The evolving male: spinner dolphin (Stenella longirostris) ecotypes are divergent at Y chromosome but not mtDNA or autosomal markers
Abstract
The susceptibility of the Y chromosome to sexual selection may make this chromosome an important player in the formation of reproductive isolating barriers, and ultimately speciation. Here, we investigate the role of the Y chromosome in phenotypic divergence and reproductive isolation of spinner dolphin (Stenella longirostris) ecotypes. This species contains six known ecotypes (grouped into four subspecies) that exhibit striking differences in morphology, habitat and mating system, despite having adjacent or overlapping ranges and little genetic divergence at previously studied mtDNA and autosomal markers. We examined the phylogeographic structure for all six ecotypes across the species range (n = 261, 17 geographic locations) using DNA sequences from three Y chromosome markers, two maternally inherited mitochondrial (mtDNA) markers, and a biparentally inherited autosomal intron. mtDNA and autosomal analyses revealed low divergence (most ΦST values <0.1) between ecotypes and geographic regions, concordant with previous studies. In contrast, Y intron analyses revealed fixed differences amongst the three most phenotypically divergent groups: S. l. longirostris vs. S. l. roseiventris vs. combined S. l. orientalis/S. l. centroamericana/Tres Marias ecotypes). Another ecotype (whitebelly), previously postulated to be a hybrid between the two phenotypically most divergent ecotypes, had Y haplotypes from both putative parent ecotypes, supporting a hybrid designation. Reduced introgression of the Y chromosome has previously been observed in other organisms ranging from insects to terrestrial mammals, and here we demonstrate this phenomenon in a marine mammal with high dispersal capabilities. These results indicate that reduced introgression of the Y chromosome occurs in a wide taxonomic range of organisms and support the growing body of evidence that rapid evolution of the Y chromosome is important in evolutionary diversification.
Introduction
The Y chromosome can play a role in speciation through involvement in the evolution of reproductive isolating barriers. Although the Y chromosome contains few genes, many of these genes are involved in male fertility (e.g., spermatogenesis) (Lahn & Page 1997; Carvalho et al. 2009) and therefore may be subject to male-specific sexual selection. Sexual selection can lead to prezygotic isolating barriers, such as behavioural isolation due to mate preferences (reviewed in Coyne & Orr 2004). Sexual selection can also lead to postzygotic isolating barriers; for example, rapid evolution of male reproductive characters due to strong sexual selection could lead to fast divergence of Y chromosome genes, resulting in hybrid incompatibilities through negative epistatic interactions (Dobzhansky 1937; Muller 1942; Wu & Davis 1993). Under this scenario, reproductive barriers would result from hybrid male sterility; this scenario would be aligned with one proposed mechanism (i.e., the ‘faster-male theory’) underlying Haldane's Rule that hybrid sterility or inviability occurs more frequently in the heterogametic sex (Haldane 1922; Wu & Davis 1993; Coyne & Orr 2004).
Various methods have been used to investigate whether the Y chromosome contributes to reproductive isolation between species. To determine whether negative epistatic interactions involving the Y chromosome are driving hybrid incompatibilities, controlled cross experiments are typically employed to introgress the Y chromosome onto a hybrid background, or to conduct genetic mapping of backcrosses (reviewed in Maheshwari & Barbash 2011). Controlled cross experiments between Drosophila species have repeatedly implicated the Y chromosome as driving hybrid incompatibilities (e.g., Coyne et al. 2004; Mishra & Singh 2007; Sweigart 2010), although incompatibilities involving the X chromosome may be more common (e.g., Masly & Presgraves 2007). Few controlled cross experiments have investigated hybrid incompatibilities in vertebrates; of these few studies, most have focused on incompatibilities between house mouse subspecies (Mus musculus), and most have focused on the X chromosome (e.g., Oka et al. 2004; Good et al. 2008). However, one experiment which introgressed an M. m. domesticus Y chromosome onto an inbred mouse strain that normally contains an M. m. musculus Y chromosome resulted in a disruption of male primary sex determination, providing evidence for hybrid incompatibilities involving the Y chromosome (Eicher & Washburn 1986; Washburn et al. 2001).
Another method used to investigate the role of the Y chromosome in generating barriers to reproductive isolation is a comparison of levels of introgression for Y chromosome markers vs. other genetic markers across natural hybrid zones. If one or more genes on the Y chromosome were driving reproductive isolation in the early stages of speciation, we would expect lower levels of introgression for Y chromosome markers compared to mtDNA or autosomal markers. Lower Y chromosome introgression across hybrid zones has been observed for insects (e.g., Drosophila, Llopart et al. 2005), small terrestrial mammals (e.g., house mice, Dod et al. 1993; and rabbits, Geraldes et al. 2008), and elephants (Roca et al. 2005). In most cases, reduced Y chromosome introgression is attributed to epistatic interactions involving the Y chromosome, although other explanations can be invoked, including assortative mating (e.g., Roca et al. 2005), hybrid male sterility resulting from X chromosome incompatibilities (reviewed in Coyne & Orr 2004), and demographic effects (reviewed in Petit & Excoffier 2009).
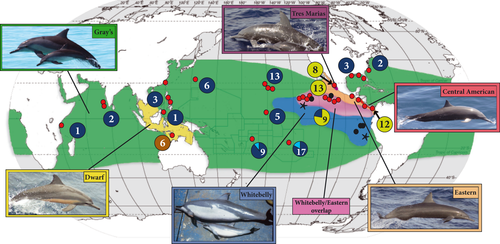
The spinner dolphin (Stenella longirostris) provides a novel framework for investigating the potential influence of the Y chromosome on reproductive isolation. Unlike the terrestrial examples cited in the previous section, S. longirostris has high dispersal capabilities and is globally distributed in tropical and subtropical waters. Six ecotypes (grouped into four subspecies) have been described within this species based on phenotypic differences including male-specific morphologies which could be directly influenced by genes on the Y chromosome, e.g., testis size (Perrin 1990, 2009; Perrin et al. 1999) (Fig. 1, Table 1). These differences in male morphologies are likely to correspond to alternate mating systems, with three ecotypes having a polygynous mating system with strong sexual selection possibly linked to male morphological characters (eastern, Tres Marias, and Central American ecotypes), and at least two ecotypes having a polygynandrous system with possibly lower sexual selection on male morphology (whitebelly and Gray's, and possibly dwarf) (Fig. 1, Table 1) (Perrin & Mesnick 2003). Surprisingly, these phenotypically divergent ecotypes exist despite having adjacent or overlapping distributions (Fig. 1). Furthermore, phenotypic divergences persist despite little genetic divergence between ecotypes at mitochondrial DNA (mtDNA) control region sequences, mtDNA Restriction Fragment Length Polymorphisms (RFLPs), microsatellite markers, and allozymes; studies using these markers found moderate divergence between dwarf vs. other ecotypes, but low or nonsignificant divergence between eastern tropical Pacific (ETP) ecotypes (Central American, Tres Marias, eastern, and whitebelly, Fig. 1) (Sharp 1981; Landino 1987; Dizon et al. 1991; Garcia-Rodriguez 1995; Galver 2002). This lack of concordance between phenotypic, geographic and genetic variation raises questions regarding the evolutionary mechanisms underlying phenotypic divergence in spinner dolphin ecotypes.
| Central American | Tres Marias | Eastern | Whitebelly | Gray's | Dwarf | |
|---|---|---|---|---|---|---|
| Morphology | ||||||
| Female body length (cm) | 193–211 | 186.2 (~165–210) | 171.3 ± 5.99 | 175.6 ± 6.82 | 180.0 ± 10.95 | — |
| Male body length (cm) | 212–216 | — | 176.1 ± 6.12 | 179.8 ± 7.31 | 186.7 ± 4.23 | 144.8 ± 7.88 |
| Coloration | Uniform | Uniform | Uniform | Intermediate | Tripartite | Tripartite |
| Sexual dimorphism | Strong | Strong | Strong | Intermediate | Weak | Weak |
| Mean testis weight (g) | ? | ? | 843 | 1354 | ? | ? |
| Reproductive Seasonality | ? | ? | Unimodal | Bimodal | ? | ? |
| Mating system | PG | PG | PG | PGA | PGA | PGA |
| Habitat | ||||||
| Coastal/pelagic | Coastal | Coastal | Pelagic | Pelagic | Coastal | Coastal |
| Thermocline depth | Shallow | Shallow | Shallow | Deep | Deep | Deep |
| Primary productivity | High | High | High | Intermediate | Low | Low |
Here, we investigate the role of the Y chromosome in phenotypic divergence and reproductive isolation between spinner dolphin ecotypes by conducting population genetic analyses across all six ecotypes using DNA sequences from three Y chromosome markers, two maternally inherited mtDNA markers and a biparentally inherited autosomal intron. Based on previous analyses, we expected to find low genetic divergence between all ecotypes for mtDNA and autosomal markers. Higher levels of divergence between ecotypes for Y chromosome markers would provide evidence that this chromosome is subject to sexual selection driving reproductive isolation and phenotypic divergence between ecotypes.
Materials and methods
Study organism
The six described spinner dolphin ecotypes are defined by geographic distribution, colour pattern, body size, dorsal fin profile, postanal hump and skull osteology (Fig. 1, Table 1; Perrin 1990; Perrin et al. 1999; Perrin 2009). Four ecotypes (in two subspecies) occur exclusively within the ETP (Perrin 1975; Perryman & Westlake 1998): the Central American spinner (Stenella longirostris centroamericana) comprises one coastal ecotype, and the eastern spinner (S. l. orientalis) includes one coastal ecotype (Tres Marias spinner) and two pelagic ecotypes (eastern and whitebelly spinners). With the exception of the whitebelly spinner, the ETP ecotypes differ morphologically from other ecotypes by having largely uniform body coloration (other ecotypes have tripartite body coloration) and increased sexual dimorphism, with adult males having a forward-canted dorsal fin that looks like it is ‘on backwards’, large postanal hump, and upturned fluke tips (Table 1; Perrin 1975, 1990, 2009). As described in the previous section, there is also evidence that the eastern and coastal ETP ecotypes have a different mating system from the whitebelly and Gray's ecotypes (Perrin & Mesnick 2003). These mating system differences are thought to be driven by differences in levels of productivity across the ETP; the far-eastern ETP is characterized by higher levels of primary productivity than any other spinner dolphin habitat (Wyrtki 1966; Reilly 1990; Fiedler et al. 1991; Ballance et al. 1997). This high productivity may have stimulated the formation of a polygynous mating system by allowing dolphins to spend less time foraging, allowing males to spend more time competing for mates, and allowing females to spend more time developing stable social relationships with other females (Perrin & Mesnick 2003).
A fifth spinner dolphin ecotype, the dwarf ecotype (S. l. roseiventris), appears to have a range limited to the coastal waters of Southeast Asia, although it may inhabit adjacent regions as well (Perrin et al. 1999). Unlike other ecotypes, it lives in shallow coral reef habitats and feeds on reef organisms (Perrin et al. 1999). All other spinner dolphins worldwide are grouped into the sixth ecotype, the Gray's spinner (S. l. longirostris). These dolphins are primarily coastal, inhabiting continental margins and nearshore waters of islands (Perrin & Gilpatrick 1994). Additional ecotypes likely remain to be documented within the Gray's ecotype (e.g., Van Waerebeek et al. 1999); however, adequate specimens are not available to address this possibility.
The whitebelly ecotype in the ETP (see previous section) is thought to be a hybrid between the eastern and Gray's ecotypes due to intermediate distribution, morphology and pattern of reproductive seasonality (Barlow 1984; Perrin 1990, 2009; Perrin et al. 1991). Where this ecotype overlaps with the eastern ecotype, a morphological continuum exists in colour patterns and sexual dimorphism, and the two ecotypes sometimes occur in the same group (Table 1; Perrin et al. 1991).
Sample collection
A total of 261 specimens from 17 geographic regions were used for this study (Table 2). Specimens from American Sāmoa, Guam/Saipan, and two specimens from Palmyra were provided by the National Marine Fisheries Service (NMFS) Pacific Islands Fisheries Science Center. All other specimens excluding the Hawai'i and Moorea specimens were provided by the NMFS Southwest Fisheries Science Center (SWFSC) Genetics Archive in the form of extracted genomic DNA, corresponding to SWFSC accession numbers listed as Supplementary Material (S1). A subset of 40 specimens was randomly chosen from 505 specimens from the Hawaiian Archipelago (Andrews et al. 2010), and a subset of 40 specimens was randomly chosen from 70 specimens from Moorea (Oremus et al. 2007). Most specimens were skin biopsies collected from free-ranging animals. Eastern and whitebelly specimens consisted of liver, skin, heart, kidney, muscle or stomach tissue from bycatch in the tuna fishery. Dwarf specimens came from bycatch in a shark gillnet fishery. The ecotype designation of specimens was determined by geographic location of sampling and/or field observations of morphology. For each of our eastern and whitebelly specimens, detailed morphological data are available regarding coloration patterns and dorsal fin shape from a previous morphological study (Perrin et al. 1991). This previous study found a morphological continuum between eastern and whitebelly ecotypes (as described in the previous section), and assigned a numerical code to each specimen which ranged from 3 to 10, with three assigned to specimens with characteristics most typical of the eastern ecotype, and 10 assigned to specimens with characteristics most typical of the whitebelly ecotype (Perrin et al. 1991). For our study, we chose specimens that exhibited the most divergent morphologies, i.e., codes 3 or 4 for eastern ecotypes, and codes 9 or 10 for whitebelly ecotypes.
| Ecotype/Region | mtDNA | Autosomal intron | Y chromosome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | k | h | π | k | h | π | n | k | h | π | |
| Central American | 23 | 18 | 0.976 | 0.0106 | 7 | 0.711 | 0.0032 | 12 | 1 | 0 | 0 |
| Tres Marias | 29 | 24 | 0.985 | 0.0125 | 11 | 0.742 | 0.0026 | 8 | 1 | 0 | 0 |
| Eastern | 27 | 23 | 0.986 | 0.0124 | 13 | 0.623 | 0.0022 | 13 | 1 | 0 | 0 |
| Whitebelly | 23 | 20 | 0.988 | 0.0117 | 9 | 0.702 | 0.0026 | 9 | 2 | 0.389 | 0.001 |
| Dwarf | 14 | 7 | 0.846 | 0.0065 | 5 | 0.696 | 0.0015 | 6 | 1 | 0 | 0 |
| Gray's: Hawai‘i | 36 | 9 | 0.620 | 0.0047 | 8 | 0.63 | 0.0029 | 13 | 1 | 0 | 0 |
| Palmyra | 9 | 7 | 0.944 | 0.0743 | 5 | 0.667 | 0.0036 | 5 | 1 | 0 | 0 |
| Moorea | 38 | 16 | 0.942 | 0.0124 | 10 | 0.663 | 0.0023 | 17 | 2 | 0.309 | 0 |
| Sāmoa | 15 | 12 | 0.971 | 0.0124 | 8 | 0.685 | 0.0018 | 9 | 2 | 0.222 | 0 |
| Guam | 10 | 7 | 0.911 | 0.0125 | 5 | 0.679 | 0.0019 | 6 | 1 | 0 | 0 |
| Taiwan | 4 | 3 | 0.833 | 0.0072 | 4 | 0.821 | 0.0022 | 0 | NA | NA | NA |
| Philippines | 6 | 6 | 1.00 | 0.0157 | 4 | 0.786 | 0.0033 | 3 | 1 | 0 | 0 |
| Indonesia | 5 | 4 | 0.900 | 0.0084 | 2 | 0.571 | 0.0009 | 1 | 1 | NA | NA |
| Maldives | 6 | 4 | 0.867 | 0.0147 | 6 | 0.803 | 0.0029 | 2 | 1 | 0 | 0 |
| Zanzibar | 9 | 5 | 0.889 | 0.0139 | 6 | 0.758 | 0.0021 | 1 | 1 | NA | NA |
| Gulf Mexico | 5 | 3 | 0.700 | 0.0090 | 7 | 0.911 | 0.0045 | 3 | 1 | 0 | 0 |
| NW Atlantic | 2 | 2 | 1.00 | 0.0011 | 2 | 0.5 | 0.0008 | 2 | 1 | 0 | 0 |
| Total | 261 | 110 | |||||||||
DNA sequencing
Genomic DNA from Hawai'i and Moorea samples was extracted as described by Andrews et al. (2010) and Oremus et al. (2007). Genomic DNA from specimens provided by the PIFSC was extracted using the Hotshot method (Meeker et al. 2007). DNA sequences were obtained through Polymerase Chain Reactions (PCR) of genomic DNA for six genetic markers, including portions of the mtDNA control region, mtDNA cytochrome b (cytb), intron I of the muscle actin gene (an autosomal marker) and three Y chromosome introns. We used mtDNA control region primers KRAdLp 1.5t-pro (Andrews et al. 2006) and dLp5 (Pichler et al. 2001); cytb primers LGL765 and 610 (Bickham et al. 1995; Harlin-Cognato et al. 2006); and Y chromosome intron primer sets DBY7, DBY9 and SMCY7 (Hellborg & Ellegren 2003) (Table S1, Supporting information). Y chromosome PCRs were conducted for male specimens when morphological data regarding sex were available (i.e., for eastern, whitebelly and some Philippines specimens), and for all specimens for which sex was unknown. For specimens which amplified for DBY7 but failed to amplify for DBY9 or SMCY7, internal primers were designed for another PCR and sequencing reaction (Table S1, Supporting information).
All PCRs were performed in 20 μL volumes containing 1X MangoMix (Bioline, Taunton MA, USA) and 0.2 μm each primer. Cycle conditions for mtDNA control region PCRs were as described in Andrews et al. (2006). PCR cycle conditions for mtDNA cytb, actin intron, DBY9 external and internal primer sets and SMCY7 internal primer set were as follows: 95 °C for 1 min, followed by 35 cycles of 94 °C for 30 s, annealing temperature for 30 s, and 72 °C for 30 s, followed by a final 72 °C extension for 10 min. Annealing temperatures were 55 °C for cytb, 60 °C for actin intron, 52 °C for DBY9, and 63 °C for internal primer sets of DBY9 and SMCY7. Cycle conditions for DBY7 and the external primer set of SMCY7 were as follows: 95 °C for 1 min, followed by 20 cycles of 94 °C for 30 s, a touchdown from 65 °C to 55 °C for 30 s decreasing by 0.5 °C/cycle, and 72 °C for 30 s; followed by 15 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s; followed by a final 72 °C extension for 10 min. PCR products were sequenced with an ABI3730 automated sequencer using the forward primer for control region, cytb and Y introns, and both forward and reverse primers for the actin intron. GenBank Accession numbers for these sequences are KC160952–KC161183. For 96 of the specimens used in our study, mtDNA control region sequences were already available on GenBank (accession numbers are listed as Supplementary Material S2).
Sequences were aligned and edited manually using Geneious 5.4.2 (Biomatters Ltd, Auckland City, NZ, USA). For actin intron sequences, heterozygous nucleotide positions were determined using the Geneious Heterozygote plugin and by eye. Alignments of forward and reverse sequences revealed three non-overlapping indels for the actin intron: two indels were one bp long and a third indel was 21 bp long. Only one individual had more than one indel; this individual had two indels, and for this individual, alleles were resolved by cloning. For all other individuals, alleles were resolved using Bayesian methods implemented in the program Phase 2.1 (Stephens et al. 2001; Stephens & Donnelly 2003). Subsequent actin analyses were conducted with datasets coded in two different ways: one dataset excluded indels, and the other dataset coded indels as separate nucleotide characters at the ends of sequences (Simmons & Ochoterena 2000; Pearce 2006). For the second dataset, indels were coded as transversions and were therefore weighted accordingly for analyses which used a nucleotide substitution model.
Genetic diversity & population structure
Because the mtDNA control region and cytb loci are strongly linked, and the three Y chromosome introns are also strongly linked (Charlesworth & Charlesworth 2000), genetic diversity and structure analyses were conducted for both concatenated and non-concatenated mtDNA and Y chromosome datasets.
Nucleotide (π) and haplotypic (h) diversities were calculated for each geographic region and ecotype for each genetic marker using Arlequin 3.11 (Excoffier et al. 2005). The nucleotide substitution models used to calculate genetic distance were the models available in Arlequin that most closely matched the best-fit models determined using AIC in jModeltest 0.1.1 (Posada 2008).
Genetic similarity of haplotypes/alleles amongst ecotypes and regions was visualized using Network 4.6.1.0 (Bandelt et al. 1999). Due to a high number of ambiguities in the mtDNA control region and concatenated mtDNA networks, the star contraction and MP options were used to reduce the complexity of these networks (Bandelt et al. 1999).
Population structure amongst ecotypes and regions was examined with pairwise ΦST values in Arlequin. For these analyses, genetic distance was calculated using the best-fit nucleotide substitution models available in Arlequin. Significance of pairwise ΦST values was tested using 20 000 permutations. Samples from the NW Atlantic and Taiwan were excluded from these analyses due to low sample sizes (n < 5).
Genetic structure was also investigated with a Bayesian clustering method in Structure 2.3.2 (Pritchard et al. 2000), with sampling location as a prior and using the admixture and correlated allele frequency models. Because pairwise ΦST values indicated different patterns of structure for Y chromosome introns vs. mtDNA and autosomal loci, Bayesian clustering analyses were conducted in two ways to compare the influence of different loci on the analysis: (i) using actin intron sequences and concatenated mtDNA sequences (ii) using these loci as well as concatenated Y intron sequences for male specimens only. These clustering analyses were conducted using only ecotypes/geographic regions with at least five sequences available for all markers (Table 2). Because these datasets consisted of both diploid and haploid markers, a second allele for each concatenated haploid marker (mtDNA and Y chromosome introns) was coded as ‘unknown’ for each individual, as recommended by the author of Structure (J. Pritchard, personal cummunication). The burn-in length was 105 steps, followed by 106 steps. Five independent runs were conducted for each value of K ranging from 1 to 10.
Coalescent analyses: gene flow between eastern, whitebelly and Gray's
Due to the particularly high phenotypic divergence accompanied by low genetic divergence between the eastern, whitebelly and Gray's ecotypes, we investigated levels of gene flow between these three ecotypes using a coalescent method implemented in the program IMa2 (Hey 2010b). This method estimates the average interchange of genes per generation across all generations since the splitting of each pair of populations. All individuals from the whitebelly and ETP ecotypes were used in the analyses; however, to reduce parameter space, only 50 individuals from the Gray's ecotype were used. These Gray's specimens were chosen from the sampled regions which were the most geographically and genetically similar (according to pairwise ΦST and Bayesian clustering analyses) to the eastern and whitebelly ecotypes: French Polynesia, American Sāmoa and Palmyra Atoll. A full analysis with all three ecotypes requires the true phylogenetic tree between ecotypes. Without making that assumption, we employed a multiple pairwise comparison framework, which limits the number of parameters that are simultaneously estimated, helping to ensure convergence within each analysis. This method has been shown to recover the same patterns seen in full analyses (Hey 2010a). Three different coalescent analyses were run, one for each pair of the three ecotypes, each run using the concatenated mtDNA, actin intron and concatenated Y-intron sequences. We used a conservative mutation rate estimate for the mtDNA control region of 2.79004 × 10−6 mutations per year as in Oremus et al. (2007), with an estimated range between 1.79004 × 10−6 and 3.79004 × 10−6. Several preliminary runs were used to hone prior values and check for consistency. Final MCMCMC chains were run for burn-in until flat trend lines appeared (at least 15 million steps), and afterward 150 000–300 000 genealogies per locus were saved. Nested models for each analysis were used in conjunction with likelihood ratio tests to examine if migration estimates were significantly different from zero and each other.
Results
Diversity, pairwise ΦST, and haplotype network results were similar for concatenated vs. non-concatenated datasets for mtDNA and Y chromosome markers, and also for actin intron datasets excluding indels vs. coding indels as transversions. Here, we present the results for the concatenated datasets and the actin dataset coding indels as transversions. Pairwise ΦST results for non-concatenated datasets are presented in Supplemental Material (Tables S2–S4, Supporting information).
We resolved fragment sizes of 884 bp for concatenated mtDNA, 629 bp for actin intron, and 1065 bp for concatenated Y introns. DNA sequence alignments revealed 142 haplotypes and 135 variable sites for mtDNA; 33 alleles and 36 variable sites for actin intron when indels were not coded; 35 alleles and 39 variable sites for actin intron when indels were coded; and four haplotypes and five variable sites for Y introns (two variable sites for both DBY7 and SMCY7, and one variable site for DBY9). The program Phase 2.1 resolved actin intron genotypes with >80% posterior probabilities for all except five individuals when indels were coded as transversions.
The best-fit nucleotide substitution models were TIM3 (Posada et al. 2003) + I + gamma = 0.777 for concatenated mtDNA, TPM2uf (Kimura 1981) for concatenated Y introns, and TIM2 (Posada et al. 2003) for actin intron. The nucleotide substitution models available in Arlequin that most closely matched these best-fit models were Tamura & Nei (1993) + gamma = 0.777 for concatenated mtDNA, Tamura (1992) for Y introns and Tamura & Nei for actin intron; these models were used to calculate genetic distance for diversity and pairwise ΦST analyses.
Genetic diversity & population structure
Haplotype and nucleotide diversities were generally higher across geographic regions and ecotypes for mtDNA (h = 0.620–1.00, π = 0.0047–0.0743) than for actin intron (h = 0.571–0.911, π = 0.0009–0.0045) (excluding NW Atlantic and Taiwan due to n < 5, Table 2). Although diversity values for geographic regions/ecotypes varied across these markers, some general trends emerged: the Philippines had higher diversity values than other regions/ecotypes, and Hawai'i and the dwarf ecotype had low diversity values (Table 2). For Y introns, most ecotypes and geographic regions had no genetic diversity due to the presence of a single fixed haplotype at each locus, with the exceptions of the whitebelly ecotype which had two haplotypes; and Moorea and American Sāmoa which had two haplotypes (Table 2).
Multiple shortest median-joining networks were produced in the mtDNA analysis, but all were similar in structure, including the one randomly chosen to report here (Fig. 2a). Actin intron and Y intron analyses each produced a single network (Fig. 2b,c). Networks for mtDNA and actin intron showed little segregation of haplotypes/alleles by geography or ecotype (Fig. 2a,b). Exceptions to this pattern were the Hawai'i population and dwarf ecotype: each of these groups had some haplotypes/alleles that were not shared between ecotypes or regions, but all of these were closely related to haplotypes/alleles of other groups.
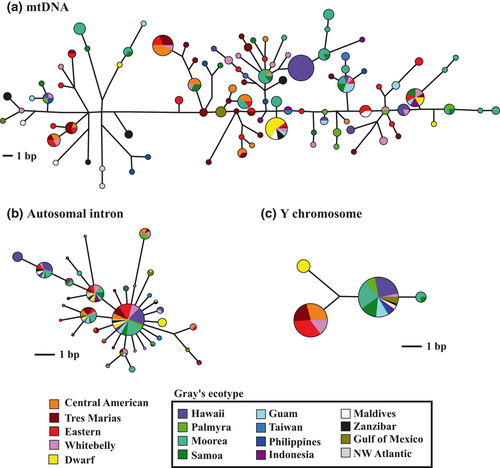
In contrast to mtDNA and autosomal networks, the Y intron network revealed strong segregation of haplotypes by ecotype (Fig. 2c). Dwarf specimens all shared a single haplotype; all specimens of the Gray's had a second haplotype, with the exceptions of three specimens from Moorea and one specimen from American Sāmoa, which had a third haplotype; and all specimens of the ETP ecotypes had a fourth haplotype, with the exception of two out of nine whitebelly specimens, which had the most common Gray's haplotype (Fig. 1). Taken together, the results from the Y introns indicate strong segregation of Y chromosome haplotypes amongst the Gray's, dwarf and ETP ecotypes, but almost no segregation of Y chromosome haplotypes amongst geographic regions within the broadly distributed Gray's ecotype.
Pairwise ΦST analyses gave overall similar results for mtDNA and autosomal markers, revealing low genetic divergence between most geographic regions and ecotypes, with many ΦST values <0.1 and nonsignificant (excluding comparisons involving the dwarf ecotype and Hawai'i population, see below) (Table 3a). Comparisons between the four ETP ecotypes resulted in consistently low or nonsignificant levels of genetic differentiation across mtDNA and autosomal markers. Most pairwise ΦST values between the coastal Central American vs. pelagic ecotypes in the ETP were significant but low (<0.11), and pairwise ΦST values between all other ETP ecotypes were nonsignificant. Comparisons of the Gray's ecotype vs. other geographic regions and ecotypes also revealed low or nonsignificant ΦST values, with some pairwise comparisons nonsignificant despite large geographic distances separating sample locations. Two exceptions to the general pattern of low or non-significant pairwise ΦST values were the dwarf ecotype and Hawai'i population. Most pairwise ΦST values involving these two groups were >0.15 and statistically significant. Therefore, pairwise ΦST results indicated an overall weak concordance between genetic and phenotypic divergence for mtDNA and autosomal markers.
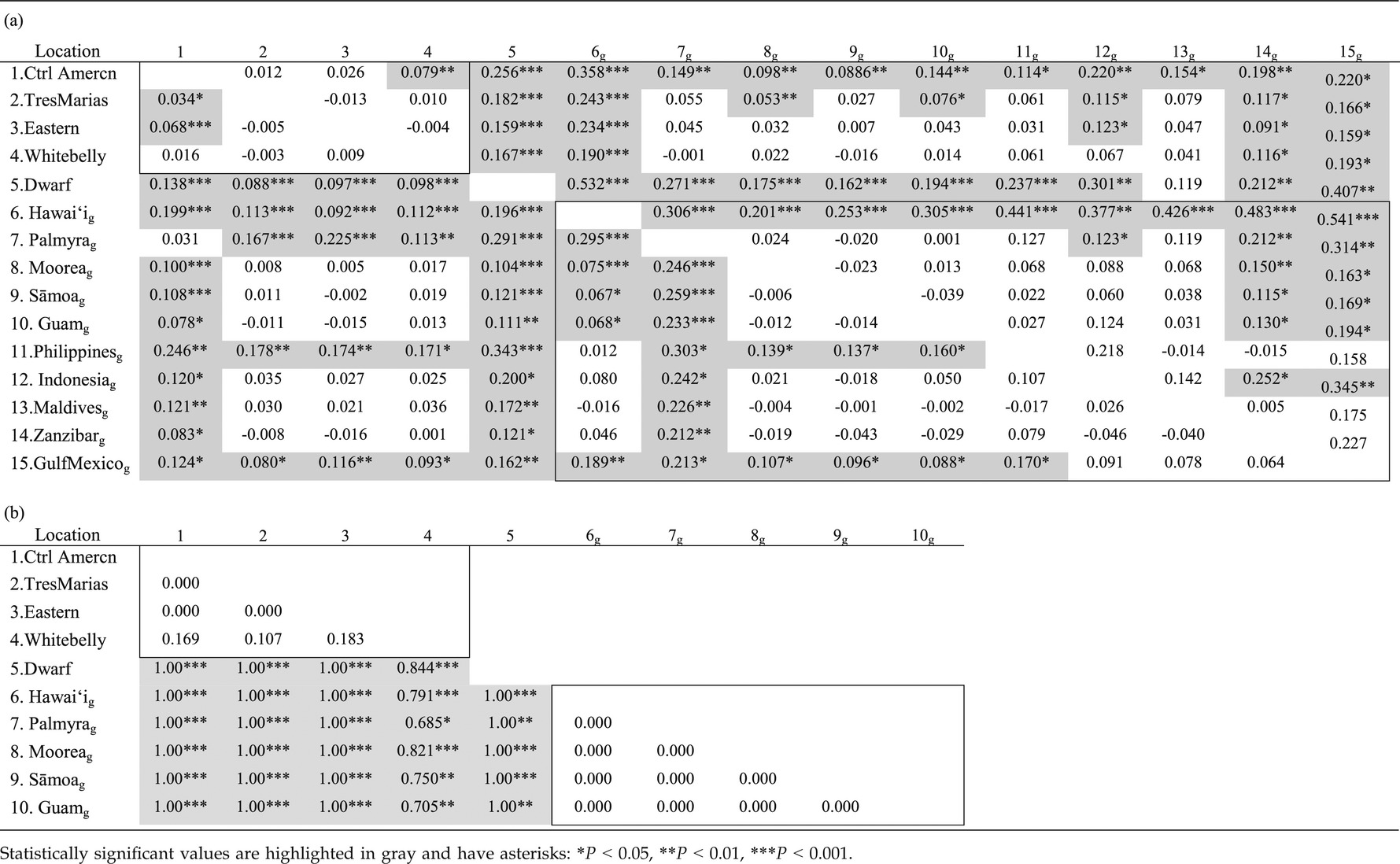
In contrast to mtDNA and autosomal markers, analyses of Y intron sequences revealed statistically significant ΦST values for all pairwise comparisons between the most morphologically divergent ecotypes (Gray's, ETP ecotypes, and dwarf), but nonsignificant ΦST values for comparisons between geographic regions within the Gray's ecotype (Table 3b). With the exception of comparisons involving American Sāmoa, Moorea, and the putative hybrid whitebelly, all ΦST values were zero or one due to the presence of a single haplotype within most ecotypes and geographic regions.
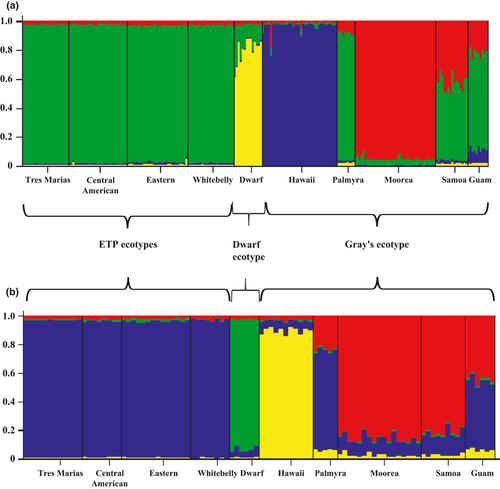
For Bayesian clustering (Structure) analyses, average posterior probabilities were consistently highest at K = 4 groupings both for analyses including mtDNA and autosomal markers, and for analyses including all markers for male specimens (Fig. 3). For both analyses, visual inspection of the output indicated three consistent population clusters: (i) ETP ecotypes, (ii) dwarf ecotype and (iii) the Hawai'i region of the Gray's ecotype. For the mtDNA/autosomal marker analysis, the Moorea region of the Gray's ecotype constituted a fourth cluster, providing evidence for fine-scale population divergence of Moorea not revealed by pairwise ΦST analyses (see previous section). The other Gray's regions either clustered with the ETP ecotypes (i.e., Palmyra region) or exhibited admixture between the ETP ecotypes and Moorea region (i.e., American Sāmoa and Guam). Results from the analysis including all markers for male specimens were similar to those for the mtDNA/autosomal analysis, with the exception that regional populations of the Gray's ecotype clustered less with the ETP ecotypes and more with each other.
Coalescent analyses indicated gene flow significantly greater than zero amongst all three ecotypes analysed (eastern, whitebelly, and Gray's), with the exception of gene flow from the Gray's to the eastern ecotype. Gene flow was higher between the whitebelly and eastern ecotypes (M = 30.1–57.9) than between the Gray's and other ecotypes (M = 0.58–3.22), where M is the mutation-scaled migration rate in forward time (Table 4).
| Ecotype 1 | Ecotype 2 | M from 1 to 2 | M from 2 to 1 |
|---|---|---|---|
| Gray's | Eastern | 0.58 (0.1–3.5) | 0.82 (0.1–5.3)a |
| Whitebelly | Gray's | 3.22 (0.7–10.9)a | 1.6 (0.2–6.9)a |
| Whitebelly | Eastern | 30.1 (8.5–77.10)a | 57.90 (23.9–171.7)a |
- a Denotes values that were significantly different than zero when tested with a nested model likelihood ratio test. Numbers in parentheses represent 95% Confidence Intervals.
Discussion
Contrasting phylogeographic patterns amongst genetic markers
In this study, Y chromosome introns revealed strikingly different phylogeographic patterns than did the mtDNA or autosomal intron. Our mtDNA and autosomal analyses revealed low levels of genetic divergence amongst ecotypes, including those recognized as separate subspecies. With the exception of comparisons involving the dwarf ecotype, most pairwise mtDNA and autosomal ΦST values between ecotypes were <0.1, and many were nonsignificant. These values are concordant with those of the previous studies which used 12 microsatellites, seven allozymes, mtDNA RFLPs (using six restriction enzymes which produced patterns of 2–13 fragments), and mtDNA control region sequences (440 bp), and found no significant divergence between eastern and whitebelly ecotypes; low or non-significant divergence between coastal vs. pelagic ETP ecotypes (ΦST < 0.2), and moderate divergence between dwarf vs. Gray's ecotype populations (ΦST = 0.2) (Sharp 1981; Landino 1987; Dizon et al. 1991; Galver 2002).
In contrast, concatenated Y chromosome analyses revealed fixed genetic differences between the most morphologically divergent ecotypes: (i) dwarf, (ii) Gray's and (iii) ETP ecotypes excluding the whitebelly form. The whitebelly spinner shared Y chromosome haplotypes with the other ETP ecotypes as well as the widespread Gray's ecotype, concordant with previous hypotheses that it is a hybrid between the eastern and Gray's ecotypes (Perrin 1990). Therefore, in contrast to mtDNA and autosomal marker analyses, Y chromosome analyses revealed a strong concordance between genetic divergence and phenotypic divergence amongst ecotypes.
MtDNA and autosomal markers: weak concordance between phenotypic & genetic divergence
The weak genetic distinction amongst phenotypically divergent spinner dolphin ecotypes at mtDNA and autosomal markers has puzzled biologists for decades (Sharp 1981; Landino 1987; Dizon et al. 1991; Galver 2002). Discordance between genetic and morphological divergence in other taxa is usually attributed to natural selection exerting a stronger influence than gene flow on morphological variation (e.g., Gíslason et al. 1999; Taylor & McPhail 1999; Milá et al. 2009; DiBattista et al. 2012). For spinner dolphins, strong natural selection has also been postulated to explain the low or non-significant genetic divergence between ecotypes at mtDNA and autosomal markers (Dizon et al. 1991; Galver 2002). The phenotypic divergence of the dwarf ecotype is thought to result from adaptation to the unique coral reef habitat and prey of this ecotype (Perrin et al. 1999). Similarly, habitat-driven selection may be responsible for phenotypic divergence between nearshore vs. offshore ecotypes in the ETP.
One of the most striking results from this study (and previous studies) is the lack of significant mtDNA or autosomal divergence between the two pelagic ecotypes within the ETP (eastern vs. whitebelly), despite particularly strong phenotypic differences (Table 1). This result does not necessarily indicate frequent mating between these ecotypes; it is possible that the reproductive isolation is extremely recent or that analytical methods employed thus far lack the statistical power to measure the low ΦST values expected when gene flow is restricted amongst large populations (Whitlock & McCauley 1999). Population sizes in the ETP are very large (estimated at 450 000 for eastern and >1 000 000 for whitebelly spinners, Wade & Gerrodette 1993; Gerrodette & Forcada 2005) compared with other ecotypes and regions such as the island-associated populations in Hawai'i (estimated ~1000 at one island in Hawai'i, Norris et al. 1994) and Moorea (estimated 135, Oremus et al. 2007). However, several additional lines of evidence point to interbreeding between eastern and whitebelly ecotypes. As described in the previous section, a morphological continuum exists between these two ecotypes, and the two ecotypes sometimes occur in the same group. In addition, migration between eastern and whitebelly ecotypes estimated with coalescent-based analyses was greater than zero, and these estimates make no assumptions regarding population size or equilibrium (Hey 2010b).
If ongoing gene flow occurs between the eastern and whitebelly ecotypes, then the morphological divergence between these ecotypes must be driven either by phenotypic plasticity or by divergent selective pressures restricting gene flow for some, but not all loci (i.e., porous genomic boundaries, Wu 2001; Gavrilets & Vose 2005). Phenotypic plasticity seems unlikely given the magnitude of the morphological and life history differences between ecotypes. If divergent selective pressures are responsible, these pressures may involve sexual selection resulting from different mating systems (Wade & Arnold 1980). Morphological and behavioural analyses indicate that the eastern ecotype has a primarily polygynous mating system, whereas the whitebelly and Gray's (and possibly dwarf) ecotypes have a primarily polygynandrous mating system, as indicated by lower average testis size and distinctive male morphological traits in the eastern ecotype compared with the whitebelly and Gray's ecotypes (Table 1; Norris et al. 1994; Perrin & Mesnick 2003).
Within the Gray's ecotype, multiple factors could be responsible for the weak genetic structure across oceans, including ongoing dispersal or recent colonization. The Gray's populations in Hawai'i appear to be more genetically isolated than any other populations included in our study, and the remoteness of the Hawaiian Archipelago is a likely explanation. The small effective population sizes of Hawaiian populations may also contribute to high ΦST values due to the stronger influence of genetic drift on small populations.
Y chromosome markers: strong concordance between phenotypic & genetic divergence
Several scenarios could explain the contrasting results obtained from the Y chromosome compared with mtDNA and autosomal markers:
Low Ne for the Y chromosome
The high level of Y marker divergence between phenotypically divergent ecotypes could be influenced by the greater effect of genetic drift due to a lower Ne for the Y chromosome. Both mtDNA and the Y chromosome have haploid, uniparental inheritance, but the latter may have a lower Ne than mtDNA markers as a result of any form of sexual selection that is stronger for males than females — for example, polygynous mating systems with high variance in male reproductive success are expected to reduce Ne for Y chromosome markers compared with mtDNA markers (Caballero 1995; Karl 2008). However, under a scenario in which one genetic marker has a lower Ne than another, we would not expect to find the strong biogeographic discordance between markers that we observe for the Y chromosome vs. mtDNA for spinner dolphins (Toews & Brelsford 2012). For example, if greater divergence of the Y chromosome markers were primarily a function of a lower Ne, we would not expect to see strong genetic isolation of the Hawaiian Archipelago within the Gray's ecotype for mtDNA without any such evidence of divergence for the Y chromosome (Fig. 1). Conversely, we see strong genetic divergence between ecotypes for Y chromosome markers but no such evidence of divergence amongst the ecotypes for mtDNA. Therefore, genetic drift resulting from unequal Ne seems an unlikely explanation for the contrasting patterns of genetic structure for Y markers vs. mtDNA and autosomal markers.
Female-biased dispersal
Strong male philopatry combined with female dispersal between ecotypes could also explain the greater divergence for Y markers. Little information exists to assess whether female-biased dispersal occurs between spinner dolphin ecotypes. Direct tests for female-biased dispersal would require genetic analyses using multiple bi-parentally inherited markers (although these statistics have low power, Goudet et al. 2002), or mark–recapture analyses. However, female-biased dispersal is rare amongst mammals (reviewed in Lawson Handley & Perrin 2007) and has not been documented in delphinids (reviewed in Möller 2011). Furthermore, photographic identification studies of the Gray's ecotype indicated low group cohesion for both males and females in Hawai'i (Norris et al. 1994; Karczmarski et al. 2005) and Moorea (Oremus et al. 2007), and equal rates of dispersal between island habitats for males and females in the Northwestern Hawaiian Islands (L. Karczmarski, personal cummunication). Therefore, the very strong male philopatry which would be required to produce fixed differences in Y marker haplotypes between ecotypes seems unlikely.
Assortative mating
Under this scenario, males and females of both ecotypes occur in a hybrid zone, but reproductive dominance of males of one ecotype prevents introgression of the Y chromosome from the competitively weaker males into the ecotype with the dominant males. For example, the morphologically distinct males of the eastern ecotype may outcompete males of the Gray's ecotype in the whitebelly hybrid zone for mating with females of both ecotypes, resulting in a predominance of eastern Y chromosomes in the hybrid zone, but introgression of mtDNA and autosomal genes between ecotypes. This scenario would be similar to that seen amongst African forest and savanna elephants, in which discordance between mtDNA vs. Y chromosome and autosomal markers is thought to result from competitive reproductive dominance of larger savanna males over forest males (Roca et al. 2005). This hypothesis could be tested by examining genetic measures of individual reproductive success within groups of each ecotype.
Hybrid male sterility or inviability between ecotypes
This postzygotic isolating mechanism would be consistent with Haldane's Rule, in which the heterogametic sex is the first to acquire hybrid sterility or inviability due to genetic incompatibilities occurring on a hybrid background (Haldane 1922). The primary explanations for Haldane's Rule are (i) the Dominance Theory, in which recessive genes on the X chromosome are exposed in the heterogametic males (Muller 1942; Coyne & Orr 2004); and (ii) the Faster-Male Theory, in which spermatogenesis is a sensitive process that is easily disrupted in hybrids, or sexual selection causes faster evolution of male-expressed than female-expressed genes (Wu & Davis 1993; Coyne & Orr 2004). Controlled cross experiments are typically used to investigate whether epistatic interactions drive hybrid incompatibilities; however, these types of experiments are probably not feasible for spinner dolphins. A more practical method for this species may be to search for evidence of pairwise associations between alleles at different loci across the genome (e.g., as in Teeter et al. 2009).
Given the data available to date, the strong Y chromosome divergence between ecotypes seems most likely driven by sexual selection on male-specific characters, whether through assortative mating, hybrid male sterility/inviability or a combination of these factors. Further evidence for sexual selection as a driver of reproduction isolation amongst spinner dolphin ecotypes comes from the morphological and behavioural evidence for different mating systems between ecotypes. Therefore, we hypothesize that the contrasting patterns of genetic structure between spinner dolphin ecotypes for Y chromosome vs. mtDNA and autosomal markers result from porous genomic boundaries, in which sexual selection involving male-specific characters prevents gene flow between ecotypes for the Y chromosome, but other regions of the genome move between ecotypes.
Relatively few studies of species with XY sex determination have examined population genetic structure at the Y chromosome. For mammals, this dearth of Y chromosome studies is largely due to technical difficulties in developing markers for this chromosome, as well as low levels of polymorphism (reviewed in Greminger et al. 2010). Of the studies which have used Y markers, some have found evidence for elevated population genetic divergence at Y markers compared with other markers. For example, higher population genetic divergence for Y chromosome markers (relative to mtDNA markers) was observed in chimpanzees and bonobos, and behavioural observations indicate that this pattern is driven by female-biased dispersal for both of these species (Eriksson et al. 2006; Langergraber et al. 2007). Higher population genetic divergence at Y chromosome markers than mtDNA and/or autosomal markers was also observed in several mammalian species which do not exhibit female-biased dispersal, including rabbits (Geraldes et al. 2008), mice (Tucker et al. 1992), shrews (Balloux et al. 2000), and elephants (Roca et al. 2005). For most of these species, elevated Y chromosome divergence is thought to result from hybrid incompatibilities or sexual selection between isolated evolutionary lineages with limited dispersal. Here, we report the first case of Y chromosome divergence in adjacent ecotypes for a large, highly mobile marine mammal for which female-biased dispersal is unlikely. This finding diminishes the factors of geographic isolation and female-biased dispersal as necessary prerequisites to the segregation of Y chromosome types. We suggest that the evolving male chromosome may be a ubiquitous facet of diversification, functioning across a broad range of evolutionary scenarios.
Acknowledgements
We thank the following for assistance with genetics laboratory work, analysis and data interpretation: Roxanne Haverkort, Michelle Gaither, other members of the ToBo Lab, Fred Allendorf, Phil Morin, Tomo Eguchi, Barbara Taylor. For assistance in sample collection, we thank Bud Antonelis, Whitlow Au, Lisa Ballance, Robin Baird, Jason Baker, Scott Baker, Jay Barlow, Marie Chapla, Mark Deakos, Louella Dolar, Ania Driscoll-Lind, Chris and Cari Eggleston, Beth Flint, Tim Gerrodette, Annie Gorgone, Nancy Hoffman, Dave Johnston, Randall Kosaki, Danielle Kreb, Marc Lammers, Keith Larson, Amarisa Marie, Charles Moore, Jan Östman-Lind, Robert Pitman, Michael Poole, Charley Potter, Susan Rickards, Rob Shallenberger, Dave Smith, Robert Smith, Russel Sparks, Jen Tietjen, Cynthia Vanderlip, Jeff Walters, Daniel Webster, Bernd Würsig, Chad Yoshinaga, Hawai'i Department of Land and Natural Resources, Division of Forestry & Wildlife and Division of Aquatic Resources, U.S. Fish & Wildlife Service, Papahānaumokuākea Marine National Monument, Hawaiian Islands Humpback Whale National Marine Sanctuary, NMFS Southwest Fisheries Science Center, NMFS Pacific Islands Fisheries Science Center, Oceanic Society, the crew of the NOAA R/V Hiialakai and Texas Institute of Oceanography. Partial funding was provided by: National Science Foundation Graduate Research Fellowship Program; National Geographic Society; Pacific Marine Life Foundation; National Fish and Wildlife Foundation; Anonymous Foundation; University of Hawai'i Sea Grant College Program; University of Hawai'i Ecology, Evolution and Conservation Biology Program; Algalita Foundation; Sea Vision Foundation; American Museum of Natural History; New Zealand Marsden Fund. Research at Midway Atoll was partially sponsored by the Oceanic Society. Most genetic analyses were conducted in the ToBo lab at the Hawai'i Institute of Marine Biology, which is supported by grants from the HIMB-NWHI Coral Reef Research Partnership (NMSP MOA 2005-008/6882), University of Hawai'i Sea Grant College Program, National Science Foundation (OCE-0454873 to B.W.B.; EPS-0554657 to University of Hawai'i; OCE-0623678 to R.J.T.). The views expressed herein are those of the authors and do not necessarily reflect the views of NSF, NOAA, the State of Hawai'i or any of their sub-agencies. This is contribution #1527 from the Hawai'i Institute of Marine Biology and SOEST #8782.
References
K.R.A., R.J.T., B.W.B. and W.F.P. designed the research project. M.O. and L.K. contributed genomic DNA. W.F.P. contributed expertise on spinner dolphin biology, including morphological assessments of ecotypes. W.F.P. is part of the National Marine Fisheries Service Southwest Fisheries Science Center, which contributed genomic DNA. K.R.A. generated molecular data and conducted most analyses. J.B.P. conducted coalescent analyses. Molecular labwork was conducted in the laboratory of R.J.T. and B.W.B. K.R.A. led the writing of the paper.
Data accessibility
Sampling data: Table S5.




