Symbiodiniaceae-Derived Fatty Acids Are Stored Differentially Across Giant Clam Species and Organs
Funding: This work was supported by the CNPq Research Productivity Grants 304274/2018-6 and 301554/2019-6.
ABSTRACT
Giant clams are invertebrates that form mutualistic associations with Symbiodiniaceae dinoflagellates. Despite their ecological significance, gaps persist regarding our understanding of their trophic ecology. Specifically, it is unknown whether Symbiodiniaceae-derived photosynthates are metabolized differently according to species and organ. Therefore, we maintained Tridacna derasa and T. noae for 3 months in a well-lit recirculated system without food supply. Samples were taken from eight organs and underwent lipid extraction and fatty acid esterification before analysis of three symbiont-derived fatty acids (stearidonic acid, docosapentaenoic acid, and docosahexaenoic acid—SDA, DPA, and DHA, respectively) using gas chromatography. Results show considerable variation in fatty acids concentration among species and organs. SDA was found in higher concentrations in T. noae, especially in the adductor muscle. DPA was detected in low concentrations across T. noae organs and absent for T. derasa. DHA did not vary significantly among species and organs. Our findings indicate that Symbiodiniaceae supply clams with fatty acids, which are stored differentially according to species and organs. This demonstrates that these compounds are translocated to multiple organs throughout the complex giant clam anatomical system, in contrast to simpler hosts like corals. These results advance our understanding of the physiological dynamics of the mollusk-algae association.
1 Introduction
Animal-algal symbioses are critical for sustaining coral reefs, as they are involved in multiple key ecological processes, such as primary production, nutrient cycling, and calcium carbonate deposition (Hatcher 1990; Furla et al. 2000; Fox et al. 2018; Brandl et al. 2019; Rädecker et al. 2021). Cnidaria, Mollusca, Platyhelminthes, Porifera, and Xenacoelomorpha are some of the animal phyla found in coral reefs that are known for harboring microalgae within their tissues (Stat, Carter, and Hoegh-Guldberg 2006; Venn, Loram, and Douglas 2008). Among the most conspicuous photosymbiotic animals found in reef environments are giant clams. Members of the Tridacninae subfamily, giant clams are bivalve mollusks notorious for their larger shells that may reach up to 137 cm in total length (Fitt, Fisher, and Trench 1986; Streamer, Griffiths, and Thinh 1988; Mies 2019; Rossbach et al. 2021). Their photosymbiotic nature has allowed for them to be successful colonizers of shallow and well-lit Indo-Pacific coral reefs (Harzhauser et al. 2008).
Giant clams hold significance not just in ecological terms but also from a socioeconomic perspective. They serve as prey item for several organisms, while also providing structural complexity and habitat for numerous epi- and endobiotic invertebrates such as crustaceans, sponges, and gastropods (Cabaitan, Gomez, and Aliño 2008; Neo et al. 2015). They are also relevant primary and secondary producers because of their mixotrophic nature, as they generate organic carbon biomass through their symbiotic relationship with Symbiodiniaceae, and additionally filter-feed substantial volumes of organic matter-rich seawater (Klumpp and Griffiths 1994; Klumpp and Lucas 1994; Mies 2019). Finally, giant clams serve as a protein-rich food source for local communities across numerous Asia-Pacific islands, countries, and territories (Lucas 1994; Van Wynsberge et al. 2016; Mies, Dor, et al. 2017). Therefore, the trophic ecology and dynamics of giant clam assemblages is particularly relevant from multiple angles.
Differently from corals, which harbor symbionts intracellularly, giant clams maintain Symbiodiniaceae cells in an extracellular branched tubular system found extended from the stomach to the hypertrophied mantle, termed “zooxanthellal tube system,” or ZTS (Norton et al. 1992). The association between giant clams and Symbiodiniaceae involves the translocation of photosynthates from the symbiont to the host (Klumpp and Griffiths 1994), which include glucose, glycerol, and amino acids (Rees et al. 1993; Burriesci, Raab, and Pringle 2012; Mies, Van Sluys, et al. 2017). This association forms a mutually advantageous interaction as the host supplies essential compounds for cellular synthesis to the dinoflagellate, including nitrogen, phosphorus, and CO2, while receiving the photosynthetic products in return (Klumpp, Bayne, and Hawkins 1992; Leggat, Rees, and Yellowlees 2000; Mani et al. 2022). This association is deemed obligatory for the hosts in the long term, as their survival is entirely dependent on the presence of these symbionts in their tissues (Norton et al. 1992; Mies, Scozzafave, et al. 2017; Ip et al. 2022). The importance of this symbiotic relationship for giant clams is evident in their evolutionary divergence from typical clams and cockles. Unlike their small, infaunal counterparts, giant clams have adapted to become epibenthic organisms dependent on light, resulting in their notably larger size and hypertrophied outer mantle (Mies 2019).
Despite advances in giant clam investigations, several gaps remain regarding their trophic ecology. An unaddressed topic is the physiological pathway of compounds generated and translocated by symbionts. It is widely acknowledged that the main photosynthates are glucose and glycerol, but some reports have showcased the transference of fatty acids in this process as well (Sutton and Hoegh-Guldberg 1990; Burriesci, Raab, and Pringle 2012; Mies, Chaves-Filho, et al. 2017). Fatty acids are particularly useful because some are metabolically stable compounds produced by Symbiodiniaceae but not by their animal host, despite the fact that many of these fatty acids are essential to them (Graeve, Kattner, and Hagen 1994; Zhukova and Aizdaicher 1995; Papina, Meziane, and Van Woesik 2003). Therefore, they may serve as trophic markers, indicating the translocation of photosynthetically fixed carbon from Symbiodiniaceae to their hosts (Mies, Chaves-Filho, et al. 2017; Mahmoud et al. 2018). Fatty acid trophic markers have been used to reconstruct intricate marine food webs involving multiple marine taxa (Dalsgaard et al. 2003; Ruess et al. 2005; Budge, Iverson, and Koopman 2006), including other mixotrophic reef organisms such as scleractinian corals (Treignier et al. 2008; Dodds, Black, and Roberts 2009; Naumann et al. 2015; Mies et al. 2018). However, while it has been established that organic compounds, such as fatty acids, produced by symbionts are transported through giant clam tissues via the hemolymph (Rees et al. 1993), it remains unclear whether these fatty acids are stored differentially across tissues and organs of giant clams.
Within this context, this work investigated the presence of symbiont-derived fatty acids in the tissues of two giant clam species, Tridacna derasa and T. noae, to assess whether they are stored in varied concentrations across multiple organs. Examining the destination of symbiont-derived fatty acids in giant clams holds significant importance for different reasons. Firstly, giant clams exhibit unique and more resilient responses to climate change and other disturbances compared to other photosymbiotic reef organisms, including bilateral bleaching and extended survival even in the near absence of symbionts (Rouzé and Hédouin 2018; Mies 2019; Zhou et al. 2019; Ip et al. 2022). Thus, comprehending the mechanisms of photosynthate transport, modulation and storage throughout their anatomical structure may shed light on the factors contributing to their unique responses. Additionally, giant clams serve as a relevant fishery resource, with various organs consumed by fishing communities (Lucas 1994; Mies, Dor, et al. 2017; Purcell, Gossuin, and Ceccarelli 2020; Eurich et al. 2023). Therefore, the fatty acid content within these organs, being a nutritional element for humans, also holds relevance for public health (Vianna, Zeller, and Pauly 2020).
2 Materials and Methods
2.1 Specimens, Organs and Sampling
Eight Tridacna derasa (24.4 ± 1.3 cm in total shell length, expressed as mean ± SD) and eight T. noae (10.5 ± 2.2 cm) individuals were imported live from Indigo Seafood in Palau and Xanh Tuoi in Ho Chi Minh City, Vietnam, respectively (Figure 1). The giant clams were maintained in two separate tanks (200 L each) connected to a recirculated aquaria system containing 2000 L of artificial seawater (Red Sea), which was produced according to the manufacturer instructions. Physicochemical parameters (temperature, salinity, pH, NH3+/NH4+, NO2−, NO3−, and PO4−3) were maintained using several pieces of equipment placed in the recirculated system. Temperature was maintained at 27°C with a 300-W submersible heater, salinity was maintained at 34 ppt, pH at 8.1, and NH3+/NH4+, NO2−, NO3−, and PO4−3 were maintained below detection through the use of a Vertex Omega protein skimmer and 50% weekly water changes. Lighting was provided by a LED 100-W fluorescent lamp producing 350 μE m−2 s−1 at the surface water level, at a photoperiod of 12:12 (L:D).
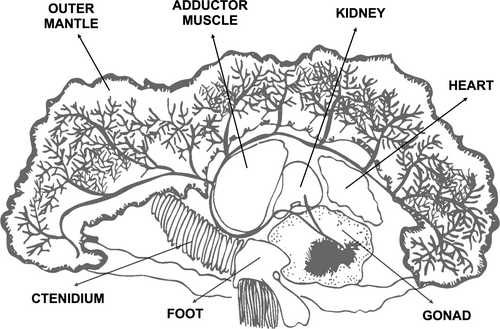
After 3 months under a no-feeding regime in the recirculated system, all 16 giant clams were euthanized according to the protocol in Gilbertson and Wyatt (2016), where they were first immersed in 5% ethanol and then and 10% formalin. Samples were then taken from eight different organs: adductor muscle, ctenidium, adhesive epithelium, foot, gonad, heart, kidney, and outer mantle (Figure 2). Symbiont cells were isolated only from the mantle tissue by grinding and centrifuging for 2 min at 6000 g. For the other organs, symbiont cells were not apparent, so any trace concentrations, if present, could not be removed. In these cases, minor contamination with fatty acids derived from the symbiont may have occurred.
2.2 Fatty Acid Extraction and Gas Chromatography
Lipid extraction was performed in a similar way to that described in Mies et al. (2018), with minor modifications. Organ samples were homogenized with sodium sulfate (Na2SO4) using a mortar and pestle, and then transferred to test tubes. Lipids were extracted using 2 mL of dichloromethane: n-hexane (1:1; v:v) with the aid of a vortex mixer for 1 min. Prior to extraction, 100 μL of tricosanoic acid (C23:0) was added as an internal standard to all samples. Fatty acids were esterified using 4 mL of sulfuric acid in methanol (2%) for 30 min at 40°C. Subsequently, 5 mL of a solution of distilled water saturated with NaCl was added, and the top organic phase containing the fatty acid methyl esters was retrieved. Fatty acid methyl esters were quantified on a 6890 Agilent gas chromatograph coupled to a flame ionization detector (GC-FID) with a DB-FATWAX (UI) capillary column measuring 30 m × 0.250 mm (0.25 μm film thickness). Samples were injected in split mode (5:1) with 4 μL injection volume. Hydrogen served as the carrier gas with a constant flow rate of 1.4 mL min−1. Injector and detector temperatures were set to 250°C and 280°C, respectively. The oven temperature was initially set to 180°C for 2 min, then increased to 210°C at a rate of 2°C min−1 and held for 15 min, and then set to 250°C at a rate of 20°C min−1 and maintained for 3 min. Peaks were identified by comparing retention times with those of Marine Source PUFA N°1 (Sigma). Quantification was based on an eight-point concentration curve for each compound, considering internal standard recovery. We examined the concentration of three fatty acids known to be translocated from Symbiodiniaceae to their host organisms (Papina, Meziane, and Van Woesik 2003; Seemann et al. 2013; Mies, Chaves-Filho, et al. 2017; Mies et al. 2018): stearidonic acid (18:4ω3), docosapentaenoic acid (22:5ω3), and docosahexaenoic acid (22:6ω3).
2.3 Statistical Analyses
To assess differences in the concentrations of stearidonic acid, docosapentaenoic acid, and docosahexaenoic acid (SDA, DPA, and DHA, respectively) in the organs of T. derasa and T. noae, we performed two-way analyses of variance (ANOVA) using giant clam species (T. derasa and T. noae) and organ (adductor muscle, ctenidium, adhesive epithelium, foot, gonad, heart, kidney, and outer mantle) as fixed factors, and the concentration of each of the three fatty acids as the response variable. Whenever statistical differences were detected (p < 0.05), a Tukey's HSD test was performed. Data were all previously checked for normality and homoscedasticity using Shapiro–Wilk and Levene's tests, respectively.
3 Results and Discussion
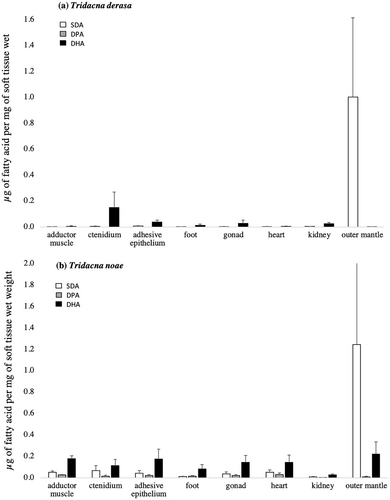
Our results show that the most abundant Symbiodiniaceae-derived fatty acid in the organs of giant clams is SDA, which was found in concentrations of ~1.0 μg mg−1 of giant clam wet tissue. DHA was found in intermediate concentrations, while DPA was almost absent from most organs. SDA was found in greater concentrations within the outer mantle of both species. However, in comparison with Tridacna derasa, T. noae exhibited higher concentrations of SDA in various organs such as the adductor muscle, adhesive epithelium, ctenidium, gonads, and heart (Figure 3). Both species and organ, as well as the interaction between them (Table 1), significantly influenced SDA concentration. This compound varied distinctively among species, and, among organs, with the outer mantle containing higher SDA content compared with the adhesive epithelium, foot, gonads, and kidney (p = 0.041, 0.023, 0.027, and 0.008, respectively).
| F | Degrees of freedom | p | |
|---|---|---|---|
| SDA | |||
| Species | 6.53 | 1 | 0.011 |
| Organs | 5.55 | 7 | < 0.001 |
| Species × organs | 4.69 | 7 | < 0.001 |
| DPA | |||
| Species | 14.16 | 1 | < 0.001 |
| Organs | 0.54 | 7 | 0.800 |
| Species × organs | 0.25 | 7 | 0.928 |
| DHA | |||
| Species | 1.49 | 1 | 0.223 |
| Organs | 0.79 | 7 | 0.589 |
| Species × organs | 1.78 | 7 | 0.091 |
- Note: Statistically significant results are marked in bold.
SDA serves as a trophic marker derived from dinoflagellates, including Symbiodiniaceae (Papina, Meziane, and Van Woesik 2003; Mies, Chaves-Filho, et al. 2017; Kihika et al. 2022), yet it is also present in various other dinoflagellate and phytoplankton species (Graeve, Kattner, and Hagen 1994; Zhukova and Aizdaicher 1995). Because giant clams are mixotrophic, the source of SDA in their tissues may be ambiguous. Clams obtain nutrition from two different dinoflagellate sources by obtaining photosynthates translocated from Symbiodiniaceae and also by filter-feeding on phytoplankton, which includes other dinoflagellate groups (Mies, Scozzafave, et al. 2017). However, it has been reported that SDA derived from Symbiodiniaceae are typically metabolized and consumed within approximately 10 days, becoming no longer detectable in the host organism after that period (Mies et al. 2018). Therefore, because (i) giant clams cannot synthesize de novo any of the three fatty acids investigated (Dalsgaard et al. 2003; Mies, Chaves-Filho, et al. 2017), and (ii) our experiment maintained the clams for 3 months without access to plankton, relying solely on photosynthates for carbon, we can conclude that the SDA originated from their symbionts. Unlike corals, where DHA is the main fatty acid translocated by Symbiodiniaceae (Mies, Chaves-Filho, et al. 2017), giant clams predominantly exhibit SDA. This suggests either a different mechanism of fatty acid transfer when compared to corals, or an alternate storage mechanism employed by giant clams.
DPA was not detected in the organs of T. derasa and found in lower concentrations in T. noae when compared to the other two fatty acids investigated (Figure 3). However, DPA was detected in all T. noae organs, in reasonably similar concentrations. Statistical differences were detected among species, but not organs nor the interaction between species and organs (Table 1). DPA is rarely detected within the tissues of Symbiodiniaceae hosts, making it a strong indicator of carbon translocation from dinoflagellates, which have high concentrations of this fatty acid, to their giant clam hosts (Papina, Meziane, and Van Woesik 2003; Mies, Chaves-Filho, et al. 2017; Mies et al. 2018; Pupier et al. 2021; Dalsgaard et al. 2003).
Our findings indicate that DPA is likely processed and/or stored differentially across clam species, as data show the presence of DPA in T. noae but its absence in T. derasa. The causes for this are unclear but may be related to the identity and diversity of the symbiont assemblage in the giant clam species investigated. Host organisms are known to harbor a diverse assemblage of Symbiodiniaceae, with marked inter- and intraspecific variations often detected (LaJeunesse 2005; Finney et al. 2010; DeBoer et al. 2012; Thornhill et al. 2014; Ikeda et al. 2017). For example, giant clams alone are known to host more than 30 different Symbiodiniaceae phylotypes distributed across four different genera (Symbiodinium, Breviolum, Cladocopium and Durusdinium), and often more than one phylotype is detected within a single host individual (Mies 2019). This may possibly indicate that T. noae and T. derasa form symbioses with distinct phylotypes that may differ in their composition and translocation of DPA to their host. Because of the functional diversity within Symbiodiniaceae (Stat, Morris, and Gates 2008; Suggett, Warner, and Leggat 2017; Swain et al. 2017), it is likely that their identity plays a major role in our findings. The possibility that distinct phylotypes influenced our findings is increased because (i) T. noae and T. derasa share only two phylotypes (Symbiodinium tridacnidorum and Cladocopium goreaui—A3 and C1, respectively), and (ii) T. noae is known to harbor a much greater diversity of Symbiodiniaceae phylotypes than T. derasa (Mies 2019). However, it is worth noting that symbiont diversity remains understudied for both species.
DHA was detected in all organs of both species. In T. derasa, higher concentrations of DHA were detected the ctenidium, adhesive epithelium, foot, gonad, and kidney, making it the predominant fatty acid among those examined (Figure 3). In the remaining organs, it was found in trace concentrations. DHA was detected in higher concentrations in T. noae, and it was the most abundant Symbiodiniaceae-derived fatty acid in all organs with the exception of the outer mantle (Figure 3). However no statistical differences were detected among species or organs (Table 1).
DHA is a critically important fatty acid for marine organisms due to its role in cell membrane composition, susceptibility to oxidative stress, and nutritional relevance (Halliwell and Gutteridge 1985; Dalsgaard et al. 2003; Morita et al. 2006; Okuyama, Orikasa, and Nishida 2008). This fatty acid is often stored in the gonads of multiple marine invertebrate species (Wu et al. 2007; Carboni et al. 2013; Laudicella et al. 2023), which may explain why higher concentrations were detected in the gonads of both T. derasa and T. noae. Similarly, DHA is frequently found in significant quantities in the adductor muscle of other bivalve species, including giant clams, (Caers et al. 1999; Bergé and Barnathan 2005; Liu et al. 2019; Mahmoud et al. 2018), consistent with our findings for T. noae. However, it is important to note that the content of DHA in bivalve organs can vary significantly, often seasonally, depending on specific environmental conditions and genetic factors (Fernández-Reiriz et al. 1999). Lastly, the symbiont phylotype may also play a role in the amount of DHA translocated to the host (Mies, Chaves-Filho, et al. 2017).
The fatty acid profiles between the two giant clam species seem considerably distinct, in accordance with previous findings reporting distinctive lipid profiles for T. crocea and T. squamosa (Liu et al. 2019). This possibly reflects differences in the production, utilization, and transfer mechanisms by the symbiont to the host, similar to observations reported for corals (Yamashiro et al. 1999; Teece et al. 2011; Imbs et al. 2014). This may suggest that the relationship between host and symbiont plays a crucial role in determining the prevalence of specific fatty acids. Other species within the Tridacna genus exhibit ecological features that are quite different from those of T. derasa and T. noae. For instance, T. squamosa, which is typically found in deeper waters, houses symbiont phylotypes that are adapted to low-light conditions (Jantzen et al. 2008; Mies 2019). This may directly affect the efficiency of photosynthesis and photosynthate production, as shown in previous studies which detected that light spectrum and intensity may influence on symbiont function and biochemical composition (Klumpp, Bayne, and Hawkins 1992; Jantzen et al. 2008). This highlights the significant role of the environment in shaping the fatty acid content of giant clams and also that further investigations into their fatty acid profile could benefit from assessing symbiont identity and diversity as well.
The concentration of fatty acids stored in the organs T. derasa and T. noae differs considerably, particularly between the mantle and the other organs. The higher concentrations of fatty acids detected in the outer mantle, where the majority of dinoflagellates reside and engage in photosynthate translocation to their giant clam host (Norton et al. 1992; Rees et al. 1993; Mies 2019), may explain why its fatty acid profile was distinct from that of the other organs. This organ, in housing the ZTS and facilitating symbiont transportation to and from other organs (see Norton et al. 1992), plays a crucial role in distributing symbiont cells throughout the host organism and even guiding them to the digestive system, where unwanted symbiont cells can be expelled in feces (Maruyama and Heslinga 1997; Morishima et al. 2019). Hence, the outer mantle and its capacity for symbiont storing and shuffling (Mies et al., unpublished data) distinguish it significantly from other organs. These remaining organs, deprived of light exposure, lack any documented ability to shuffle symbionts, potentially accounting for their differing fatty acid compositions. This distinctive lipid composition of the outer mantle is corroborated by other studies (Dubousquet et al. 2016; Mahmoud et al. 2018; Liu et al. 2019).
Our findings show that Symbiodiniaceae dinoflagellates supply giant clams with fatty acids that are stored differentially according to species and organs. This indicates that these compounds are either initially translocated to the mantle and then distributed to other organs or are directly transferred by symbionts within those organs. It also demonstrates that these compounds are moved throughout the giant clam system, which is far more complex anatomically when compared to other hosts such as corals. These results advance our understanding of the physiological dynamics of the mollusk-algae symbiotic relationship. This is particularly timely given the threats posed by climate change and overfishing to these essential associations, which are key elements in coral reef environments.
Author Contributions
M.M. and P.Y.G.S. designed the experiment. M.M. performed the experiment. S.T., M.C.B., and P.Y.G.S. contributed to infrastructure/material/technical support. J.R.N. analyzed the data, and all authors contributed to the manuscript.
Acknowledgements
We would like to thank Lucas Canela and Lucas Gonçalves for aiding in the maintenance of the giant clams. M.C.B. and P.Y.G.S. acknowledge CNPq Research Productivity Grants 304274/2018-6 and 301554/2019-6, respectively. We are also grateful to the reviewers for their time dedicated to reviewing the manuscript.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data are available in this manuscript; additional information will be provided upon request.




