Fishers as Potential Dispersal Agents for Corals: Balancing the Impact of Small-Scale Fisheries on a Pillow Coral Bed
Funding: This study were collected as part of the ENVIRO_NETS and EcoSEPIA projects, which were financed by the Greek Operational Programme for Fisheries and Sea (2014–2020).
ABSTRACT
The Mediterranean Sea is home to the endemic pillow coral, Cladocora caespitosa, which thrives on stony or rocky substrates to form coral beds or banks. A C. caespitosa hotspot habitat was identified in the eastern Thermaikos Gulf, with densely populated, large colonies of over 0.5 m in diameter. Local small-scale fishers intentionally avoid operating in this area in order to prevent damage to their nets. The substrate in the adjacent fishing ground consists of a heterogeneous assemblage of soft substrates, mainly sandy sediments and patches of seagrass meadows, and scattered colonies of C. caespitosa. Nonetheless, the colonies were shown to be fragmented, and most were smaller than in the hotspot area. This indicates that this area is not the native environment of C. caespitosa, but rather transported from the hotspot region. Static nets, primarily trammel nets but also gillnets, were shown to capture C. caespitosa colonies, which are subsequently discarded by fishers and returned to the seafloor. It is thus anticipated that small-scale fishers contribute to the spread of the C. caespitosa population and the transfer from the hotspot area to the entire sandy zone, serving as potential dispersal agents. Indeed, the majority of C. caespitosa in the sandy region had living polyps present on them, and in many instances, the colonies attained the characteristic spherical shape. The role of small-scale fishers as dispersal agents of C. caespitosa colonies closely resembles the fragment-based transplant approach, applicable for the restoration of coral banks.
1 Introduction
The pillow coral, Cladocora caespitosa, is a symbiotic and colonial scleractinian coral that is endemic in the Mediterranean Sea (Kružić and Benković 2008). It can be found in different habitats both in shallow and deeper waters, frequently settling on rocky substrates, seagrass beds, and in locations with moderate water velocity. It can also occur as free-living on coarse, soft bottoms, frequently in association with algal rhodoliths (Kersting et al. 2017). When abundant, the coral typically forms “beds” consisting of small, subspherical, bush-shaped colonies (10–30 cm in diameter) (Peirano et al. 1998). The largest colonies in beds may coalesce to form reef-like structures known as “banks” which can reach a height of several decimetres and cover a surface area of several square meters (Kružić and Požar-Domac 2003; Peirano et al. 1998). Its adaptability to various depths and substrates and its role as an ecosystem engineer providing living habitat with enhanced complexity to the benthic environment (Schiller 1993) make it an important component of the Mediterranean marine ecosystem.
C. caespitosa is a hermaphroditic species that releases egg and sperm bundles into the water column for external fertilization (Kružić et al. 2008). Alternatively, it can reproduce asexually through extratentacular and intratentacular budding (Kružić et al. 2008) or fragmentation. During the latter process, peripheral parts of large colonies are torn by wave action in shallow water sites or by boring cryptofauna in deeper sites, and normally develop to create a new colony near the original colony (Kružić et al. 2008; Schiller 1993). This type of asexual reproduction serves as the foundation for many coral reef restoration approaches. Translocation or transplantation of whole or fragmented colonies has been successfully used to restore damaged or threatened coral reefs (Kotb 2016), and it has recently been tested for C. caespitosa populations in the south Tyrrhenian Sea (Roveta, Coppari, Calcinai, Gioia, et al. 2023) and the Central and Southern Adriatic Seas (Cardinale and Danovaro 2024). Such restoration actions may be required when coral reef deterioration reaches levels that are difficult to reverse with traditional management and conservation strategies (Chavanich et al. 2015).
C. caespitosa has limited capacity to recover from stressful conditions, making it vulnerable to various threats that jeopardize its survival (Antoniadou et al. 2023; Roveta, Coppari, Calcinai, Di Camillo, et al. 2023). It has been included in the IUCN red list since 2008 and assigned as endangered since 2015 (Otero et al. 2017) owing to increasing evidence of population declines in the Mediterranean. Climate change is a major concern since rising water temperatures cause coral bleaching or polyp necrosis frequently resulting in mass mortality events (e.g., Antoniadou et al. 2023; Jiménez et al. 2016). The spread of non-native invasive green alga (e.g., Caulerpa racemosa; Kružić and Benković 2008), as well as physical damage from coastal construction and recreational activities, such as anchoring and trampling, pose further concerns (Kersting et al. 2023). Among anthropogenic threats, fishing practices and the use of destructive fishing gear constitute a serious risk to C. caespitosa because they can physically harm the colonies degrading the habitat and making it more difficult for the species to recover and thrive (Chavanich et al. 2015; Enrichetti et al. 2019; Silvestrini et al. 2024).
Small-scale fisheries (SSF), in particular, may have a significant impact on C. caespitosa because their operating area is within its shallow bathymetric range (Kersting and Linares 2012; Zunino et al. 2018) and because important fishing gears including bottom-set nets (trammel-nets and gillnets) and netting traps can interact with the seafloor and the sessile marine biota (Silvestrini et al. 2024). Indeed, Voultsiadou et al. (2011) observed that C. caespitosa was captured in almost 30% of hanging-net operations (gillnets and trammel nets) by SSF vessels in Thermaikos Gulf (N. Aegean), whereas the species was not present in deeper bottom-trawl hauls. For the same region, Ganias et al. (2021, 2023) report considerable bycatch rates for C. caespitosa in trammel nets and gillnets, while the species was absent from netting trap operations. These findings indicate that the species is particularly vulnerable to bottom-set net operations, owing to its shallower bathymetric distribution and the capture mechanism of these fishing gears, which sweep the sea floor primarily during net retrieval. While these studies assessed the catchability and discard rates of C. caespitosa in bottom-set nets, they did not report on the extent to which SSF has an impact on its population, such as the level of physical damage, the status, density, and distribution of coral colonies.
The aim of this study was to assess the impacts of the SSF on the population of C. caepitosa in Thermaikos Gulf focusing on bottom-set nets, which are the main fishing gears employed by local fishermen. Although the presence of a significant C. caepitosa population in this extensively fished location has been confirmed, no effort has been made to assess the fisheries' influence on its population. In order to accomplish this, a comprehensive dataset including catch data, input from fishermen, and on-site observations was utilized to accurately delineate the distribution of C. caepitosa beds and the potential presence of banks. This study further examines an alternate theory that investigates if fishing can have a positive impact on coral populations, with fishermen playing a role as dispersal agents. Considering that fragmentation is a natural method of asexual reproduction in this species, we question the notion that small-scale fishermen could potentially contribute to population growth by collecting and discarding specimens in areas beyond their original habitat.
2 Methods
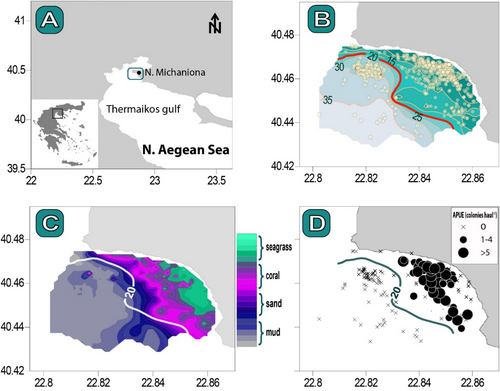
The study was conducted in eastern Thermaikos Gulf, off the fishing port of Nea Michaniona (Figure 1A). The area is an important fishing ground for a small-scale fishery fleet with over 30 commercial fishing vessels. These vessels typically use static nets to catch the common cuttlefish Sepia officinalis from mid-winter to early summer, the red mullet Mullus surmuletus from late spring to mid-fall, and flatfish, primarily the common sole Solea solea, during the entire year.
For the needs of the study, we used data from 698 commercial bottom-net hauls, carried out between January 2019 and July 2023 (Figure 1B). All fishing hauls were carried out by three collaborating fishermen who permitted our research team to board and collect all relevant information during the fishing activities. The primary data employed in the current study were the characterization of the seabed by fishers, based on their prior experience from fishing over each location, as well as the quantity and weight (0.1 g) of Cladocora caespitosa specimens caught during fishing hauls. Because these two forms of data had to be independent, seabed characterization always preceded net retrieval to prevent the fisher from being influenced by the possibility of capturing C. caespitosa. Alongside this basic information, all pertinent data from the fishing hauls was recorded, including the location, kind and characteristics of the fishing gear and mean fishing depth. The catch of C. caespitosa was expressed as the biomass (CPUE; g/haul) or abundance (APUE; colonies/haul) per fishing effort standardized to the mean gear length (1900 m) and mean soak time (19 h).
From October to December 2022, remotely operated underwater vehicle (ROUV) casts were utilized to undertake in situ observations of the seabed in the shallower section of the survey region (Figure 2A,C) with the goal of comparing these observations to characterizations of the seabed by fishers (see Appendix A). For the ROUV casts, we used a chartered fishing vessel and a QYSea FiFish V6 drone. The casts were carried out as visual census transects along the footrope of 100 m long fishing nets at a depth of 3–15 m. This method was particularly effective for following the footrope, especially on days with low visibility. The footage from each transect was processed using KINOVEA software (kinovea.org) to define the seabed's properties.
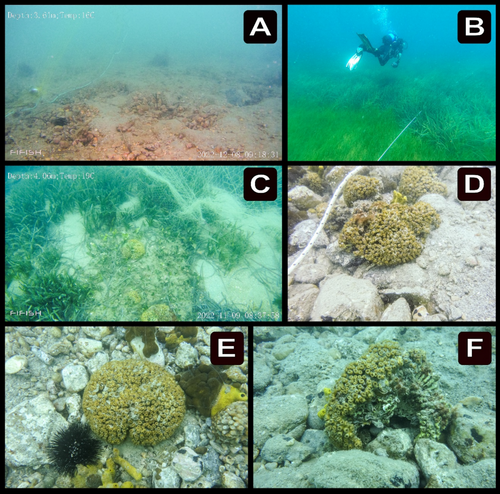
Furthermore, a scientific dive survey of 20 visual census line transects of 50 m each was conducted with the primary goal of determining the density of C. caespitosa in different sites within the coral zone (see Results Section 3; Figure 2B,D). During these dives, we either photographed all of the C. caespitosa specimens we came across (Figure 2D) or captured continuous video footage. The area of each transect was calculated by multiplying the dive length by 2 m, the average observation breadth of the divers. C. caespitosa density was estimated at each station by dividing the number of colonies by the transect's surface.
3 Results
According to the fishermen, there were four types of substrate in the survey area: seagrass, mud, sand, and coral beds. Because these substrates were typically intermingled, substrate type in each fishing haul was recorded using hierarchical scoring, with the major substrate type scored first as the principal type. The spatial distribution of the principal substrate types showed that the survey region is divided into three distinct zones (Figure 1C): a shallower area dominated by seagrass, a deeper area characterized by mud, and an intermediate zone comprising both sandy substrates and coral beds. Considering that coral beds were evenly distributed throughout this zone without any specific spatial pattern, such as gradients or clustered distributions, this intermediate area was designated as the coral zone.
The fishing survey revealed a clear spatial correlation between the catches of C. caespitosa and the distribution of the coral zone (Figure 1D). Consequently, the hauling stations were categorized into the three spatial zones described above. The average APUE and CPUE of C. caespitosa in the coral zone were 1.97 colonies haul−1 (s.e 0.25) and 316.5 g haul−1 (s.e. 64.07), respectively. There were fewer catches of C. caespitosa over seagrass beds (average APUE: 0.43 colonies haul−1; s.e. 0.13), while no captures of C. caespitosa were found in the deeper muddy zone (i.e., above 20 m; Figure 1D).
Fishermen's recordings were juxtaposed with in situ observations acquired via ROUV casts at identical locations as the fishing hauls. The comparison demonstrated a substantial level of concordance (87% for seagrass substrate and 70% for sandy substrate), validating the precision of fishers' assessments about substrate type (more detailed analysis of this comparison is provided in the APPENDIX). This comparison also illustrated that, in the majority of instances, the principal substrate type comprised over 70% of the bottom surface in the fishing haul areas. The local fishermen in the surveyed region accurately identified C. caespitosa as the characteristic element of the coral beds, regardless of whether they were presented with photos or live specimens. Furthermore, our in situ observations revealed that regions identified as coral beds consistently exhibited a high incidence of C. caespitosa.
The ROUV casts and the diving survey confirmed the increased prevalence of scattered C. caespitosa colonies within the coral zone and revealed that the seabed consisted of a heterogeneous assemblage of soft substrates, including sandy sediments interspersed with detritus, shell fragments, and boulders, as well as scattered patches of Posidonia oceanica and Cymodocea nodosa meadows (Figure 2). C. caespitosa colonies in this location were often small (maximum 200 cm in diameter), bush-like or fragmented (Figure 2E,F), and were found either solitarily or in association with other organisms. Specifically, the colonies were often observed with sponges including Aplysina aerophoba, Chondrosia reniformis, and Chondrilla nucula, as well as sea anemones such as Anemonia viridis and Condylactis aurantiaca. The diving survey indicated that the average density of C. caespitosa within the coral zone was 0.056 colonies m−2.
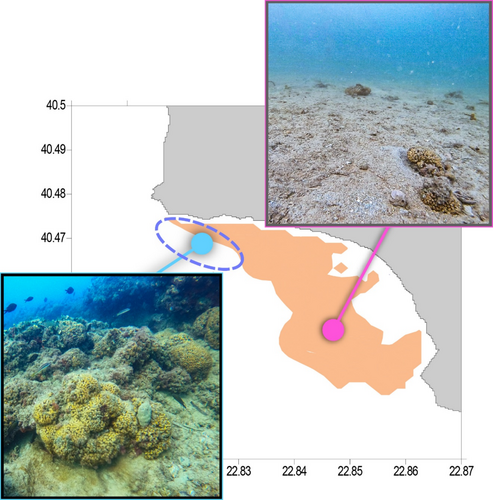
The underwater survey also identified an area with an enhanced prevalence of C. caespitosa, exhibiting a density of 0.754 colonies m−2, which is 13.5 times greater than the previously described coral zone (Figure 3). This site exhibited massive colonies over 0.5 m in diameter on a stony or rocky substrate (Figure 3), so designated as the C. caespitosa hotspot area. This region, situated in the northwest portion of the survey area, was distinctly outside the operational zone of the SSF vessels during our fishing survey (Figure 3); the fishermen deliberately avoided it because of its hard substrate to minimize damage to their gear.
4 Discussion
Cladocora caespitosa primarily thrives in areas with stony or rocky cliffs and substrates, allowing it to create coral beds or banks. C. caespitosa colonies were found in the shallower sandy section of the survey area till the start of the deeper muddy zone, which is in agreement with its reported preference for depths between 3 and 20 m (Kersting and Linares 2012; Mastrototaro et al. 2023; Zunino et al. 2018). Additionally, the presence of sandy substrate combined with biogenic material offers a more solid surface for the establishment of coral colonies. Conversely, corals refrain from settling on muddy substrates since they are buried in them. Despite the widespread presence of C. caespitosa in the sandy/coral zone, one of our primary concerns was determining if this zone functioned as the primary habitat within the survey area.
The dive survey revealed a hotspot habitat for C. caespitosa in the northwestern section of the survey region (Figure 3), characterized by densely inhabited colonies exceeding 0.5 m in diameter. Nevertheless, no additional data was gathered concerning colony morphology, corallite development, and the total surface area occupied by the colonies, as conducted by Kružić and Benković (2008) for C. caespitosa banks in the Adriatic Sea. These constraints made it difficult to determine whether the C. caespitosa hotspot area meets the criteria for a coral bank as delineated by Kružić and Požar-Domac (2003) and Peirano et al. (1998). Even so, the size and density of the colonies in the hotspot area were clearly distinguishable from the wider sandy zone where the fishing survey was conducted. Indeed, this location falls beyond the operational range of the commercial fishing hauls. In addition, according to fishers' description of the substrate on which the fishing took place, out of the 698 hauls analyzed in the study, only two were conducted on a rocky substrate. This outcome demonstrates that fishermen intentionally avoid operating in areas with rocky bottoms in order to prevent damage to their nets.
Although C. caespitosa was present in the sandy zone, the colonies exhibited distinct differences from those in the hotspot area. To be more precise, the majority of the specimens were fragmented (see also Ganias et al. 2023), while the intact colonies were relatively smaller in size compared to the hotspot region. This was demonstrated by the specimens retrieved from the fishing hauls, as well as the underwater survey conducted mainly through scuba diving but also using ROUV casts. The general perception was that the sandy zone was not the native environment of C. caespitosa, but rather that these colonies were transported to this place from the hotspot region.
Ganias et al. (2023) suggest that the transfer of C. caespitosa colonies can occur due to the combined effects of hydrodynamics and human activities (see also Zunino et al. 2018). However, hydrodynamics may not be a significant factor since the survey region is protected from strong ocean currents that may potentially remove the well-established C. caespitosa colonies from their substrate. During our dive survey, we have consistently observed C. caespitosa colonies settling on specific landmarks, such as abandoned anchors or tyres, over extended periods of time, sometimes exceeding 2 years. Hence, we can affirm that the spread of C. caespitosa in the sandy region is totally attributed to human activity and is exclusively linked to the use of bottom-set nets. In this scenario, small-scale fishers may have served as dispersal agents for the C. caespitosa colonies expanding its population from the hotspot area throughout the sandy region of the study area (Figure 3). It is noteworthy that the majority of C. caespitosa in the sandy region, even the smaller fragments, had living polyps present on them, while in numerous instances, the colonies attained the characteristic spherical shape.
How did small-scale fishers contribute to the spread of C. caespitosa population, and how was the transfer from the hotspot area to the entire sandy zone accomplished? During our survey, we constantly noticed that both gillnets and trammel nets were effective in capturing C. caespitosa specimens. Furthermore, a distinct spatial pattern was observed in C. caespitosa catches, as both APUE and CPUE were significantly higher in the sandy zone—also referred to by the fishers as the ‘coral zone’—compared to seagrass beds and the deeper muddy zone. Analysis of catch data from the fishing survey revealed that C. caespitosa colonies were uniformly captured and discarded by the fishers within the sandy zone. The Nea Michaniona harbor has served as a thriving fishing port for almost a century, following the settlement of Asia-Minor migrants (Frangoudes 2010), and the fishing pressure has remained continuously strong with minor fluctuations (Moutopoulos and Stergiou 2012). Thus, over the past century, there has been a consistent pattern of capturing and discarding C. caespitosa colonies from local SSF fishers, while the considerable length of their fishing nets (~1.5 km on average) accounts for the significant distance between the collection and disposal sights of each coral colony. It is also plausible to assume that smaller fragments containing living polyps, which are easier to capture, may have a greater impact on the growth of coral colonies. Some of these colonies, if left undisturbed for prolonged periods, can develop the distinctive spherical shape (Kružić and Benković 2008) which explains the prevalence of a smaller fraction of entire spherical colonies in the sandy zone.
The natural role of small-scale fishers as dispersal agents of C. caespitosa specimens closely resembles the fragment-based transplant approach, a well-established method used to restore coral banks. This can be accomplished by either utilizing dislodged colonies found alive on the bottom or by using fragments separated from wild donors. The transplantation of intact or fractured colonies has proven to be effective in establishing a self-sustaining reef in areas where the original reef has suffered damage. The cultivation of fragments obtained from wild corals serves several functions apart from restoration, such as generating colonies for aquarium trade or study (Shafir et al. 2006). This practice helps alleviate the pressure on wild populations. Nevertheless, wild donors may experience the elimination of substantial segments (Henry and Hart 2005; Shafir et al. 2006). In order to address this problem, the utilization of small colony fragments (known as coral nubbins) has been demonstrated as an effective strategy for coral gardening (Musco et al. 2017; Shafir et al. 2006).
The current findings about the potential advantageous impact of fishermen on the conservation of C. caespitosa must be approached with caution and not misconstrued, as fisheries are recognized as one of the primary anthropogenic threats to C. caespitosa and corals in general (Enrichetti et al. 2019). Illegal trawling and dredging can physically harm coral colonies and destroy their habitats, whereas small-scale fisheries may have localized effects on bioconstructors such as C. caespitosa because fishing gear such as longlines, trolling lines, and traps can damage coral colonies by turning them upside down (Kružić et al. 2025). During the present survey, it was observed that when larger coral colonies were tightly entangled in the net, the fishers smashed them and discarded the fragments either in the fishing field or at the bottom of the fishing port. Especially in the latter situation, and in order to prevent the scattering of fragments onto the concrete or into the unfavorable and inhospitable conditions of the port, fishermen may temporarily store the colonies in submerged fishing cages. Indeed, during the course of this study, few fishers were advised to place discarded colonies in submerged rectangular fishing traps, and it was observed that nearly all members of the colony, including various forms of epibiotic organisms, were able to survive for a duration of at least 10 days, which is longer than the time between fishing operations. Hence, fishers may be motivated to collect these colonies and later release them into their preferred habitats, particularly on rocky or stable sandy substrates, while avoiding muddy substrates, as part of their regular fishing activities. This could represent an extra and purposeful role of fishers acting as dispersal agents for the coral, thus assisting in the conservation of the local population. To improve success rates, it is crucial for fishers to get instruction on the biology and importance of these organisms, specifically their status as endemic and endangered species. Additionally, the hotspot area should be officially designated as off-limits to fishing activities, with the exception of nondestructive commercial activities such as recreational diving. This would provide fishers with the opportunity to generate additional revenue through fishing tourism or diving tourism.
Acknowledgments
The author wishes to thank Chrysanthi Antoniadou, Alexandra Karatza, Katerina Charitonidou, and Dimitris Lachouvaris for their support in sampling. Two anonymous reviewers are thanked for their useful feedback, which allowed to explain the goals and considerably improve the quality of the manuscript.
Ethics Statement
The fishing procedure during the monitoring of commercial fishers followed national laws, ethics, and regulations. Necessary permits and permissions to carry out the samplings of this research were obtained from the Fisheries Department of the Regional Unit of Central Macedonia (document number: 549031/4080).
Conflicts of Interest
The author declares no conflicts of interest.
Appendix A
The ROUV survey was conducted by carrying out 32 casts using a QYSea FiFish V6 drone from September 30 to December 8, 2022, encompassing the shallower region (3–15 m) of total study area. The casts were carried out as visual census transects along the footrope of 100 m long fishing nets. The footage from each cast was examined, and the ratio of each substrate type (seagrass, sand, mud) was determined.
The following map features a contour plot depicting the fraction of seagrass substrate type derived from the ROUV survey. The contour plot (Figure A1) was produced via the Kriging gridding method within Surfer software (v.25.1.229).

To calculate the agreement proportion between the Fisher's evaluation of the substrate type and the ROUV survey, only the fishing hauls within the bounds of the contour plot were considered. It was further posited that the two sets were congruent when a haul with seagrass substrate occurred in a region with ≥ 50% seagrass or when a haul with sand substrate occurred in a region with < 50% seagrass. The outcomes of the agreement exercise are summarized in Table A1: Of the 182 hauls conducted within the ≥ 50% seagrass area, 163 (87%) were classified as seagrass substrate type by the fishers. Furthermore, 67 of the 96 hauls (70%) conducted within the < 50% seagrass area were classified as sandy substrate type by the fishermen.
| ROUV survey substrate | Fishing survey substrate | |||
|---|---|---|---|---|
| Seagrass | Sand | Coral | Mud | |
| ≥ 50% seagrass | 163 | 19 | 2 | 3 |
| < 50% seagrass | 23 | 67 | 2 | 4 |
Open Research
Data Availability Statement
Data will be made available on reasonable request.




