Are long-lived trees poised for evolutionary change? Single locus effects in the evolution of gene expression networks in spruce
Abstract
Genetic variation in gene expression traits contributes to phenotypic diversity and may facilitate adaptation following environmental change. This is especially important in long-lived organisms where adaptation to rapid changes in the environment must rely on standing variation within populations. However, the extent of expression variation in most wild species remains to be investigated. We address this question by measuring the segregation of expression levels in white spruce [Picea glauca (Moench), Voss] in a transcriptome-wide manner and examining the underlying evolutionary and biological processes. We applied a novel approach for the genetic analysis of expression variation by measuring its segregation in haploid meiotic seed tissue. We identified over 800 transcripts whose abundances are most likely controlled by variants in single loci. Cosegregation analysis of allelic expression levels was used to construct regulatory associations between genes and define regulatory networks. The majority (67%) of segregating transcripts were under linkage. Regulatory associations were typically among small groups of genes (2–3 transcripts), indicating that most segregating expression levels can evolve independently from one another. One notable exception was a large putative trans effect that altered the expression of 180 genes that includes key regulators of protein metabolism, highlighting a regulatory cascade affected by variation in a single locus in this conserved metabolic pathway. Overall, segregating expression variation was associated with stress response- and duplicated genes, whose evolution may be linked to functional innovations. These observations indicate that expression variation might be important in facilitating diversity of molecular responses to environmental stresses in wild trees.
Introduction
Long-lived and sessile organisms such as trees require long-term resistance and environmental adaptability to survive in a context where environmental changes can outpace their generation time. White spruce is a keystone species of the North American taiga whose populations are affected by global environmental change (Peng et al. 2011). Their potential for adaptation is likely to have wide-sweeping impacts on boreal animal and plant communities and the ecosystem services they provide. The ranges of spruce trees and other conifers cover large climatic gradients while subpopulations can be adapted to their local environments (Aitken et al. 2008; Mimura & Aitken 2010; Savolainen et al. 2011). These populations may draw upon alternative molecular solutions to respond to local environmental conditions (Prunier et al. 2011, 2012), a phenomenon also seen in annual plants (Fournier-Level et al. 2011). Variation in gene expression contributes to phenotypic diversity in cellular and physiological processes, including responses to environmental stresses and, therefore, may sustain the adaptability of conifer populations. Despite the fact that it could help predict their responses to a changing environment (Aitken et al. 2008), the extent of expression variation in wild conifers remains to be investigated.
Scans covering whole transcriptomes have identified hundreds of heritable gene expression changes in organisms ranging from budding yeast to primates (Brem et al. 2002; Schadt et al. 2003; Hubner et al. 2005; Kirst et al. 2005; Whitehead & Crawford 2006; West et al. 2007; Drost et al. 2010; Romero et al. 2012). A standard method to study the genetic architecture of expression variation (the number of loci involved, size of the genetic effects and degree of linkage) in model organisms has been to map gene expression variation to genetic loci (Gilad et al. 2008; Kliebenstein 2009). Evolutionary inferences can then be drawn based on, for instance, the identification of molecular pathways and gene features contributing to expression variation (Landry et al. 2006, 2007; Gan et al. 2011) or adaptive expression differences (Fraser et al. 2010). Furthermore, the genetic description of expression variation provides information about the interconnectedness of variable expression traits, which is a key in evaluating the evolutionary potential of populations. If expression variation in different genes is controlled by independent mutations, their evolution can take different paths and thus provide alternative adaptive solutions. If, however, expression variation in most genes is due to a few genetic variants with wide-ranging effects, the diversity in independent evolutionary paths is reduced. In addition, the linkage between beneficial and detrimental expression variation would likely not permit positive selection on favourable variants. The examination of the genetic architecture of expression variation requires the definition of the level of cosegregation between expression traits (Keurentjes et al. 2007; Ayroles et al. 2009; Drost et al. 2010). In both model and nonmodel organisms, this involves segregating populations that allow the tracking of genetic effects on expression variation. Novel approaches need to be developed in nonmodel organisms, such as forest trees, where it is unfeasible to generate the inbred lines or controlled crossed progeny that facilitate traditional analysis (Gilad et al. 2008).
The conserved seed morphology of the gymnosperms may provide an unexplored approach for the genetic tracking of gene expression variation. The gymnosperm seed contains a haploid tissue, the megagametophyte (Fig. 1c), which is derived from one of the four megaspores produced during meiosis. Megagametophytes inherit maternal alleles in a 1:1 ratio (Fig. 1b), allowing analysis of each allele in distinct haploid meiotic products (O'Malley et al. 1996). Heritable expression variation that is linked to a single major causal variant can be identified by screening for expression levels that segregate in the haploid progeny. No breeding is required because expression variation is detected between segregating maternal alleles. The method can thus be applied to any wild individual. No information on segregant genotypes is needed, given that the presence of two allelic forms can be inferred from expression differences that segregate in Mendelian ratios. Furthermore, megagametophytes were reported to have the largest number of expressed genes in a survey of white spruce tissues (Raherison et al. 2012), indicating that the megagametophytes allow expression variation to be analysed in the majority of genes. The megagametophyte is also relevant for studying adaptation to a changing environment because it serves as an important life-transition stage that affects embryo viability and thus contributes to fitness.
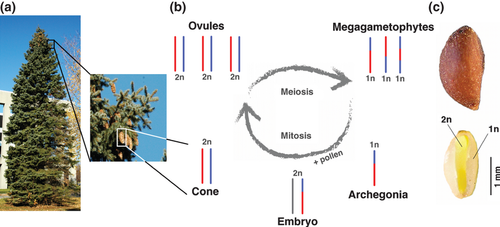
Here, we report the first study that applies the megagametophyte approach to gene expression analysis in a transcriptome-wide scale. Our objectives were to first determine the extent of segregating gene expression variation using the megagametophyte system. This would allow a determination of the feasibility of the approach and a characterization of gene expression variation most directly affected by genetic variants. Second, we aimed to study the linkage between expression traits, which would allow to determine the level of connectedness between variable expression traits and subsequent evolutionary prospects. Third, along with functional categories, we investigated the evolutionary events that could be associated with segregating expression variation. One prominent source of differentially expressed gene variants is gene duplication (Gu et al. 2004; Landry et al. 2007), and expression differences due to gene duplications can have significant contribution to adaptation both in short and in long timescales (Kondrashov 2012). Gene duplication rates (Lipinski et al. 2011), duplicate gene half-lifes (Lynch & Conery 2003) and evolutionary paths of duplicate genes (Carreterro-Paulet & Fares 2012) seem to vary significantly between organisms, and it is unclear whether gene duplication is a significant source of expression variation in conifers.
Materials and methods
We focused our study on two undomesticated and field-grown mother trees representing the same East Canadian population. The wild mother trees (tree A and B) served as references in genetic mapping studies and correspond to study trees 77 111 and 80 112 in Pelgas et al. (2011). The trees were grown in even-aged plantations near Cap Tourmente (tree A, +47° 4’ 1.28”, −70° 49’ 4.03”) and Aubin (tree B, +46° 40’ 15.27”, −71° 29’ 34.54”), Québec, Canada. We obtained controlled crossed seed material from the Canadian Forest Service seed bank in Québec City, QC, Canada (Dr J. Beaulieu, Laurentian Forestry Centre). Seed lots were collected in a single year (tree A: 1994, tree B: 1996) from a single tree and stored at −20 °C until use for the study. Surface-sterilized seeds were stratified by submersion in sterile water at 4 °C for 24 h, followed by storage at 4 °C under 100% RH for 28 days and then dark incubation at 26 °C for 4 h to start germination. The megagametophyte was removed from the seed under a dissecting microscope, separated into two halves of equal size, flash-frozen on liquid nitrogen, and stored at −80 °C until used for RNA extraction.
Microarray analysis
We randomly selected 18 megagametophytes from each parent tree for transcriptome profiling. We performed independent replication of sample preparation, microarray hybridization and fluorescent dyes for 16 and 18 of the samples from trees A and B, respectively. mRNA was independently extracted from the separate megagametophyte halves using magnetic oligo-d(T) beads, transcribed in vitro, labelled using two different dyes (one for each replicate sample set) and randomly hybridized on microarrays comprising oligonucleotide gene-specific probes matching 23 804 unique white spruce transcripts (Beaulieu et al. 2011; Raherison et al. 2012). The two mRNA samples from each megagametophyte were hybridized onto two independent microarrays using two different dyes. The microarray data were deposited in the GEO database (accession GSE35337) with detailed methods and protocols.
Segregation analysis
Analyses were carried out separately for the two microarray dyes. Fluorescence data were background corrected and normalized using the normexp and quantile methods of the Limma package (Smyth 2005) in R (Ihaka & Gentleman 1996). Other normalization methods were investigated, but were rejected due to their reduced power in identifying segregating expression variation (Data S1). Information from spots that had abnormal morphology, that were overlaid by dust particles, or that were saturated in more than four samples were ignored in further analyses.
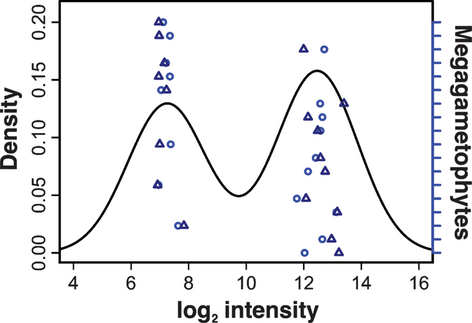
We defined transcripts with heritable expression variation that segregated according to Mendel's first law as Mendelian Expression Traits (METs). Mendelian Expression Traits were predicted to correspond to bimodal expression distributions in which the two modes were observed in a 1:1 frequency (falling within the 95% IC of 1:1 segregation, i.e. between five and 13 observations per mode when N = 18), based on differential transcript abundance in haploid progeny (Fig. 2). The number of modes in fluorescence distributions of each microarray spot was determined with a combined expectation-maximization clustering and mixture modelling approach as implemented in the Mclust function (Fraley & Raftery 2006; Hsieh et al. 2007) in R, allowing a maximum of two modes. Spots with bimodal fluorescence distributions were divided into those with among sibling observed frequencies of distribution modes falling between five and 13 and to those whose frequencies fell outside this range and thus could be exhibiting the contribution of more than one locus.
Transcripts meeting the 1:1 criterion were designated as displaying a MET, given that an identical segregation pattern was observed in both replicates. The replicated measurements were used to determine the stringency of our MET identification criteria. Because noise in microarray data may cause replicated samples to be incorrectly assigned to expression distribution modes, three mismatches were allowed between the mode annotations of the replicate datasets (Fig. S1). Data for each gene were independently randomized between the 18 samples for each replicate dataset while conserving information on spot quality. The mean number of METs that could be identified in 100 randomized datasets was compared to the number of METs that had been obtained by running the analysis on nonrandomized data, thereby allowing a given number of mismatches in each case. Allowing three mismatches gave an empirical ‘false discovery rate’ < 5%. The number of analysed (expressed) genes was defined as the number of genes whose mean intensity over all microarrays was higher or equal than the mean intensity of the lowest identified MET transcript.
Quantitative RT-PCR validation of METs
We selected 23 genes exhibiting METs in tree A and whose expression differences covered a large part of the observed range (Table S1). We measured the expression of these genes in 15 previously analysed megagametophytes with quantitative RT-PCR. 250-ng of aaRNA was reverse transcribed using SuperScript II reagents (Invitrogen, Carlsbad, CA, USA), and random hexamer primers and transcripts were quantified using the QuantiTect SybrGreen reagents (Qiagen, Hilden, Germany) on a LightCycler 480 qPCR instrument (Roche, Indianapolis, IN, USA). Fluorescence data were analysed using the linear regression of efficiency method (Rutledge & Stewart 2008), and target gene expression levels were normalized to an endogenous housekeeping gene (ribosomal protein L3, GenBank: BT115036) by calculating a log2 ratio between the target and housekeeping gene. Low amplification efficiency (<80%) was detected for one of the genes, which was not considered for further analysis. The expression levels of each gene were attributed to one of two clusters according to the microarray-determined expression modes for given samples, and the clusters were tested for difference in mean relative expression level using the Welch two-sample t-test (corrected for multiple testing with the Benjamini–Hochberg false discovery rate) (Benjamini & Hochberg 1995) (Fig. S2). METs were considered confirmed if the segregating expression clusters determined by microarrays differed in mean expression level when measured by quantitative RT-PCR with a P-value threshold of 0.05 (Table S1).
For independent validation, mRNA levels of (i) 18 of the genes analysed previously and that validated the microarray results; (ii) two genes analysed previously that did not validate; and (iii) two other genes for which METs were not detected were directly measured starting from independently stratified, germinated and dissected megagametophytes of the same mother tree. mRNA was extracted as described previously, and 10-ng of mRNA was reverse transcribed using oligo-d(T) primers. Expression levels were measured and normalized as explained previously. Evidence for METs was tested with the Mclust function allowing two cluster centres.
Cosegregation analysis
Coregulated transcripts were defined using a simple cosegregation analysis in the haploid segregants. The segregation patterns of each gene's expression modes were compared to the patterns of all other MET genes using a custom R script. Two mismatches were allowed between segregation patterns. This was determined following a similar approach that was used to identify METs; segregation patterns were randomized in each gene, and the number of cosegregation patterns that were observed by chance was compared to the number of cosegregation calls that were obtained using the nonrandomized data. Because of intense computational requirement, spot quality information was ignored in the analysis. A mean of 100 data permutations was used to calculate the final ‘false discovery rate’.
A control for cosegregation calling was performed. Each METs segregation pattern had to be within two mismatches from all other segregation patterns included in a cosegregating group. Some cosegregating partners of a focal MET can be left of a group if they are not within two mismatches from all other group members. As a consequence, the number and size of cosegregating groups may depend on the order in which the METs segregation patterns were compared. We randomized the order in which MET segregation patterns were compared and counted the number and median of groups. A mean of 100 permutations was reported for the number of cosegregating groups and for the median number of genes included in cosegregating groups.
GO analysis
We analysed functional gene annotations of the genes exhibiting METs to identify gene classes more or less prone to expression variation. Arabidopsis thaliana gene annotations were assigned to each identified MET gene based on the white spruce gene catalogue (Rigault et al. 2011). GOslim biological process annotations for all expressed genes (see above) in each tree and the MET genes were then downloaded from the TAIR database (www.arabidopsis.org), and the number of expected versus observed annotation counts was compared using the hypergeometric test. The P-values were adjusted for multiple testing using the Benjamini–Hochberg false discovery rate correction (Benjamini & Hochberg 1995).
Paralog definition and dS
We tested whether the MET genes were associated with gene duplications. EST cluster sequences corresponding to MET genes and their predicted protein sequences were downloaded from the white spruce gene catalogue (Rigault et al. 2011). These sequences were searched against the whole white spruce transcript sequence catalogue with blastp (Altschul et al. 1997) using a word size of five and default parameters. Sequences were identified as paralogs if their protein sequences aligned for more than 30 amino acids with more than 80% alignment identity. The protein sequences of paralog pairs were aligned using ClustalW2 (Larkin et al. 2007) and default parameters. Codon sequences were then forced to fit the protein alignment using PAL2NAL (Suyama et al. 2006), and the number of synonymous substitutions per synonymous site (dS) was calculated using the codeml function using the Phylogenetic Analysis by Maximum Likelihood (PAML) -software (Yang 1997) and the substitution model of Yang et al. (1998). Only dS values less than 1.5 were considered in statistical testing. The difference in proportions of duplicated METs and duplicated white spruce genes was tested using the two-proportion z-test.
Analysis of budding yeast data
Normalized Saccharomyces cerevisiae data were downloaded from the GEO website (GDS1115 & GDS1116) (Brem & Kruglyak 2005). An analysis protocol of identical steps as with the white spruce data was run on 18 segregants, each replicated on two channels and two arrays. A comparative analysis was performed on a compiled dataset obtained personally from the authors of the original study (Brem & Kruglyak 2005). This was a compiled dataset of two replicate channels, and thus the analysis steps were run on two sets of 18 different segregants. Positive segregation of two alleles was called if both sets exhibited bimodal expression patterns consistent with segregation of two alleles in one locus.
Results
Megagametophyte analysis permits the measurement of segregating expression variation
We identified 841 and 807 transcripts (30 and 22 expected by chance) whose expression levels segregated in a 1:1 ratio in the haploid progeny and thus corresponded to METs in white spruce trees A and B, respectively. A total of 111 METs were shared between the two trees. The differences between expression distribution modes ranged from 1.04- to 64.8-fold with a median of 2.1. We estimated that roughly 20 000 genes are expressed in the megagametophytes, based on lowest detected MET expression (2). Segregation of transcript abundances in genes exhibiting METs was confirmed by quantitative RT-PCR analysis in 82% of the transcripts (N = 22) when assayed in the transcriptome-profiled samples of tree A and in 80% (N = 20) when measured in an independent set of megagametophytes from the same parent (Table S1). Two negative control genes showed no segregation of expression levels in the additional megagametophyte set. In addition to the METs, we identified 420 and 451 segregating transcripts that did not follow a 1:1 ratio. This pattern is consistent with the possible contribution of two or more loci. Finally, when applied to published budding yeast data (Brem & Kruglyak 2005), our procedure identified METs in 1.8% of assayed genes (63 transcripts, two expected by chance), consistent with the 1.86% found by eQTL analysis (Brem & Kruglyak 2005). This result suggests that our analysis procedure is able to detect Mendelian expression traits in a manner comparable with eQTL analyses.
Cosegregation of METs reveals distinct transcription networks
Haploid segregant analysis allowed us to study the cosegregation among METs. Cosegregating METs represent allelic expression levels in different genes that were always found to be correlated between individual megagametophytes and are thus expected to be affected by the same segregating genetic factors. These factors can be either a common trans effect, such as a transcription factor or other upstream regulator, or a cis effect affecting all loci, such as shared regulatory elements or multigenic copy number variation (Fig. 3).
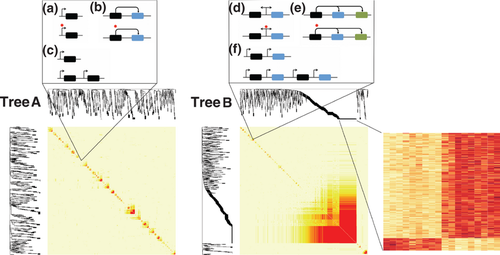
Most of the METs (65% & 67%, trees A & B, respectively) cosegregated with at least one other Mendelian expression trait. The METs formed 147 coregulated groups in tree A (eight groups were expected by chance) and 111 groups in tree B (eight expected by chance). In tree A, the standard deviation in the number of cosegregating groups and the median number of METs included in groups was 2.9 and 0.22, respectively, which indicates that the order in which segregation patterns were compared did not greatly affect the number or size of cosegregating groups. The coregulated groups included members with opposite gene expression patterns in 63% and 54% of the cases, respectively, indicating that most genetic effects were not associated with specifically higher or lower expression levels. The median numbers of METs in a given group were 3 and 2, indicating that most single-gene trans or common cis effects act on a limited number of genes. A notable exception was observed in tree B, which contained a coregulated group that accounted for more than 22% of all discovered METs (180 transcripts, Fig. 3). Consistent with expectations for a trans hotspot (Brown et al. 2008; Wu et al. 2008), the group is associated with similar functional annotations, overrepresented in protein metabolism (34 observed vs. 24 expected, P = 4.5 × 10−2) and underrepresented in stress response (nine observed vs. 23 expected, P = 5.6 × 10−3).
We analysed cosegregation between the 111 shared METs to determine shared regulatory groups between white spruce individuals. In total, 22% (24/111) of the shared METs cosegregated with at least one other shared MET in both trees, forming seven coregulated groups. Gene membership in only two of these groups was totally shared in trees A and B. In addition, in two cases, shared METs could be coexpressed in either the same or the opposite directions, depending upon the individual. These results suggest that while the same genes may exhibit expression variation in two individuals, the underlying genetic variants may be different or have opposite effects. This may be the case if the genes exhibiting a switch in effect directions are affected by different trans variation in the two individuals, for example.
METs are associated with distinct biological processes and gene duplications
We analysed the representation of Gene Ontology (Ashburner et al. 2000) categories in the METs to define gene groups whose expression is more prone to vary due to direct Mendelian effects. GO annotations were assigned to 55% and 58% of the METs in trees A and B, respectively, based on their sequence similarity to Arabidopsis thaliana (Rigault et al. 2011). Overrepresentation was observed in stress response [tree A: 123 observed vs. 71 expected, P = 8 × 10−9; tree B (excluding large cosegregating group): 88 observed vs. 61 expected, P = 1.5 × 10−3], while genes associated with developmental processes were underrepresented (tree A: 48 observed vs. 70 expected, P = 6.3 × 10−3).
We investigated whether genes exhibiting METs could be associated with gene duplications. The proportions of duplicated genes in the MET datasets were significantly higher than in the white spruce gene catalogue in general (pgenome = 0.108 vs. tree A: pMET = 0.187, P = 3 × 10−12; vs. tree B: pMET = 0.142, P = 3 × 10−3, two-sample z-test of proportions), indicating an association between gene duplication and Mendelian expression variation in white spruce. We also observed an overrepresentation in gene functions associated with stress response in the duplicated MET genes in tree A (40 observed vs. 21 expected, P = 2.8 × 10−4), pointing towards an interplay of Mendelian expression variation, gene duplication and stress response genes.
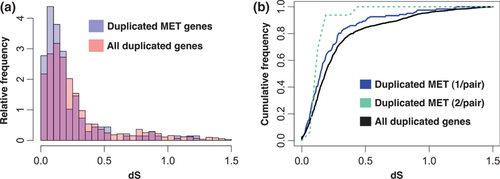
Next, we investigated the timescale of gene duplication events that contribute to Mendelian expression variation. We calculated the relative time of duplication of MET genes based on the silent-site divergence from their most probable protein pairs. The ratio of synonymous substitutions to synonymous sites was significantly smaller in duplicated MET genes of tree A compared to expressed duplicate genes in the white spruce genome on average [mean(dS<1.5)MET = 0.21 vs. mean(dS<1.5)genome = 0.28, P = 8 × 10−4, Wilcoxon–Mann–Whitney U-test, Fig. 4a]. This indicates that METs are preferentially found in younger duplicated genes. The duplicated gene pairs in which METs were observed for at least one paralog were divided into two groups; those in which we observed METs for both paralogous copies and those in which just one gene of a duplicate pair exhibited METs. The majority (88%) of duplicate pairs exhibited METs in only one gene paralog. This indicates that the evolutionary paths of most gene duplicates diverge; while expression levels are nonvariable in one copy, they are polymorphic in the other. Asymmetric expression evolution where expression variation is constrained to only one copy of a duplicate pair is often interpreted as evidence for unequal selection on the two copies (Innan & Kondrashov 2010). Those paralog pairs in which both genes did exhibit METs were very young, suggesting that symmetric expression evolution, which is consistent with relaxed selection on both copies (Innan & Kondrashov 2010), is restricted to young duplicates (Fig. 4b).
Discussion
We developed a method for tracking segregating gene expression variation in haploid meiotic products of wild gymnosperm individuals. We identified expression variation associated with variants in single loci (METs) using microarray data from megagametophytes of two wild white spruce trees. METs were for most part specific to one or the other genotype, consistent with an abundance of segregating alleles in the population. As we concentrated on two unrelated individuals, more in-depth studies on the diversity of METs in white spruce would be needed to determine the level to which METs are shared within and among populations. We also applied our procedure for the identification of segregating expression variation to gene expression data from three model species (Arabidopsis thaliana, Saccharomyces cerevisiae & Rattus norvegicus) to gain an interspecific perspective of the frequencies of segregating expression traits (Data S1). METs were twice as frequent in white spruce as in the other species, which could be accounted by differences in expression network connectedness, for example (Figure S4). Our megagametophyte analysis allowed a robust identification of METs, supported by technical replication and quantitative RT-PCR validation. However, future studies using megagametophytes might benefit from the analysis of a larger number of segregant genotypes, as it may allow the tracking of more complex inheritance patterns of expression traits.
To understand the effect of single Mendelian alleles on whole gene networks, we investigated whether METs were associated with many independently segregating mutations or with few mutations having widespread effects. Most METs (67% and 65%) cosegregated with at least one other MET in both studied trees. Combining the number of cosegregating groups and the number of independently segregating METs, over 400 independent mutations causing segregating gene expression levels could be inferred in each of the trees. The numbers of METs that were included in coexpressed regulatory groups were roughly similar to observed trans proportions in eQTL studies on Zea mays (Swanson-Wagner et al. 2009), A. thaliana (West et al. 2007) and budding yeast (Brem et al. 2002), yet most of the groups did not exhibit directional biases on gene expression levels predicted for trans effects (West et al. 2007). Most coregulated groups were relatively small (2–3 members), which indicated a trend towards generally short trans cascades and, to a large extent, independent segregation of Mendelian expression variation. This aspect of MET cosegregation is important for their possible evolutionary implications. Given that most regulatory associations were between small numbers of METs, natural selection can, to some extent, act on their phenotypes in an independent manner. It is therefore possible that the diversity in METs might contribute to molecular evolution by providing alternative phenotypes. Again, a more fine-grained dissection of these cosegregation patterns will benefit from the analysis of a larger number of progeny. Furthermore, assessing whether METs may have evolutionary impacts will require elucidation of their effects on fitness in haploid and diploid tissues as well as their possible association with adaptative traits. We also note that the predominant effect sizes of regulatory variants within the white spruce population remain to be determined, considering that we observed widely varying effects of single regulatory variants, affecting chiefly fewer than four genes in both trees but up to 180 genes in tree B.
Many genes that are associated with transcription and translation were included in the group of 180 genes segregating in tree B, such as putative homologs of A. thaliana histone acetyltransferase GCN5 and three eukaryotic translation initiation factors (eIF5A, eIF2B & eIF4A). Their null mutants exhibit striking phenotypic effects on growth and development in A. thaliana (Vlachonasios et al. 2003; Feng et al. 2007). The putative eIF factors were particularly interesting as trans controlling genes, because the highly conserved eIF proteins are essential for proper mRNA 5′ capping and translation in eukaryotes (Hernández et al. 2010). In budding yeast, at least eIF2B and eIF5A have been directly associated with a transcriptional/translational cascade that includes GCN5 through GCN4 (Georgakopoulos & Thireos 1992; Hinnebusch 1997; Kuo et al. 2000; Yamamoto et al. 2005). GCN4, which is a master regulator of amino acid biosynthesis, recruits GCN5 to specific gene promoters, leading to histone hyperacetylation and transcriptional activation of amino acid biosynthesis genes (Kuo et al. 2000). We observed that high allelic expression levels of GCN5 and eIF genes cosegregated with high expression levels of many genes that are involved in protein metabolism. They also cosegregated with low levels of expression of genes involved in protein degradation and associated with the vacuole, which is indicative of a generally lower level of protein turnover. Our results suggested that a conserved regulatory link exists between eIF's and amino acid metabolism genes in gymnosperms. No plant GCN4 has yet been characterized (Hey et al. 2010).
A large trans effect on protein metabolism has also been observed in budding yeast and was caused by a single nucleotide frameshift (Brown et al. 2008), indicating that such strong trans effects can have very simple molecular bases. Our findings illustrate the power of our approach to identifying putative Mendelian trans regulators, an ability that has a wide range of applications in studying gymnosperm populations, phenotypes and gene networks. Notably, because our approach identifies gene expression variation due to simply inherited Mendelian variants, the results of MET analysis can be readily combined with genetic mapping or marker analysis, along with physiological responses. The approach offers simple and direct ways to study the frequencies of Mendelian variants in wild individuals and their possible association with locally adapted populations.
We identified functional and evolutionary gene categories that were associated with Mendelian expression traits. We found that METs were preferentially associated with genes that were involved in stress responses and relatively rare in genes involved in developmental processes. Nearly half of the identified MET transcripts had no sequence similarity to A. thaliana, indicating that diversity is also present in genes without close A. thaliana homologs. Our results indicate that METs might contribute to the diversity of stress responses in white spruce. Moreover, genes exhibiting METs were associated with gene duplications, with recent duplication events being more likely to contribute to Mendelian expression variation than old ones. We investigated the distribution of Mendelian variation between duplicated gene pairs and observed that MET genes generally followed an asymmetric evolution in which one gene of a duplicate pair exhibits expression variation. Our results supported the view that a change in evolutionary paths as a function of age is common in duplicated genes (Gu et al. 2005; He & Zhang 2005; Zou et al. 2009) and suggested that the accumulation of Mendelian expression differences might be coupled with relaxation of selection pressure through single-gene duplication events. The retention of paralogs with asymmetric expression has been associated with functional innovation in the copy whose expression is under lower constraint (Zou et al. 2009). Our observations included METs in asymmetrically expressed paralogs that had high dS values and that may have been retained through this mechanism.
Taken together, our results agreed with observations of a link between expression variation and gene duplication in selfing annual plants (Zou et al. 2009) and model organisms (Gu et al. 2004; Landry et al. 2006) and have highlighted the role of gene duplication as a prominent source of evolutionary novelty that facilitates gene diversity especially in stress responses. This mechanism might be an important source of diversity on a local scale, which could play a role in local adaptation through alternative genes and alleles, as suggested by recent studies in annual plants (Fournier-Level et al. 2011) as well as spruce trees (Prunier et al. 2011, 2012).
In summary, we showed that gymnosperm megagametophytes facilitate the tracking of segregating expression traits within single individuals and that their haploid nature can provide a powerful system for estimating the total number of loci that are associated with such traits. Megagametophytes can facilitate direct analyses of any number of unrelated individuals or meiotic products, allowing the study of gene network evolution also in long-lived, out bred species with large genomes. The relatively high frequency of segregating expression traits, their diversity between individuals and their tendency to be associated with stress response genes could provide the means for phenotypic diversity and facilitate adaptation in white spruce.
The impracticality of standard approaches is a common problem that hampers genetic analyses in nonmodel species, yet expanding our scope to wild species is necessary to advance the field of ecological and evolutionary genomics (Pavey et al. 2012). The megagametophyte approach is a step towards enabling such analyses in a large number of wild plant species.
Acknowledgements
Funding to JM was provided by Genome Canada and Genome Quebec for the Arborea II and projects as well as NSERC (Natural Sciences and Engineering Research Council of Canada). CRL is a CIHR New Investigator and his research in ecological genomics is funded by a NSERC Discovery Grant. The authors thank R. Brem for providing a S. cerevisiae dataset, members of J. MacKay laboratory for technical assistance and R. Sederoff, N. Aubin-Horth and W. Parsons for helpful comments on the manuscript.
References
J-P.V. designed and performed the experiments, analyzed the data and drafted the manuscript. This study is part of his PhD research towards understanding heritable expression variation in conifer trees. J-P.V. is broadly interested in evolutionary and ecological genomics in model and non-model species. C.R.L. and J.J.M. provided advice and guidance on the design of experiments, data analysis and interpretation of results, and contributed to the writing of the manuscript. C.R.L. is interested in evolutionary systems biology and ecological genomics. J.J.M. is interested in forest genomics and investigates transcriptional regulation as a component of adaptive diversity and metabolic plasticity.
Data accessibility
Gene expression data: Gene Expression Omnibus accession GSE35337.
R scripts: included in online supporting information.




