until lyophilization.
Although we sampled soils in two different tundra types, our methodological approach was not intended to compare the ascomycete communities in the dry vs. the moist tundra. Instead, we aimed to investigate the effect of warming on ascomycete communities in both tundra types separately to eliminate all potentially contributing factors other than the warming treatment itself.
DNA isolation, PCR and sequencing
Genomic DNA was extracted from approximately 0.4–1 g of dry soil (that corresponded to the maximal amount of soil that could be loaded to the tube) using NucleoSpin® Soil kit (Macherey-Nagel Gmbh & Co., Düren, Germany), according to the manufacturer's protocol. Because of the relatively small amount of the soil that could be processed in one tube (approximately 0.2–0.5 g), for each sample DNA extraction was carried out twice and replicates were combined. The DNA concentration of the samples was normalized. PCR amplification and Ion Torrent sequencing of the ITS2 region (approximately 250 bp) of the nuclear ribosomal rDNA repeat were carried out with primers fITS7 (Ihrmark et al. 2012) and ITS4 (White et al. 1990) (see Table S1 Supporting Information for the primer sequence information) and as described in detail in both Geml et al. (2014) and Morgado et al. (2014). ITS is the universal DNA barcode marker for fungi and has been used in a wide variety of taxonomic and ecological studies (e.g. Bruns et al. 1991; O'Brien et al. 2005; Geml et al. 2014 and references therein). The ITS4 primer was labelled with sample-specific Multiplex Identification DNA-tags (MIDs, see Table S1 Supporting Information for the complete MID list). The amplicon library was sequenced using an Ion 318™ Chip by an Ion Torrent Personal Genome Machine (PGM; Life Technologies, Guilford, CT, USA) at the Naturalis Biodiversity Center. The raw sequence data (FASTQ files) are available at Dryad (doi:10.5061/dryad.2fc32).
The initial clean-up of the raw sequence data was carried out using the online platform Galaxy (https://main.g2.bx.psu.edu/root), in which the sequences were sorted according to samples and sequence regions of primers and adapters (identification tags) were removed. We used a parallel version of mothur v. 1.32.1 (Schloss et al. 2009) for subsequent sequence analyses following the protocol described in detail in Geml et al. (2014). The FASTQ files were converted to FASTA and QUAL files, and the sequences were subjected to quality filtering, whereby each sequence was screened for thresholds for the average Phred score of Q ≥ 25 in a sliding window of 50 bp (qwindowaverage = 25; qwindowsize = 50), no ambiguous bases (maxambig = 0) and homopolymers not longer than 8 bp (maxhomop = 8). Sequences shorter than 150 bp or longer than 400 bp were omitted from further analysis (minlength = 150, maxlength = 400). Because next-generation sequencing libraries generally vary in size, we normalized the number of sequences for all samples, as recommended by Gihring et al. (2012), to ensure that estimators across all samples were comparable. For this purpose, we randomly subsampled the number of trimmed and quality-filtered reads to the size of the smallest library (of 56 483 sequences). The resulting sequences were clustered into operational taxonomic units (OTUs) with 97% ITS sequence similarity using OTUPIPE 1.1.9 (Edgar et al. 2011). Simultaneously, the putatively chimeric sequences were removed by de novo and reference-based filtering using the curated data set for fungal ITS sequences (http://www.emerencia.org/chimerachecker.html) of Nilsson et al. (2011). We assigned sequences to genera based on pairwise similarity searches using USEARCH (Edgar 2010) against the quality-checked UNITE fungal ITS sequence database containing identified fungal sequences. Of the total fungal OTUs, the ones assigned to the phylum Ascomycota were selected for further analysis. Representative sequences of these ascomycete OTUs have been submitted to GenBank with the following Accession no KJ826608-KJ828710. The depth of the OTU coverage across the treatments was examined by Good's coverage (as in Brown et al. 2013) and by rarefaction analysis using the Vegan package (Oksanen et al. 2012) in r software for statistical computing (R Core team 2013).
Comparing ascomycete fungal communities across the sampling sites
We first compared the communities among all sites by performing one-way cluster analysis, using Euclidean distances and Ward's group linkage method in pc-ord v.5.32 (McCune & Grace 2002). The effect of warming on community compositions was estimated using nonmetric multidimensional scaling (NMDS) on a primary presence/absence matrix of plots by OTUs, also, in PC-Ord, following the protocol described in detail in Geml et al. (2014). Because of uncertainties regarding the reliability of read count as an estimator of species abundance (Amend et al. 2010), we carried out two sets of ordination analyses: (i) based on presence/absence and (ii) taking into account OTU abundance values. Given the very high sequencing coverage we achieved, ‘presence’ was defined as ≥5 OTUs on a per sample basis following the recommendations of Lindahl et al. (2013) to minimize false positives (e.g. OTUs that are common in one sample, but may be low-abundant contaminants in the others). The secondary matrix consisted of treatment (control or warming) and number of ascomycete OTUs per taxonomic order (see Table S3 Supporting Information for ordination matrices). We also tested whether the warmed and control ascomycete communities were statistically different across the sites using a multiresponse permutation procedure (MRPP) and permutation-based nonparametric manova (Anderson 2001) and determined any preferences of individual OTUs for specific experimental treatment using indicator species analyses (Dufrêne & Legendre 1997), also in PC-Ord. Additionally, for the most diverse orders of Ascomycota, Venn diagrams were generated with the BioVenn web tool (Hulsen et al. 2008) to visualize the distribution of OTU composition across the experimental treatments. In addition, the significance of the observed differences in the OTU richness across the different experimental treatments was tested by Student t-test.
Analysis of ecological functions
We estimated the proportions of different ecological groups among the OTUs that showed strong (|r| ≥ 0.5) positive or negative correlation with warming in the NMDS analyses. Ecological functions for these OTUs were selected based on the information for the isolation source for the reference sequences (with at least 97% similarity) presented in GenBank. For the OTUs with similarity levels of 95–96% to the reference sequence, the ecological function was set as ‘putative’, and in case, there were no sequences with at least 95% similarity in the database, the ecological function for the OTU was set as ‘unknown’. Fungi isolated from nonliving materials (i.e. litter, rocks, marble, feathers, decaying wood, rotten fruits and mushrooms) were defined as ‘saprotrophic’, and different types of mycorrhizal and root endophytic fungi were considered ‘root-associated’. The group of ‘endophytes’ involved the fungi isolated from asymptomatic photosynthetic tissues of plants, lichens and bryophytes. Mycobionts of lichens were grouped as ‘lichenized’ fungi. For the OTUs that matched only to the sequences obtained from the arctic soils, the putative ecological function was defined as ‘soil’. We calculated the proportion (%) of OTUs representing various ecological groups across the control and warmed plots. The percentages were arcsine-transformed and differences between the treatments were tested by Student's t-test.
Results
Sequence data analysis
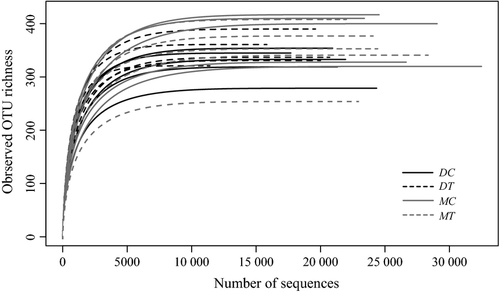
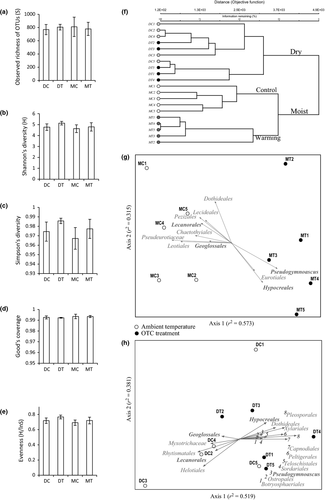
The Ion Torrent data set contained 4 046 811 sequences with the median length of 303 bp. After the initial filtering step, we obtained 2 068 216 sequences characterized by the sufficient length (150–400 bp), reasonable quality scores (Q > 25) and homopolymers with no more than eight nucleotides. Sequence data were subsampled according to the size of the smallest sequence library of 56 483 sequences. Total number of 1 129 660 sequences obtained for twenty samples had the mean read length of 255 ± 56 bp (± SD); OTU clustering of these sequences resulted in 5638 nonsingleton OTUs. The rarefaction curves approached the saturation plateau for all of the samples, suggesting an equally deep OTU recovery across the treatments (Fig. 1). We observed no correlation between warming treatment and OTU richness (Fig. 2a). Similarly, there was no difference between Shannon's and Simpson's diversity indexes (Fig. 2b, c) and evenness values (Fig. 2e) among the control and warmed sites. Good's coverage estimators (99.3 ± 0.14 across all the treatments) indicated that the deep sequencing allowed for a very high OTU coverage (Fig. 2d).
We identified 2103 OTUs belonging to the phylum Ascomycota. Among them, only about 20% showed ≥97% similarity with their closest relatives in reference databases and therefore could putatively be regarded as conspecific with the reference sequences. We detected 35 taxonomic orders, with most of the OTUs belonging to Helotiales (480 OTUs), Chaetothyriales (243), Lecanorales (91), Pleosporales (73), Verrucariales (56), Hypocreales (51), Capnodiales (48), Leotiales (45), Coniochaetales (43), Eurotiales (25) and Pezizales (25), followed by numerous orders with <20 OTUs. We detected representatives of 311 genera. For example, observed genera of putative root endophytes included Cladophialophora (114 OTUs) – the genus with the highest obtained OTU richness in the data set – Meliniomyces (66), Phialocephala (39), Cadophora (19), Phialophora (14) and Leptodontidium (12). The genera of putative lichenized fungi with the high OTU richness included Lecidea (98 OTUs), Cladonia (40), Peltigera (13) and Allocetraria (10). Relatively high OTU richness was observed for the saprotrophic genus Capronia (94 OTUs) and ericoid mycorrhizal fungi of the genera Rhizoscyphus (89) and Pseudogymnoascus (83). Additionally, the group of aquatic hyphomycetous fungi was represented by a high OTU number, with the affinities to the following genera: Alatospora (51 OTUs), Articulospora (35), Helicodendron (25) and Spirosphaera (20).
Moist tundra communities
For the moist tundra site, we obtained a data set of 1253 ascomycete OTUs. OTU richness did not vary significantly with the treatment (t8 = 0.85; P = 0.42), although the total number of OTUs in the control plots, 901 (mean value 378 ± 47 OTUs per plot), was slightly higher than the richness observed for the warming treatment (802 OTUs, mean value 350 ± 58 OTUs per plot).
The orders with the highest observed OTU richness – Helotiales and Chaetothyriales – comprised approximately 38% and 15% of the OTUs in control communities, and respectively approximately 38% and 10% of the OTUs in warmed communities. The rest of the OTUs belonged to numerous taxonomic orders with lower numbers of OTU hits: for the control communities Leotiales (5%) and Pleosporales (4%) followed the OTU richness ranking, and for the warmed plots Hypocreales (5%), Pleosporales (4%), Eurotiales (3.4%) and Leotiales (3.2%) orders followed the richness ranking of the ascomycete communities.
Because the results of the presence/absence and abundance-based NMDS analyses were very similar, we present the former below and the latter in the (Fig. S1, Table S3, Supporting Information). We found a strong effect of warming on the community composition of ascomycetes, as shown by one-way clustering (Fig 2f), MRPP (P = 0.0025; size effect A = 0.134) and manova (F = 3.99; P = 0.01) analyses and depicted by the NMDS (final stress = 7.10) plot (Fig 2g). Temperature treatment accounted for 37.4% of the variation in ascomycete community compositions (manova). Richness in seven orders and one family of uncertain taxonomic placement negatively correlated (|r| > 0.5) with warming: Leotiales (r = −0.874), Chaetothyriales (r = −0.767), Pezizales (r = −0.752), Lecanorales (r = −0.718), Lecideales (r = −0.659), Dothideales (r = −0.571), Geoglossales (r = −0.569) and Pseudeurotiaceae (r = −0.952). On the other hand, two orders and one genus with unknown systematic position (Incertae sedis) showed positive correlation with increased temperatures: Eurotiales (r = 0.632), Hypocreales (r = 0.761) and Pseudogymnoascus (r = 0.876).
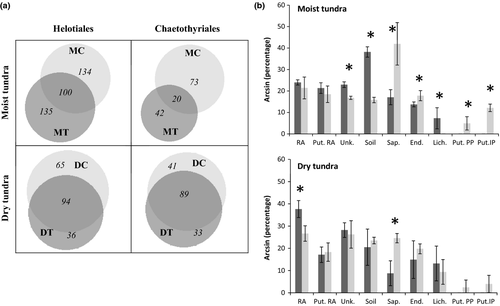
The effect of experimental warming on OTU composition of the two orders with highest OTU richness – Helotiales and Chaetothyriales - was also displayed in the Venn diagram (Fig. 3a). In both orders, we observed a relatively high proportion of OTUs unique to a temperature treatment. Although OTU richness in the order Helotiales was not affected by the treatment, and thus, not depicted on NMDS plot, Venn analysis suggested a visible effect of the warming treatment on OTU composition in this order.
Pearson's correlation analysis for the individual OTUs resulted in 153 OTUs associated with the control conditions and 143 OTUs correlated with the warming treatment. Identification of these OTUs to the species level, however, was hampered by the scarcity of publicly available identified sequences of closely related taxa. From the 153 OTUs negatively correlated with warming, only 16 were identified to the species level. Of the 143 OTUs positively correlating with the OTC treatment, 59 OTUs were identified to species, and 27 of them related to a single genus – Pseudogymnoascus.
296 OTUs that correlated with one of the treatment were used for the analysis of ecological functions (Fig. 3b). Student t-test analysis revealed a significant increase in the richness of saprotrophs, endophytes and putative pathogens of plants and insects with warming. At the same time, the richness of lichenized ascomycetes declined significantly.
Indicator Species analysis unravelled 33 OTUs characteristic to control plots and 42 OTUs indicator for the warmed plots across the moist tundra type (Table S2). With the obtained similarity levels, we identified to species two OTUs as indicators for the control plots – Alatospora acuminata (AY204589) and Aureobasidium pullulans (EU272483). 15 OTUs were associated with the warming treatment, among them, ten showed high sequence similarity and affined to species of Pseudogymnoascus (AJ608972; EF540755) (Table S2).
Dry tundra communities
Data analysis indicated a highly diverse community of 1159 ascomycete OTUs in the dry tundra. We did not observe significant change in OTU richness under the experimentally elevated temperatures (t8 = 1.22; P = 0.26). In total, 849 OTUs were found across the ambient temperature plots (mean value 329 ± 29 OTUs per plot), and 831 OTUs were obtained for the OTC communities (mean value 351 ± 28 OTUs per plot). Most of the OTUs (approximately 80%) were identified to taxonomic order, while the percentage of the OTUs identified to the level of the species was much lower (17%). The orders Helotiales and Chaetothyriales were characterized by the highest OTU richness across the control and warmed sites in the dry tundra, representing 24% and 19%, and 20% and 18.8% of the OTUs, respectively.
Cluster analysis did not reveal a clear distinction between the overall compositions of ascomycete communities in the control and warmed sites (Fig 2f). Similarly nonsignificant results were obtained by MRPP analysis (P = 0.10; A = 0.24) and by NMDS (final stress = 0.19) (Fig. 2h). Treatment explained only 8% of the variation, as revealed by manova statistics (P = 0.087; F = 1.46). Similarly, the proportion of shared OTUs of Helotiales and Chaetothyriales among the ambient temperature and warmed plots was greater than in the moist sites (Fig. 3a). However, we were able to observe changes in the OTU richness of several taxonomic orders in correlation (|r| > 0.5) with the temperature treatment (Fig. 2h). Similar results were obtained in the analysis of relative abundance of ascomycete taxa (see Fig. S1 for the ordination plot and correlation values). OTU richness in the following taxonomic groups negatively correlated with warming: Rhytismatales (r = −0.786), Helotiales (r = −0.732), Lecanorales (r = −0.6) and Geoglossales (r = −0.51) and the family Myxotrichaceae (Incertae sedis) (r = −0.651).
Ten taxonomic orders exhibited higher OTU richness under the simulated warming. These orders included Botryosphaeriales (r = 0.509), Ostropales (r = 0.546), Sordariales (r = 0.588), Teloschistales (r = 0.632), Hypocreales (r = 0.639), Dothideales (r = 0.735), Peltigerales (r = 0.807), Xylariales (r = 0.821), Capnodiales (r = 0.847) and Pleosporales (r = 0.92). Correlation between OTU richness and warming was also shown for the genus Pseudogymnoascus (r = 0.584), (Fig. 2h).
On the level of individual OTUs, Pearson's correlation analysis resulted in 179 OTUs that negatively correlated with warming. Of these, only 25 OTUs could be identified to species and 14 OTUs related to a single species, Rhizoscyphus ericae. On the other hand, 235 OTUs correlated positively with the experimentally warmed sites. We identified 45 OTUs to the species level and obtained a diverse group of ascomycete species, with one dominant genus, Pseudogymnoascus, represented by 11 OTUs.
414 OTUs with high Pearson's correlation values were checked for possible ecological functions (Fig 3b). Student's t-test analysis showed a significant decrease in the richness of root-associated fungi and increase in saprotrophic species with warming.
Few OTUs were characteristic for either control or warming treatment, as revealed by the indicator species analysis. One OTU that matched with the reference sequence for Pertusaria excludens (SH117228.05FU) was indicator for the control plots (Table S2); nine OTUs were characteristic for the warmed sites, and among the nine, five related to the following species: Cladosporium cladosporoides (AJ300335), Fuscicladium catenosporum (EU035427), Pseudogymnoascus vinaceus (AJ608972), Venturia alpina (EU035446) and Venturia polygoni-vivipari (EU035466) (Table S2).
Discussion
Warming alters ascomycete communities
We found significant shifts in ascomycete community composition induced by eighteen years of summer warming. According to our research hypothesis and in agreement with the results of the previous vegetation studies, we observed greater changes in the ascomycete community compositions in the moist tundra compared with the dry tundra. Stronger responses of the plant communities in the moist tundra may correlate with greater responses in soil fungal communities due to tight associations that arctic fungi form with living and/or dead parts of specific vascular plants (Hobbie et al. 2009; Bjorbækmo et al. 2010; Dahlberg & Bültmann 2013).
It is difficult to compare the findings of our study with previous works addressing the effects of experimental warming on soil fungal communities (Allison & Treseder 2008; Papanikolaou et al. 2010; Gutknecht et al. 2012; Hayden et al. 2012; Anderson et al. 2013; Jumpponen & Jones 2014), because most of these projects studied different vegetation types, used different warming methods (e.g. greenhouse, infrared lamps) for much shorter periods (up to 6 years) and used different molecular techniques best suited for biomass and abundance estimations. For example, Jumpponen & Jones (2014) and Papanikolaou et al. (2010) found no effect of warming on community composition in temperate grasslands and agricultural soils, respectively, similarly to what we observed in the dry tundra. On the other hand, Allison & Treseder (2008) found that 3-year warming had significant effects on the composition of the active fungal community (approximately 90% basidiomycetes, 5% ascomycetes) at an Alaskan boreal forest site, which is consistent with our results from the moist tundra. It is interesting to note that the samples analysed by Allison & Treseder (2008) originate from the acidic black spruce (Picea mariana) vegetation type that generally shows substantial floristic resemblance in the understory to the low arctic moist tussock tundra (e.g. Hollingsworth et al. 2006).
With respect to the observed richness of different taxonomic groups, Jumpponen & Jones (2014) found that warming did not affect the richness in most ascomycete classes, except for the Lecanoromycetes that declined with warming. In our study, the order Lecanorales (involving lichenized ascomycetes) was one of the two orders that showed significant decrease in OTU richness in the warmed plots of both tundra types. Nonsignificant or inconsistent effect of OTC and infrared warming on total fungal abundance and biomass were also reported by Hayden et al. (2012), Papanikolaou et al. (2010) and Gutknecht et al. (2012). On the other hand, Allison & Treseder (2008) reported a significant positive effect of warming on the taxonomic richness of active fungi, particularly in ascomycetes that showed an increase in relative abundance from 5 to 14.4%. Neither total ascomycete richness nor total abundance across the treatments (Table S3) changed in our study after 18 years of warming, although there were several groups with higher number of OTUs in the warming treatment plots (e.g. Eurotiales, Hypocreales and Pseudogymnoascus).
Responses in shrub-associated ascomycetes
The formerly observed, warming-induced expansion of shrubs was expected to favour plant pathogenic, mycorrhizal and root endophytic fungi. Reported larger shrub density and biomass (Mercado-Diaz 2011) was assumed to reflect increased root biomass (Sullivan et al. 2007), which in turn, may broaden niches for the root-associated ascomycetes. Analysis of ecological functions revealed a significant increase in the proportion of endophytic ascomycetes and plant pathogens among the OTUs correlated with warming treatment in the moist tundra, suggesting that warming-induced increase in plant growth may provide more suitable microhabitats for shrub-associated ascomycetes. Possibly, increase in OTU richness and abundance in the genus Pseudogymnoascus is also related to its ability to form ericoid mycorrhiza (Vohnik et al. 2007) with arctic shrubs. In our data set, several Pseudogymnoascus OTUs were at least 98% similar to sequences derived from plant roots or photosynthetic tissue (AJ608972).
Unexpectedly, among the OTUs that correlated with the warming treatment in the moist tundra data set, we observed no significant change in the proportions of root-associated ascomycetes (Fig. 3b). Moreover, in the dry tundra, the proportion of root-associated ascomycetes decreased with warming. Sharp warming-induced decrease in the richness has been also shown for ectomycorrhizal basidiomycetes (Morgado et al. 2014). It is possible, therefore, that either warming provides unfavourable conditions for root-associated fungi in general or, more likely, warming favours few root-associated taxa that may competitively exclude other groups, leading to the overall decrease in richness, despite the reported trend of warming-induced increase in total fungal biomass (Clemmensen et al. 2006).
Responses in lichenized and moss-associated ascomycetes
We expected the decline in lichenized ascomycetes and species associated with bryophytes in response to warming due to reported previously overall decrease in bryophyte and lichen coverage (Hollister et al. 2005; Walker et al. 2006). In agreement with the former observations, our analysis revealed a reduction in OTU richness in the order Geoglossales, many of which are moss-associated (Hustad et al. 2013). In both ecological and taxonomic analyses and for both tundra types, we observed the reduction in lichenized fungi (order Lecanorales). In addition, in the moist tundra, we observed the warming-induced decline in richness for at least one more order that includes lichenized fungi (Lecideales). Due to the key role of lichens in caribou food diet, the warming-induced decline in lichen coverage is of high concern, as it could affect the populations of caribou and related chains of tundra food webs (Dahlberg & Bültmann 2013).
Responses in saprotrophic and insect pathogenic ascomycetes
Leaf litter accumulation reported for the OTC plots (Hollister et al. 2005; Wahren et al. 2005; Walker et al. 2006) was expected to favour the growth of litter-inhabiting saprotrophic ascomycetes. Indeed, in the both tundra types, the proportion of saprotrophic ascomycetes increased under the warming treatment (Fig. 3b). Possibly, increased litter accumulation in the warmed plots may contribute to higher richness and abundance of several saprotrophic and psychrophilic Pseudogymnoascus species (see Table S2 for the list of OTUs that were at least 97% similar to sequences derived from nonliving materials).
Interestingly, irrespective of the initial vegetation type, OTU richness in the order Hypocreales correlated positively with the warming treatment. Analysis of the OTU affinities in this order revealed high sequence similarities to various species in the family Cordicipitaceae that comprises many insect pathogenic fungi. It is possible that the increased abundance of certain arctic insect groups due to warming (Dollery et al. 2006; Adler et al. 2007) contributes to the higher OTU richness of insect pathogenic ascomycetes. Also, previous studies in temperate and subtropical ecosystems have consistently found that OTU richness of hypocrealean fungi correlates positively with temperature, as shown in various altitudinal gradient studies (Devi et al. 2012; Geml et al. 2014). Therefore, the underlying mechanisms for the increase in richness in Hypocreales are uncertain, but it is safe to speculate that the increased temperature likely favours the decomposer capabilities of hypocrealean fungi and that other warming-induced changes (e.g. increase in the abundance of insects) may contribute to the higher OTU richness in this group as well. On the other hand, some of the OTUs that were highly similar to the sequences of well-known insect pathogenic fungi (e.g. Beauveria bassiana) had also high similarity to the sequences amplified from roots (e.g. EF093153, KC243962). Therefore, defining the ecological roles for these ascomycetes is complicated, and some of the putative insect pathogens may have a root-associated or endophytic lifestyle (White et al. 2003).
Arctic ascomycete diversity
Although the main focus of our study was to assess responses of soil ascomycete communities in dry and moist arctic tundra to long-term experimental warming, our data offer unprecedented insights in the richness of arctic fungi. It is well known that functioning of the arctic ecosystems is reliant on fungi, nonetheless, fungal communities in the arctic tundra, with the exception of ectomycorrhizal basidiomycetes, remain largely unstudied. This is particularly true for ascomycetes that comprise more than 60% of all known fungi and are often difficult to study because the vast majority of them are inconspicuous ‘microfungi’, including most of soil fungi, leaf and root endophytes, plant and animal pathogens that do not form macroscopic fruiting structures (with few exceptions, e.g. lichens). The existing regional checklists mention 1245 nonlichenized ascomycete species for the whole Arctic region (Dahlberg & Bültmann 2013) that is less than the number of nonlichenized OTUs (1967) found in our study for one location only. Although the 97% ITS sequence similarity OTUs routinely used in molecular studies, including this one, likely do not correspond one-to-one to species, they nevertheless represent the best approximation in rapid richness assessments with wide taxonomic focus, and when applied with rigorous sequence quality checks, they can provide reasonably accurate estimates of the number of species. Our analysis of OTU richness supported suggested previously high diversity within several fungal taxa, for example, Rhizoscyphus ericae, that is considered a ‘species aggregate’ (Hambleton & Sigler 2005; Grelet et al. 2010) and for which 19 species hypotheses have been proposed by UNITE experts, or Pseudogymnoascus sp. that is known to be far exceeding the number of described species (Minnis & Lindner 2013).
The total fungal richness for the Arctic was estimated by Schmit & Mueller (2007) as 11 000 species, based on plant–fungal diversity ratio of Hawksworth (2001). However, the diversity estimates provided in this study suggest that the total fungal richness in the Arctic may be even greater, particularly because fungal-per-plant species richness was shown to increase towards the poles (Tedersoo et al. 2014). In our data analysis, a relatively low percentage (<20%) of OTUs were identified to species (with >97% sequence similarity) due to the scarcity of reference sequence data. Approximately 40% of the OTUs that correlated with the treatment had highly similar, but unidentified sequences known only from other soil sequencing studies, while the proportion of OTUs that had no close reference sequences with >95% similarity was approximately 20%. It is well known that the sequence data currently available in public databases only represent a fraction of total fungal diversity: it has been estimated that >5% of all fungi are known (Blackwell 2011) and only 20% of the described species have been sequenced and, thus, can be identified using DNA (Kõljalg et al. 2013). In our data set, there was a vast inequality in the average sequence similarity values obtained for economically ‘important’ vs. ‘nonimportant’ ascomycete genera. For example, sequence similarity values to the most similar publicly available sequences for OTUs in relatively well-studied genera, that is those with economic importance in food production, medicine or biotechnology, were generally high: Penicillium (98.5 ± 1.21%), Torula (97.3 ± 2.63%), Botrytis (99.1 ± 0.31%) and Hypocrea (98.4 ± 0.9%). On the other hand, sequence similarity values were much lower for numerous species lineages that do not have any industrial application, for example Lecidea (86.5 ± 3.4%), Capronia (85.5 ± 2.16%), and therefore likely are underrepresented in public databases. Consequently, it is very difficult to speculate on the ecological roles of most of the ascomycete OTUs and to link the observed changes in ascomycete community composition to changes in ecological functions. For example, many aquatic hyphomycetous species, that have been traditionally considered saprotrophic, have been isolated from surface-sterilized roots suggesting that these fungi may be root endophytes as well (Sati et al.2009). Clearly, more research on the taxonomy, phylogenetic diversity and ecological functions of arctic fungi is needed.
Our data suggest that arctic ascomycete communities are extremely diverse and vary in composition depending on the tundra type. Their community composition is altered by warming, with a much stronger response exhibited in the moist tussock tundra compared with dry heath tundra. Yet, the lack of adequate taxonomic and ecological knowledge of fungi severely compromises our ability to disentangle causal relationships, to infer likely changes in ecological functions and to provide predictions about the Arctic. Numerous fungi in our samples likely are still undescribed, while others may remain unidentified because of the lack of reference sequences from known species. In addition, even for known species, more research is required to obtain information on their ecological functions. Addressing these questions will be helpful in predicting how arctic ecosystems respond to warming, from nutrient cycling to trophic relationships.
Acknowledgements
We thank Elza Duijm and Marcel Eurlings for help with the Ion Torrent sequencing, Toolik Lake Field Station and University of Alaska, Fairbanks, USA, for providing facilities to work with experimental devices for climate change. TAS, ES and JG were supported by NWO-ALW Open Program research grant (821.01.016) and Naturalis Research Initiative grant. Experimental work was also largely supported by NSF grants OPP AON 0856728 & 1433063, OPP IPY 0612534 & 0632184 awarded to JMW.
References
J.G. conceived the research idea, and J.G. and L.N.M. carried out the soil sampling for this study. T.A.S. extracted the DNA, carried out PCR and prepared the samples for the Ion Torrent run. J.G., L.N.M. and T.A.S. conducted the bioinformatic and statistical analyses and wrote the manuscript with input from J.M.W., M.D.W. and E.S. All authors read and approved the final manuscript.
Data accessibility
Representative sequences of all OTUs in this study were submitted to Genbank: KJ826608-KJ828710. The raw (fastq) sequence data are submitted to Dryad (doi:10.5061/dryad.2fc32).




