Ecology and evolutionary biology of fishing bats
Abstract
- A few of the >1300 bat species recognised worldwide are known to consume fish to various extents. However, empirical information about how and how much different bat species capture and consume fish is limited and is probably distorted due to the biases introduced by occasional observations.
- In this review, we aim to synthesise the knowledge so far generated on fishing bats, in order to 1) assess the incidence of fishing in different bat species; 2) discuss the evolutionary framework and origins of fishing behaviour; and 3) identify the ecological challenges of fishing and review the state of knowledge about kinetic, morphological, sensory, behavioural, and physiological adaptations related to fishing.
- Fishing bats can be clustered into three groups based on the incidence of fish consumption. Noctilio leporinus is the only species in which fishing is widespread (occurring in most populations) and common (occurring most of the time and carried out by many individuals). Myotis vivesi, Myotis pilosus, and Myotis capaccinii comprise the second group, characterised by exhibiting regular fishing behaviour that is restricted to a limited number of populations. Noctilio albiventris, Myotis daubentonii, and Myotis macropus are classified in a third group, because although the occasional presence of fish traces in faeces has been reported, regular fishing behaviour in the wild has not been confirmed.
- Fishing was developed independently multiple times in different bat lineages, probably under different selective pressures and ecological scenarios.
- Nevertheless, all fishing species face similar challenges in terms of detecting, capturing, handling, and metabolising fish, which require specific kinetic, morphological, sensory, behavioural, and physiological adaptations.
- Both basic information about fishing behaviour in different species and specific knowledge of the biological adaptation are still missing. Thus, in this review we identify gaps in the knowledge and suggest experimental approaches to overcome them.
Introduction
Fish are one of the myriads of food resources that bats consume, alongside arthropods, fruits, leaves, nectar, blood, and other vertebrates such as birds and amphibians (Altringham 1996). However, despite the near ubiquitous availability of fish, only a very limited number of bat species have been able to incorporate them into their diet, and even fewer have specialised on this particular food resource (Campbell 2011). The rarity of fishing is probably related to the number of adaptations required to hunt, handle, and digest fish efficiently – including kinetic, morphological, sensory, behavioural, and physiological adaptations – although the apparent rarity may also simply be a result of the limited research effort that has been invested in the topic. Our knowledge of the extent and biological importance of fishing, as well as the adaptations needed to consume fish, is still insufficient, because most ecological and evolutionary aspects of fishing in bats remain largely unstudied. In this review, we gather the scientific knowledge so far generated about fishing bats and identify unsolved ecological and evolutionary aspects that we consider worthy of study in the future. In the hope of stimulating future research on this fascinating topic, we also suggest study designs and methodological approaches that could be applied to address the main unsolved issues.
Methods
An extensive review of records published in peer-reviewed journals was conducted by checking scientific web portals and data bases. In our search for scientific papers, we mainly used the Thomson Reuters (Web of Science) and Scopus data bases, Google Scholar, and Google Books, using the following keywords in different combinations and languages: bat, Chiroptera, fish, fishing, Myotis, Noctilio, piscivory, trawling.
Results and Discussion
The incidence of fishing among bat species worldwide
Of the more than 1300 bat species currently known worldwide (Fenton & Simmons 2014, Voigt & Kingston 2016), not one feeds exclusively on fish, as all fishing bats also consume arthropods to varying extents. The relative incidence of each type of predation (arthropod hunting and fishing) differs, depending on the bat species, the population, and the environmental conditions.
All well-known fishing species belong to two bat genera, namely Noctilio and Myotis, and they can be categorised in three main groups according to the incidence of fish consumption: ‘widespread’, ‘limited’, and ‘not confirmed’. The greater bulldog bat Noctilio leporinus is the only species that falls within the first group (‘widespread’; Fig. 1). A large bat (>50 g; Hood & Jones 1984) with a wing morphology that avails powerful flight (Goodwin & Greenhall 1961, Fig. 2a), it echolocates using quasi-constant-frequency (QCF) calls (Fig. 3a; Hood & Jones 1984). Piscivory is both widespread within the species (occurring in most populations) and a common behaviour (occurring most of the time and carried out by many individuals). Fish traces have been found in almost all dietary studies (e.g. Benedict 1926, Gudger 1945, Goodwin & Greenhall 1961, Hooper & Brown 1968, Willig 1985, Brooke 1994, Bordignon & França 2002, Bordignon 2006a), and fish may constitute up to 90% of the diet of Noctilio leporinus (Bordignon 2006a).
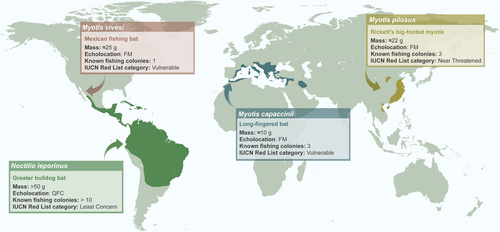

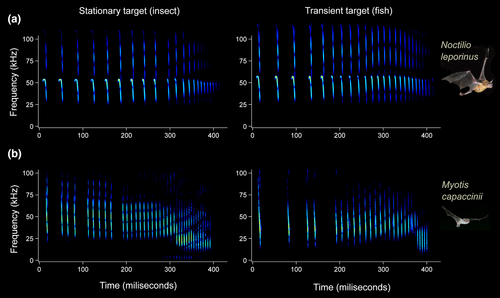
The second group (‘limited’) consists of three species within the genus Myotis that are characterised by regular but geographically restricted fish consumption. These three species, Myotis vivesi, Myotis pilosus (=Myotis ricketti), and Myotis capaccinii, are smaller (<45 g; Nowak 1994, Fig. 1) than Noctilio leporinus, and their wing morphology is better adapted for flying in cluttered environments (Ghazali et al. 2017). The frequency-modulated (FM) calls they emit for echolocation are also different from those of Noctilio leporinus (Fig. 3; Schnitzler & Kalko 2001). The main dietary source of the three species is different kinds of arthropod, and fishing behaviour is limited to a small number of colonies of each species. The diet of the fish-eating myotis Myotis vivesi, a species that is endemic to the Gulf of California and Baja California, Mexico, is mainly comprised of marine crustaceans and fish (Reeder & Norris 1954, Maya 1968, Otalora-Ardila et al. 2013). The presence of fish remains in Myotis vivesi faeces has been reported by different authors (Burt 1932, Bloedel 1955, Maya 1968, Otalora-Ardila et al. 2013), although all but Bloedel (1955) referred to the same single location where regular fish consumption was recently confirmed (Otalora-Ardila et al. 2013). Rickett's big-footed bat Myotis pilosus is also considered a piscivorous species by many authors (e.g. Aihartza et al. 2003, Campbell 2011), and although fish remains were found in its diet in three localities (Robinson 1998, Ma et al. 2003), regular fish consumption has been confirmed only in a single locality in China (Ma et al. 2006). Fish consumption by the long-fingered bat Myotis capaccinii (Fig. 2b) has been reported in three geographically separated colonies (Aihartza et al. 2003, Levin et al. 2006, Biscardi et al. 2007), although regular fishing behaviour has been reported in only one of them (Aizpurua et al. 2013).
The third group (‘not confirmed’) contains species in which the presence of fish traces within faeces has been reported, although regular fishing behaviour in the wild has not been confirmed. These species include Noctilio albiventris (Howell & Burch 1974), Myotis daubentonii (Brosset & Delamare 1966), and Myotis macropus (Robson 1984, Jansen 1987, Law & Urquhart 2000, Campbell 2011). Noctilio albiventris and Myotis daubentonii have been shown to be able to take fish flesh or carcasses from the water surface (Brosset & Delamare 1966, Siemers et al. 2001a). Finally, a number of other species that are described in the literature to capture insects directly from the water surface are likely candidates for fishing, although observational evidence remains lacking: Macrophyllum macrophyllum, Myotis adversus, Myotis dasycneme, Myotis macrodactylus, Myotis macrotarsus, Myotis ruber, Myotis stalkeri, Myotis horsfieldii, and Myotis hasseltii (Novick & Dale 1971, Campbell 2011).
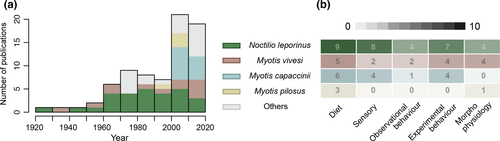
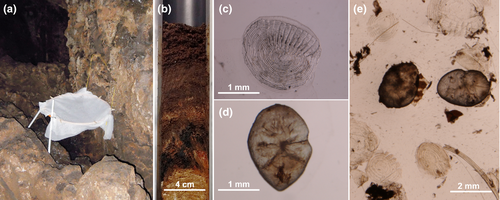
The classification we present is almost certainly incomplete and prone to sampling bias, since research effort on the species and over time has been uneven (Fig. 4). Many data come from occasional observations (e.g. Burt 1932, Howell & Burch 1974, Robson 1984), which complicates assessing the actual incidence of fishing among bats and probably artificially inflates the importance of fishing behaviour in the species in the ‘not confirmed’ group. In order to overcome this situation, the geographical extent of fishing behaviour in each species should be evaluated by conducting extensive cross-sectional studies that aim to detect the presence of fish traces in faeces from different colonies. If fish traces are found, dedicated longitudinal studies in which samples are obtained at different points in time would be necessary to assess the incidence of fishing in each particular colony. Such studies are relatively affordable in cave-dwelling bats from which faeces can be collected passively (Fig. 5a, Aizpurua et al. 2013). In special cases, vertical core samples of stratified guano deposits could be also employed to detect past fishing events (Fig. 5b); this could provide information about when bats in a given colony started fishing or how the incidence of fishing varied in the long term. Calcified fish remains such as scales and otoliths suffer limited damage when going through the intestines of a bat (Fig. 5c-e); hence, identification of macroscopic fragments in fresh faeces is usually possible (e.g. Treacy & Crawford 1981). Although they are far less abundant than scales, otoliths can be used for species-level fish identification as well as for size determination (Aizpurua et al. 2013, Otalora-Ardila et al. 2013). Visual inspection, however, is a tedious task, especially when large amounts of guano need to be analysed, and bearing in mind that fish consumption does not always ensure the presence of macroscopic remains in bat faeces (Siemers et al. 2001a). Additionally, quantitation of the incidence of fishing might be troublesome when sampling multispecies colonies using passive collection, due to the difficulty of reliably assigning faecal pellets to different bat species.
Stable isotope analyses might help overcome some of these limitations. Animal tissues are generated by assimilating atoms from dietary items, and it is possible to trace the origin and trophic position of the dietary items by studying the proportions of different stable isotopes in the tissues of predators (e.g. Aliperti et al. 2017, Neto et al. 2017). The main advantage of stable isotope analyses is their ability to integrate animal diet information over a long timescale (Nielsen et al. 2017), which means that an isotopic signal might be measurable even if a bat had not consumed fish for several days before it was captured and sampled. Stable isotope analysis is thus a good method with which to assess overall long-term fish consumption by bats (Otalora-Ardila et al. 2013). However, isotopic methods are not sensitive enough to detect infrequent or occasional use of a certain dietary item (Hedges 2004); thus, occasional fishing would not leave a detectable isotopic imprint. Another limitation of isotopic approaches is the low taxonomic resolution of prey (Kartzinel et al. 2015).
DNA metabarcoding enables the detection of fish even when no visual fish traces are found. Furthermore, compared to isotope approaches, DNA metabarcoding provides much finer taxonomic resolution, enabling species-level identification of prey items (e.g. Deagle et al. 2009, Rasmussen et al. 2009). Unlike stable isotope analyses, metabarcoding is used to generate the DNA sequences of the actual DNA molecules present in faecal samples (Deiner et al. 2017); thus, when analysing fresh faecal pellets collected from individual bats, fish would be detected only if the bat had consumed them very recently. However, metabarcoding enables reliable assignment of faecal pellets collected passively in roosts to bat species. Metabarcoding can also be used to detect fish traces in bulk guano samples or in vertical guano cores. Dedicated commercial kits exist to extract DNA from large as well as small quantities of faeces, and due to the incorporation of specialised molecular tags, multiple samples can be sequenced in parallel on high-throughput sequencing platforms (Soininen et al. 2009, Valentini et al. 2009). Selecting appropriate metabarcoding primers is crucial though (Alberdi et al. 2018). Primer sets targeting specific fish taxa or covering a broader taxonomic range of fish already exist (e.g. Miya et al. 2015, Valentini et al. 2016). It is important to note that the mere amplification of DNA – as visualised in electrophoresis gels or capillary electrophoresis machines – may not mean that the amplified fragment belongs to a fish species, since primers designed to amplify fish DNA often also amplify the DNA of other vertebrates (Miya et al. 2015). Thus, the use of multiple positive and negative controls is recommended to improve the reliability of results, and ideally, amplified fragments should be sequenced to dispel any doubt.
Evolutionary biology of fishing
Fishing probably evolved independently at least five times in two major chiropteran evolutionary lineages, namely the families Noctilionidae and Vespertilionidae (Fig. 6a). How fishing evolved in these two lineages, however, is uncertain. Occasional capture of small surface-feeder fish could have occurred while bats were hunting insects or crustaceans on the water surface (Gudger 1945, Schnitzler et al. 1994a, Kalko et al. 1998), but the development of the necessary mechanical and sensory adaptations to make fishing cost-efficient probably required very specific environmental conditions (e.g. high abundance of fish displaying a certain behaviour) through prolonged periods of time. Likewise, assessing when fishing ability was developed is complicated. Researchers have proposed that, in noctilionids, the fishing technique may have evolved around two million years ago, when the two species (Noctilio albiventris and Noctilio leporinus) diverged (Lewis-Oritt et al. 2001, Agnarsson et al. 2011, Rojas et al. 2016). The case of the Myotis species is radically different, since this genus is one of the most diverse among bats, comprising >100 species, of which only three are known to fish regularly (Fig. 6b). Due to the rapid radiation undergone by this genus (Gunnell et al. 2017), the phylogenetic relationship between species is not well established, although some preliminary studies exist (Stadelmann et al. 2004, 2007, Ruedi et al. 2013). The three fishing species are located in different phylogenetic clades within the Myotis bats, and the absence of monophyletic fishing bat groups suggests that fishing behaviour is a recently developed trait in evolutionary terms.

Despite the differences in both fish consumption and in various morphological and sensory traits, there are three main common features that characterise all fishing bats: 1) their hunting technique; 2) the morphology of their hind feet and tail; and 3) their geographical distribution. The development of the skills for hunting prey from the water surface seems a prerequisite for the development of fishing. Although many bats are capable of highly controlled flight that enables, for instance, drinking from smooth water surfaces while flying (Russo et al. 2016), few species are able to hunt floating or emerging invertebrates from the water surface using their hind feet (Campbell 2011). This technique is known as trawling (Norberg & Rayner 1987) and is employed by approximately 17 bat species distributed across four evolutionary lineages: the aforementioned Noctilionidae and Vespertilionidae, as well as Phyllostomidae and Emballonuridae (Campbell 2011, Weinbeer et al. 2013). All trawling bats have similar morphological features that seem to be adapted for hunting prey from the water surface: enlarged hind feet, hairy legs, strengthened calcars (the cartilage that extends from the ankle along the trailing edge of the tail membrane), and relatively short tails (Findley 1972, Norberg & Rayner 1987). All these adaptations are also beneficial for fishing, yet only four of the 17 trawling species are known to consume fish regularly.
The rarity of fishing might also be related to the limited availability of fish prey with suitable morphological and behavioural features that enable capture. For example, to be captured by bats, fish apparently need to be within a certain size range, exhibit high densities, and forage near the water surface. Additionally, the fishing environment requires certain properties, such as a smooth water surface, which is essential for enabling bats to detect, track, and capture fish. These factors may result in the distribution of fishing bats being mostly restricted to lowland and coastal areas, where they are more likely to find areas suitable for fishing such as marshlands, lagoons, and similar lentic water ecosystems. The time needed to develop the evolutionary adaptations that enabled fishing might have required stable environments where fish resources were available for multiple subsequent generations. This may have triggered directional selection towards the development of morphological and sensory adaptations that allowed the ecological challenges posed by fishing to be overcome. Due to increasing human disturbance, the conditions that make fishing feasible and profitable for bats – that is high densities of small surface-feeder fish in smooth-surface ponds – are now probably less common than in the past (e.g. on the Mediterranean coastline). This could be the reason why fishing shows patchy distribution in many Myotis species and has not been fixed in most of the populations within these bats. In contrast, though, the recent spread of exotic invasive fish species may render fishing more feasible; Gambusia (Levin et al. 2006, Aizpurua et al. 2013) and Tilapia (Brooke 1994) fish are known to be currently consumed by Myotis capaccinii and Noctilio leporinus, respectively.
Ecological challenges of fishing and related evolutionary adaptations
All piscivorous bats use a similar technique to hunt fish: they introduce their hind feet into the water and trawl or gaff fish swimming close to the water surface (Altenbach 1989, Schnitzler et al. 1994b, Aihartza et al. 2008, Aizpurua et al. 2014). Although the technique is, in general terms, the same as the one used for hunting insects on the water surface, fishing entails many challenges and requires an integral adjustment of the hunting technique that very few bat species have been able to achieve.
Detecting Fish
The first step in fishing is to detect the presence of fish in a given foraging area. A high density of fish at the water surface has been proposed as an important condition for triggering fishing behaviour in bats (Schnitzler et al. 1994b, Aihartza et al. 2003, 2008, Bordignon 2006b, Aizpurua et al. 2013), probably because the presence of a large number of fish swimming near the surface and capturing insects that are resting on the water surface produces acoustic cues that bats can detect using echolocation. Although attenuation of sound at the air–water interface prevents bats from recovering echoes from underwater bodies, bats are very sensitive to water surface disturbances due to the acoustic mirror property of water, whereby the surface reflects all incident pulses away while returning the neat echoes produced by any solid object on the water surface (Siemers et al. 2001b, 2005, Zsebok et al. 2013). This is one of the reasons why bats mostly capture surface-feeder fish that frequently break the water surface, rendering them detectable to echolocating bats. The limited bibliography suggests that the fish densities that Myotis bats require for triggering fishing are higher than those needed by Noctilio leporinus (Bloedel 1955, Schnitzler et al. 1994b, Bordignon 2006b, Aihartza et al. 2008); this may be related to the different sensory and morphological features of the bats.
Both Myotis bats and Noctilio leporinus use a targeted dipping strategy for fishing, that is they detect the presence of individual fish using echolocation, and then perform targeted attacks upon them (Schnitzler et al. 1994b, Aizpurua et al. 2015). However, the ability of Myotis bats and Noctilio leporinus to detect fish differs considerably due to the specific features of the echolocation calls they employ. The QCF calls produced by Noctilio leporinus allow the detection of weak echoes (Fig. 3a; Simmons et al. 1979, Schnitzler & Kalko 2001) and transient cues from long distances (Suthers 1967), which could enable detection of distant indirect stimuli generated by fish, such as concentrically expanding water ripples (Übernickel et al. 2016) or splashes (Schnitzler et al. 1994b, Übernickel et al. 2013). The broadband FM calls of Myotis bats (Fig. 3b), in contrast, allow shorter detection distances and are not suitable for perceiving faint target stimuli such as echoes produced by ripples, unless they come from very close range (Halfwerk et al. 2014). In fact, the only experiment that investigated the reaction of Myotis fishing bats to different types of stimuli reported that simulated ripples did not trigger any attack, and fish were only detected when parts of their bodies (lips or fins) emerged over the water surface (Aizpurua et al. 2015).
Besides acoustic properties, body size and wing morphology of bats might also limit fishing strategy. Field observations suggest that, unlike Myotis bats, Noctilio leporinus uses a random dipping strategy as well as the targeted dipping strategy to capture fish. Noctilio leporinus is able to perform >10-m-long rakes (Schnitzler et al. 1994b), which suggests that the bats are not dipping to target a specific fish, but aim to gaff fish that might be within their raking line. Such behaviour has also been reported in areas without fish stimuli at the time, but where, seemingly, fish had successfully been hunted previously (Bloedel 1955, Schnitzler et al. 1994b). Random dipping is probably exclusive to Noctilio leporinus because of two specific features of this species. First, the powerful flight enabled by its large body size and wing morphology allows it to perform very long rakes across the water surface, and second, the acoustic features of its echolocation calls enable it to detect fish cues from long distances. A random dipping strategy might not be cost-effective for smaller Myotis bats (Aihartza et al. 2008, Aizpurua et al. 2015), which have less powerful flight and lower long-range detection capacity.
Our knowledge of the mechanisms bats use to detect fish and decide on the foraging strategy is still limited, because research has been focused mainly on two species: Noctilio leporinus and Myotis capaccinii. Additionally, the effect of fish density triggering fishing behaviour and its link to the foraging strategy chosen by bats remains unclear. To shed light on these issues, fish density manipulation experiments could be conducted, in which fishing attempts are monitored using infrared high-speed video cameras and ultrasound recordings. Laboratory set-ups would allow experimenting under reduced environmental noise conditions, although the behaviour displayed by the animals may differ from in wild conditions, and the low number of animals usually employed in captivity experiments (e.g. one in Altenbach 1989, three in Übernickel et al. 2013, Geberl et al. 2015, and six in Hulgard & Ratcliffe 2016) could provide skewed results. Ideally, laboratory experiments should be complemented by field experiments in which fish densities are manipulated by the researchers if the experimental area is enclosed, or researchers track variation in bat behaviour in response to natural fluctuation in fish density.
Identifying Fish
The fact that bats use different techniques for capturing insects and fish (Schnitzler et al. 1994b, Aizpurua et al. 2014) demonstrates that they are able to distinguish fish from insects. By taking advantage of its QCF-type echolocation calls, Noctilio leporinus is able to recognise specific echo patterns of disturbances of the water surface generated by fish and associate them with potential fish prey (Suthers 1965, Hartley et al. 1989, Schnitzler et al. 1994b). In contrast, Myotis bats need to rely on the actual fish instead of on indirect stimuli, as they are probably hampered by the features of their echolocation calls. Myotis bats do not seem to use fine morphological discrimination of prey, as they commonly gaff inedible objects such as aquatic plants from the water surface (Kalko & Schnitzler 1989, Boonman et al. 1998, Almenar et al. 2008). Indeed, an experiment carried out with Myotis capaccinii showed that bats do not decide the capture technique based on the morphological features of the prey, but based on the prey's disappearing movement (Aizpurua et al. 2015). When fish were held stationary (i.e. with the upper lip protruding from the water surface), bats performed attacks as if the prey items were insects; their typical fish-catching capture technique was only displayed when the fish submerged under the water during the capture attempt.
Tracking Fish
Once a fish is detected, identified, and targeted, bats need to track the location of the prey in order to dip in the appropriate place. Unlike insects, fish usually disappear under the water long before the bat reaches the location where the prey was detected. Although bats usually abort their attacks upon flying insects if the intended prey suddenly disappears in the early phase of the capture process (Marimuthu 1997, Geberl et al. 2015), when a fish disappears under the water, bats continue their attack towards the last tracked location of the item, so they are likely to be performing a blind capture attempt (Aihartza et al. 2008). Myotis capaccinii accomplishes fishing attempts even when the fish has disappeared 250 ms prior to prey contact (Aizpurua et al. 2015), and even longer gaps have been recorded for Noctilio leporinus (Schnitzler et al. 1994b, Übernickel et al. 2013). Although echolocation seems useless for locating fish once they have disappeared under the water, bats do not stop echolocating. Bats use echolocation to calculate their position with respect to the water surface (Greif & Siemers 2010), but the call variations observed after the disappearance of the prey suggest that echolocation might also be used to keep track of the spot where the previously detected fish disappeared (Aizpurua et al. 2015, Geberl et al. 2015). When approaching the dipping location, bats increase the rate of echolocation calls to increase the information flow (Simmons et al. 1975). QCF-type calls emitted by Noctilio are useful for discerning prey at the long range, but broadband FM components emitted by Myotis bats provide advantages for exact target location at short distances compared to QCF-type calls (Hartley et al. 1989). Therefore, Noctilio leporinus modulates its echolocation signal from long QCF-type calls to short FM-type calls in the last part of the echolocation call sequence (Suthers 1965, Hartley et al. 1989, Schnitzler et al. 1994b, Übernickel et al. 2013).
The increase in the rate of echolocation calls produced when a bat is approaching a prey is known as the terminal phase or terminal buzz (Griffin et al. 1960) and is a common sensory feature among insectivorous bats (Kalko & Schnitzler 1998). The terminal phase tends to be flexible, as its entire length varies depending on the foraging habitat, target size, and difficulty of the task performed (Schnitzler et al. 1987, Melcón et al. 2007, Russo et al. 2007, 2016). Fishing bats, like the other trawling bats, emit this characteristic echolocation call sequence when hunting insects, but they modify its features when fishing (Schnitzler et al. 1994b, Aizpurua et al. 2014). When bats are hunting insects, the terminal phase usually exhibits two distinct parts, known as buzz I and buzz II, in which buzz I is comprised of echolocation calls with broader bandwidth and higher peak frequency than the calls in buzz II. In turn, when bats prey on fish, buzz I is extended and buzz II undergoes a considerable reduction or even disappears (Fig. 3b; Aihartza et al. 2008, Übernickel et al. 2013, Aizpurua et al. 2014). Myotis bats regulate the lengths of buzz I and buzz II depending on the time of disappearance of the target (Aizpurua et al. 2015, Geberl et al. 2015). Specifically, the sooner the fish disappears under the water during the attack sequence, the longer is the buzz I and the shorter is the buzz II (Aizpurua et al. 2015). This adjustment is probably related to the acoustic properties of the calls emitted in each part of the terminal phase. Buzz I allows bats to get the final time information at a high rate about the prey position and the bat's position in relation to the water (Russo et al. 2007, Greif & Siemers 2010). The exact function of buzz II and its importance in capture actions is still controversial. Some authors argue that buzz II cannot be useful during the capture attempt (Melcón et al. 2007), because the time bats would need to process and use buzz II information would be longer than their minimum reaction time (around 50 ms). However, other researchers have reported that the terminal phase is dynamically adjusted to echo feedback received from the prey's motion features (Aizpurua et al. 2015, Geberl et al. 2015), highlighting the importance of buzz II for the capture action. The characteristic drop in the peak frequency of calls in buzz II might allow the detection angle to widen during the final part of prey pursuit, in order to counteract insects’ evasive manoeuvres (Ratcliffe et al. 2013, Jakobsen et al. 2015).
When bats are fishing, buzz II loses its importance because, unlike insects, fish do not perform evasive movements; they simply submerge and disappear (Aizpurua et al. 2014, Fig. 3b). Furthermore, by biasing the terminal phase towards buzz I when the target is under the water, bats could improve target location capacity, since buzz I calls provide higher directionality and greater echo strength than buzz II calls (Aizpurua et al. 2015, Jakobsen et al. 2015). These sensory adjustments might allow bats to focus on the point where the fish has disappeared and retrieve information about that spot by processing information obtained from the ripples fish produce when submerging. The amplitude and overall structure of the received echo tend to be stronger at broader angles; thus, the ability to perceive ripples increases as the bat gets closer to the target (Halfwerk et al. 2014). Thus, unlike bats detecting prey at long range, Myotis bats might rely on ripples to ensure they dip in the correct spot.
One of the main research challenges regarding tracking fish and prey in general is determining the true role of the terminal phase, and especially buzz II calls, with regards to understanding the rapid decision processes bats are able to undergo at the neural and behavioural level. Recent advances in sound and video recording technologies, such as multiple ultrasound-microphone arrays and high-speed video cameras, enable the detailed analysis of fast acoustic and mechanical reactions of bats, which can be studied both in captivity and in the field. Thus, bats, especially fishing species, provide an attractive animal model for the study of ultrafast information processing and decision-making, which could be extremely relevant for addressing neurobiological issues.
Capturing Fish
Bats use similar, yet different, techniques for capturing insects and fish from water, responding to the different requirements of both prey types (Schnitzler et al. 1994b, Aizpurua et al. 2014). The insect capturing technique is modified according to three specific properties of fish prey: 1) fish are under the water instead of resting on the water surface as are insects; 2) the exact location of the fish is usually unknown when accomplishing the attack, while insect prey can be tracked continuously using echolocation; and 3) fish are larger and heavier prey than insects.
When capturing insects on the water surface, bats might or might not break the water surface, depending on the size of the prey. However, when bats are capturing fish, insertion of their hind feet into the water is inevitable (Altenbach 1989, Schnitzler et al. 1994b, Aihartza et al. 2008). For instance, individuals of Myotis capaccinii touch the water surface only with their toes when hunting insects, but when fishing, they insert more than half of their hind feet into the water in most hunting attempts (Aizpurua et al. 2014). Besides, bats perform longer dips when catching fish than when capturing insects, probably to account for the uncertainty of prey location, which is impossible to detect by echolocation. The length of the drag for fishing can be twice that for capturing insects in Myotis capaccinii (Aizpurua et al. 2014), and Noctilio leporinus is able to perform rakes of several metres long (Schnitzler et al. 1994b). Along the dip, bats need to gaff and take out the heavy prey that is under the water; thus, the use of their toes and claws is necessary. This is in contrast to the technique used when catching insects, which can be captured using the tail membrane (uropatagium). Although it has been suggested that the uropatagium plays an active role when catching fish (Reeder & Norris 1954, Maya 1968, Altenbach 1989), some studies confirm that the uropatagium is only used as a pouch after capture, enabling safe transfer of the captured fish to the mouth (Bloedel 1955, Siemers et al. 2001a, Aizpurua et al. 2014). In fact, when fishing, Noctilio leporinus folds and tucks up the uropatagium between the legs, thus keeping it completely out of the water (Bloedel 1955, Altenbach 1989, Schnitzler et al. 1994b). In contrast, smaller Myotis bats are unable to fold their uropatagium, so it is partially inserted into the water (Altenbach 1989, Aihartza et al. 2008, Flores-Martínez 2009), although it does not play any role during the capture of fish (Aizpurua et al. 2014).
These modifications to the hunting technique entail a high loss of kinetic energy during capture, due to the increased drag produced by the water. Fishing bats seem to counteract this fact through morphological and behavioural adaptations. In addition to the morphological adaptations of trawling bats, all fishing bats share similar morphological features that reduce even further the drag associated with deep raking the water and grasping fish. Fishing bats are characterised by their larger hind feet armed with elongated and laterally compressed digits and sharp claws, smooth plantar surfaces with a reduced number of wrinkles and hair, and a plagiopatagium attached on the tibia instead of the feet (Findley 1972, Norberg & Rayner 1987, Fish et al. 1991). In the case of Noctilio leporinus, these morphological adaptations are extremely marked, while they appear to a lesser degree in Myotis species. The constraints imposed by the less developed morphological adaptations and their small body size have probably forced Myotis bats to develop behavioural adaptations to counteract the high loss of kinetic energy produced by long, deep dips into water and to keep the balance. Unlike Noctilio leporinus, Myotis bats use a considerably higher flight speed and a greater inclination of the body when capturing fish than when catching insects (Altenbach 1989, Flores-Martínez 2009, Aizpurua et al. 2014). Thus, fishing seems to be a particularly demanding task for Myotis bats, which might be efficient only under certain environmental conditions and using accurate capture techniques.
The limited research on this topic suggests that haptic perception might play an important role when bats are capturing fish. Fishing bats have high concentrations of tactile epithelial cells, which are essential for light touch sensation, in their uropatagia and footpads (Yin et al. 2009, Marshall et al. 2015). Bats use haptic perception provided by their hind feet and uropatagium to trigger prey capture reactions, such as folding their hind feet (Geberl et al. 2015). However, while aerial-hawking bats fold their hind feet only when a prey item has been captured, trawling bats targeting insects exhibit this behaviour even when the prey has not been captured, suggesting that trawling bats do not distinguish between touching the water surface and touching an insect prey item – probably because the haptic stimuli produced by small light insects are too weak. Fishing Myotis capaccinii, however, do not display such a movement after a failed fishing attempt (Aizpurua et al. 2016). This suggests that fishing bats are able to use haptic perception from their hind feet to discern between a fish capture and a mere water grasp, something that is probably possible because fishing bats are aware that they are capturing larger and heavier prey than small insects.
At the time of grasping the fish, fishing bats gaff the fish with their sharp claws. The small punctures observed in the body of dropped fish suggest that individuals of Noctilio leporinus grasp the fish indiscriminately in their bodies, sometimes passing their claws completely through the body of a fish (Bloedel 1955). In contrast, Myotis bats seem unable to stab fish with their claws due to their less powerful flight and smaller size. A study using high-speed video images showed that Myotis capaccinii gaffs fish at the gills using one or several toes from a single foot (Aizpurua et al. 2014).
Handling and Consuming Fish
Fishing bats can capture and consume prey fish that are up to fifty times larger than the arthropods included in their diet (Aizpurua et al. 2013). After a successful fish capture, bats rapidly move the prey to their mouths (Altenbach 1989, Schnitzler et al. 1994b, Bordignon 2006b, Aizpurua et al. 2014). While small fish can be swallowed on the wing (Bloedel 1955, Siemers et al. 2001a), bigger fish have to be consumed when bats hang up in a perch (e.g. tree branch) or in the roost (Bloedel 1955, Aihartza et al. 2008). Noctilio leporinus is believed to be able to echolocate while carrying partially masticated fish in its mouth, as it has cheek pouches (Murray & Strickler 1975), but whether and how Myotis bats manage to continue echolocating while carrying fish is unknown.
Fish are eaten head first, in line with normal scale arrangement (Maya 1968, Aihartza et al. 2008). Studies conducted with Noctilio leporinus and Myotis vivesi show that, although their mandibular shape and mouth features do not differ from those of insectivorous bats (Freeman 1984, Elizalde-Arellano et al. 2004, Santana & Cheung 2016), piscivorous bats exhibit differences in the cranial shape compared to their insectivorous counterparts (Ospina-Garcés et al. 2016, Santana & Cheung 2016). They have a relatively tall, short rostrum that is broad at the zygomatic arches, suitable for producing high bite forces at the expense of lower closing speed. Unlike in carnivorous bats though, slow closing speed might not be a constraint for piscivorous species, since they use their hind feet and claws to capture their prey (Santana & Cheung 2016). Higher bite force combined with a longer period of mastication might be essential for the ingestion of the hard and sharp bones of fish, which unless thoroughly chewed, could cause wounds in the intestinal tract of bats.
The nutritional composition of fish is also different from that of insects; it is characterised by the absence of fibre, which is abundant in insect exoskeletons (Kourimská & Adámková 2016). The digestive tract of bats is about 20-60% shorter than that of other similar-sized terrestrial mammals (Caviedes-Vidal et al. 2007). This reduction in intestinal size and volume is believed to be an adaptation to flight, the most energetically expensive form of locomotion. Moreover, bats have shorter retention times for digesta (Welch et al. 2015). In spite of these apparent limitations, insectivorous bats have very high (70–90%) digestive efficiency, in part because they compensate for the loss of absorptive area by intestinal villous amplification and higher glucose absorption (Price et al. 2015). Although still poorly studied in bats, the gut microbiota could be another factor related to their high digestion efficiency (Zepeda Mendoza et al. 2018). Bats have different gut microbial communities depending whether they feed on fruits, nectar, blood, or insects (Phillips et al. 2012, Carrillo-Araujo et al. 2015), and the gut microbial composition is known to vary in response to fibre content differences in diet (David et al. 2014). Thus, variations in gut microorganism composition may play a crucial role when incorporating a new food type with a different nutritional composition into the diet, as is the case with fish.
Acknowledging the nutritional differences between insects and fish and unveiling the physiological variation these changes trigger in bats seem to be essential for the nuanced understanding of fishing bat biology. To obtain further insights into the role of gut microorganisms, both cross-sectional studies contrasting fishing bats with phylogenetically related non-fishing bats and longitudinal dietary manipulation studies (e.g. from insect-only diet to fish-only diet) could be conducted. Molecular analysis of gut microbial communities would enable researchers to unveil whether taxonomic and functional differences exist between the gut microbiotas of fishing and non-fishing bats. If such analyses were coupled with microbiota transplants and digestive efficiency tests (Welch et al. 2015), the role of gut microorganisms in the adaptation to dietary changes could be assessed (Alberdi et al. 2016).
Conclusions
This bibliographical review shows that the rarity of fishing among bats is probably related to the many challenges bats have had to overcome to make fishing cost-effective, as well as to the specific environmental conditions bats require for fishing to become feasible. Although research conducted in the last decade has cast light on the mechanisms bats use to capture fish, our knowledge of the evolutionary importance of fishing among bats is still limited.
Therefore, the study of fishing behaviour in bats still has a long way to go. Many aspects relating to behavioural ecology, conservation biology, sensory ecology, evolutionary biology, and physiology, among other disciplines within biological sciences, can be studied in fishing bats. Additionally, solving some issues might have added value, and the research could be used to gain very transversal knowledge, such as information regarding ultrafast information processing and mechanosensory responses. All this new knowledge is essential to improve our understanding of this fascinating and challenging capacity that a few bat species have been able to develop.
Acknowledgements
We thank M. Thomas P. Gilbert for the edits and comments on the manuscript and Kirstin Übernickel for providing the sound files of Noctilio leporinus. The authors were supported by the Carlsberg Foundation, The Danish Council for Independent Research, and the Lundbeck Foundation.




