“Another Kid on the Block”: Testing the Effects of Artificial Substrates on the Recruitment of Juvenile Fishes in the Northwestern Gulf of Mexico
Funding: This work was supported by Texas Parks and Wildlife Department.
ABSTRACT
Most artificial reefs (ARs) in the Gulf of Mexico are considered intermediate to high vertical relief structures which have recruited large predatory species indicating a lack of juvenile habitat. Small, inexpensive ARs, standard monitoring units for the recruitment of reef fishes (SMURFs), were deployed at eight treatment stations at −20 m as habitat for juvenile fishes to understand and determine the effects of substrate type on the recruitment of juvenile fishes. Each treatment station contained four SMURFs with four treatments: small and large concrete rubble (~10 and ~20 cm), oyster shells, and bare. Surveys conducted from July 2017 to July 2019 yielded 39 different juvenile species, including one of the most economically important species, Lutjanus campechanus, present across all replicates. There were 5238 individual fishes collected, and the family Lutjanidae accounted for ~49% of these. The highest species richness and diversity occurred in SMURFs containing oysters or small concrete rubble while bare treatment had the lowest. Both concrete rubble and oyster shells might offer shelter to numerous body shapes and sizes of juvenile fishes as a result from a variety of crevice sizes. This study suggests that the deployment of low-relief structures with different substrate materials might affect recruitment of select species and provide further information useful to designing ARs, aiding fisheries production. Because enhancing fisheries is one of the goals of the Texas Parks and Wildlife Department Artificial Reef Program it is here recommended to continue developing other designs of low-relief to be allocated in between existing high-relief ARs that should serve as stepping stones for the survival of species of fisheries interest (e.g., L. campechanus).
1 Introduction
According to the United Nations (2017), ~40% of the world's population resides within 100 km of a coast. Indeed, Earth's shorelines are also ecological hotspots, regions hosting numerous threatened species (Myers et al. 2000). Coral reef ecosystems are estimated to harbor ~600,000 to > 9 million living species worldwide and are of critical economic importance, providing ecosystems that are beneficial to their inhabitants as well as to humans through fisheries, coastal protection, and tourism (Hoegh-Guldberg et al. 2007; Plaisance et al. 2011). Rapid human population growth and expansion accompanied by increased resource consumption has sparked concerns among scientists, government officials, and the public over the future of fisheries. Mainly due to the fact that as population augments, urbanization and economic rise increase the amount of waste, pollution and chemical runoff, and the use of destructive fishing practices that can wreak havoc and lead to the depletion of species and habitat damage in many locations (Clausen and York 2008). In addition, the compounded effect of climate change and the sometimes inappropriate institutional policies guiding fisheries and harvest sensu Finkbeiner et al. (2017) contribute to fisheries degradation. These effects have resulted in an increased deployment of artificial reefs (ARs) around the world to help buffer the effects of overfishing and habitat damage and destruction by providing additional habitat for marine life (Bohnsack et al. 1994; Clausen and York 2008).
Artificial reefs are defined as man-made structures or materials that provide abiotic and/or biotic colonization substrates for marine organisms ranging from prokaryotes and protozoa to barnacles, corals, sponges, clams, bryozoans, hydroids, etc. (Chang 1985; Jardeweski and Almeida 2006; Wiens et al. 2010). Fishermen have known for centuries that fishes congregate around sunken objects within hours of hitting the ocean floor, and evidence suggests that ARs and fish aggregating devices have been used as early as before the common era that is, since ~3000 years ago as rocks to anchor tuna nets were disposed and accumulated in the Mediterranean's bottom attracting marine invertebrates and fishes (Purpura 1989 cited in Riggio, Badalamenti, and D'Anna 2000). By 1789, ARs were used to help populate coastal fisheries (Oren 1968; Polovina 1991; Stone 1982). Primitive ARs consisted of mangrove tree branches, bamboo, tires, and concrete cubes that provided solid substrates that attracted species not usually found in soft-bottom sites (Deudero et al. 1999; Gratwicke and Speight 2005; Jardeweski and Almeida 2006; Lukens and Selberg 2004; Munro and Balgos 1995; Polovina 1991). Hard substrates are initially colonized by bacterial biofilms providing a biotic substrate for films of periphyton, algae and sessile invertebrates that function as important “habitat-formers,” and an important food source to grazing animals (Gratwicke and Speight 2005). These habitat-formers or epibenthic organisms provide subsequent biotic substrates on ARs as forage items for crustaceans, polychaetes, mollusks, and fishes (Jessee, Carpenter, and Carter 1985). Increasing the total hard surface area of a reef elevates net productivity and hence boosts the number of fishes supported (Gratwicke and Speight 2005). Thus, ARs have been used for multiple purposes ranging from recreation and diving activities fostering tourism (Lee, Otake, and Kim 2018; Lima, Zalmon, and Love 2019; Tynyakov et al. 2017), habitat restoration (Fabi et al. 2015; Komyakova et al. 2019; Lee, Otake, and Kim 2018), stock enhancement of fishes and fisheries management (Jha et al. 2022; Lee, Otake, and Kim 2018), and research (Fabi et al. 2015) among others. More recently, efforts to monitor the effectiveness of ARs and improve their design are increasing (e.g., Blount et al. 2021; Ramm et al. 2021; Vivier et al. 2021).
In the United States of America (USA), the number of permitted AR projects to mitigate loss of habitat and increase fish populations has grown exponentially since the first documented ARs in the 1830s (Bohnsack et al. 1994; Gratwicke and Speight 2005; Lukens and Selberg 2004; Polovina 1991). Most early ARs in the USA consisted mainly of scrap materials (Buchanan 1973; Lukens and Selberg 2004; Parker et al. 1974; Pybas 1997; Smith, Hensley, and Mathews 1979) until in 1989 the Texas legislature recognized the importance of these efforts and enacted the Artificial Reef Act directing Texas Parks and Wildlife Department (TPWD) to develop a state artificial plan, Texas Artificial Reef Plan (Bohnsack and Sutherland 1985; Stephan et al. 1990).
Three AR programs are used in the Texas Artificial Reef Plan. These include Rigs-to-Reef, Ships-to-Reef, and Nearshore Reefing Program (Stephan et al. 1990). The Rigs-to-Reef Program involves tipping over or sinking decommissioned petroleum platforms constructed of corrosion-resistant steel. These platforms meet the five major criteria for AR materials which include: function, compatibility, durability, stability, and availability. Together, these criteria yield high profile (i.e., vertical relief) ARs (Lukens and Selberg 2004). Typically, oil rigs support a thriving reef ecosystem while in service (Shively et al. 2003; Stephan et al. 1990). The Ships-to-Reef Program intentionally sinks decommissioned boats for the same purpose. At the time of this study, the Ships-to-Reef Program sunk 12 liberty ships, four deck barges, two tugboats, one shrimp boat, and a ~30 m Navy dive barge (Arnold 1998; Froehlich and Kline 2015). The Nearshore Reefing Program allows private citizens, organizations, and corporations to deploy stable (material remaining in its original configuration on the site), durable (resistant to the chemical and physical forces of the marine environment such as steel or concrete), and complex (have lots of spaces or openings for marine life) AR materials under TPWD's guidance (Lukens and Selberg 2004).
Given the economic and ecological importance of Red Snapper, Lutjanus campechanus (Poey, 1860) in the Gulf of Mexico, most of these ARs have been deployed specifically targeting the recovery of this species (Powers et al. 2018; Syc and Szedlmayer 2012). Lutjanus campechanus forms one of the largest fisheries in the region and are associated with natural embankments, limestone outcroppings, live bottom, and ARs (Gallaway, Szedlmayer, and Gazey 2009). This species is also observed at ARs with variable relief, with post-larval stages preferring low relief structures, moving into larger and deeper ARs as they age (Gallaway, Szedlmayer, and Gazey 2009; Gazey et al. 2008; Karnauskas et al. 2017). Because the northwestern Gulf of Mexico is broadly characterized by the rarity of hard-bottom habitat (< 0.01%) and soft sediment dominance (Mueller 2012), ARs deployed by the TPWD act as preferred L. campechanus habitat in a habitat-limited region (Froehlich et al. 2018). Numerous studies have shown L. campechanus presence, affinity, and high abundances for various types of ARs such as culverts, shipwrecks, oil platforms, concrete pyramids, etc. (Arney, Froehlich, and Kline 2017; Froehlich and Kline 2015; Froehlich, Lee et al. 2021; Froehlich, Garcia et al. 2021; Patterson et al. 2001; Powers et al. 2018; Streich et al. 2017). Most studies comparing natural to ARs have found similar community structure among the reefs but sometimes with fewer species on ARs (Carr and Hixon 1997). Nevertheless, compared to open bottom areas, ARs have been found to host up to 35 times greater biomass and fishes density (Bohnsack and Sutherland 1985).
The quantity and type of habitat structure have been shown to impact the distribution and abundance of fishes in response to different components of the habitat such as vertical relief, spatial heterogeneity and structural density that contribute to varying degrees of complexity that affect colonization, and therefore food availability and/or predation (Anderson, DeMartini, and Roberts 1989; Svane and Petersen 2001). Vertical relief refers to the height provided by the structure (Bohnsack and Sutherland 1985). Several studies have shown that most high-relief structures such as ships and rigs generally provide habitat for larger, adult fishes resulting in high predation-mortality on juveniles recruited to these habitats (Arnold 1998; Bohnsack et al. 1994; West, Buckley, and Doty 1994). As a result, most juvenile fishes (age 0 and age 1), such as L. campechanus, will usually move to available small, low-relief (1–3 m3) ARs (Syc and Szedlmayer 2012). The introduction of novel hard substrate such as ARs leads to colonization by settling larvae and spores belonging to a large number of epibenthic organisms thereby increasing the abundance of food for fishes (Anderson, DeMartini, and Roberts 1989; Svane and Petersen 2001). Therefore, incrementing complexity and heterogeneity of the area is hypothesized to increase species diversity by providing more protection per unit space, which as August (1983) indicated is advantageous especially for juvenile fishes.
Predation is one of the major factors influencing population size and structure of ecological communities, as habitat structure may interfere with effects of predation by providing individuals with higher numbers of refuges where predators are unable to reach the prey or by diminishing encounters between interacting parties (Beukers and Jones 1998). Several studies (e.g., Alvarez-Filip et al. 2009; Gratwicke and Speight 2005; Kuffner et al. 2007) mentioned rugosity of a structure or substrate, a measure of small-scale variations of amplitude in the height of a surface, as an important and significant complexity variable positively affecting fish species abundance and richness, possibly due to the increased refuge from predators. Therefore, the type of the substrate or material utilized is expected to influence the species composition and diversity of fishes in an area (Luckhurst and Luckhurst 1978). Noteworthy, recruitment in marine fishes is determined by density-dependent processes (i.e., predation, competition, growth, survival rate) during their early life stages (Fogarty, Sissenwine, and Cohen 1991). While the interactions between predator and prey can result in positive, negative or neutral interactions as a result of the population dynamics of those involved (Abrams 1987). It is often hypothesized that predators significantly can decrease the abundance of juvenile fishes attracted to ARs (e.g., Hixon and Beets 1993; Leitão et al. 2008; Valles, Kramer, and Hunte 2006). Results of these studies have practical applications in designing ARs as well as theoretical value in helping to explain the organization of reef fish assemblages as suggested by Friedlander and Parrish (1998). Further, purpose-built ARs are employed for a variety of reasons among others are designs tailoring desirable species, accounting for local sediments and hydrodynamics, increasing durability, using nontoxic or noncorrosive materials (Blount et al. 2021). Substrate-based collectors were modified by Ammann (2004) to design the standard monitoring unit for the recruitment of fishes (SMURF), which provides relative estimates of fishes' settlement without distorted post-settlement processes at an easier to collect with a reduced cost both monetary and effort-wise (Valles, Kramer, and Hunte 2006). The economic advantage to produce and deploy SMURFs was also recognized by Ammann (2004) and utilized by Arney, Froehlich, and Kline (2017) to characterize juvenile fishes of the same AR used in the present study; however, their culvert reef study had two main purposes: (1) to determine if the sampled juvenile fishes represented their adult counterparts; and (2) to determine if juvenile fishes used habitat differently due to topographic complexity and/or structural density.
The objective of this study was to deploy small, inexpensive ARs, known as SMURFs (Ammann 2004), as habitat for juvenile fishes along the northwestern Gulf of Mexico in compliance with the TPWD's Nearshore Reefing Program to understand and determine the effects of substrate type on juvenile fishes recruitment, and assess fish assemblage differences among the varying treatments (small (~10 cm) or large concrete rubble (~20 cm), oyster shells (~10 cm) and bare), throughout SMURFs reset event (see details in Section 2.3), sampling dates, and for the month of July among three consecutive years. An additional aim was to identify an optimal low-relief (< 1 m) AR substrate for the recruitment and survival of targeted species such as L. campechanus by comparing abundances and sizes.
Specific hypotheses to be tested included the following:
1. Bare SMURFs will harbor lower species richness, evenness, diversity, and abundance than treatments with concrete rubble or oyster shells given that fishes are typically found in more complex habitats. Furthermore, L. campechanus abundance will be lowest at the bare compared to SMURFs with concrete rubble or oyster shells. The size range is expected to be low given the small area of the SMURF as larger individuals do not need cover.
2. Because a reset event (detailed in Section 2.3) occurred for all SMURFs, it is expected to influence fish assemblages as the disturbance might cause some species to leave the units.
3. Fish assemblages will differ among sampling dates due to seasonal variations, spawning events, and stochastic processes, but remain similar for the month of July among the three consecutive years.
2 Methods
2.1 Study Site
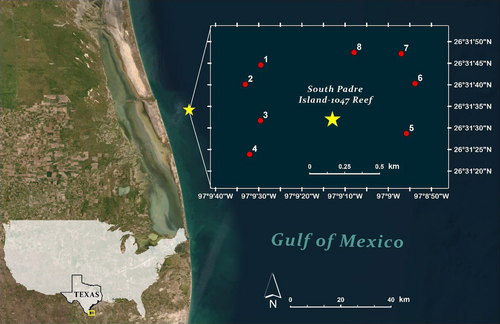
The study was conducted from July 2017 to July 2019 at the South Padre Island-1047 Reef previously known as the Port Mansfield Nearshore Reef (PS-1047, thus hereafter), located 11.3 km east of Port Mansfield, Texas (26°31′32.10″ N and 97° 9'12.91″ W, Figure 1). At the time of sampling, this region was composed of 4922 concrete culverts (1 × 3 m) ranging in density (1–190 culverts), and a sunken tugboat at a depth of 20 m deployed by TPWD and the Coastal Conservation Association of Texas (Shively et al. 2003). The bottom is flat, and the sediment is characterized generally by soft sand and mud. The site also includes four naturally occurring reefs composed of hard clay and sandstone varying in length from 5 to 200 m (Froehlich and Kline 2015).
2.2 Artificial Reef Construction and Deployment
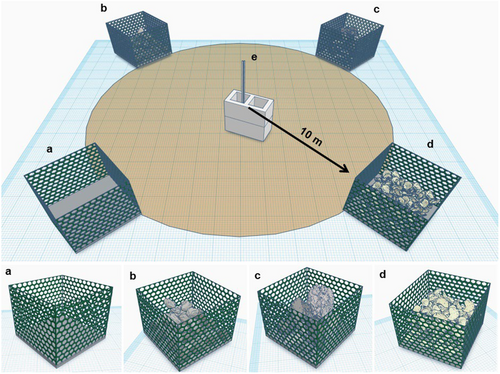
Each of the 32 SMURFs was constructed as follows: two 108 × 36 cm plastic netting with grid sizes of 4.5 × 4.5 cm were laid down perpendicular one on top of the other, a concrete base (36 × 36 cm) was then placed at the center for the purpose of weighing the unit down; the remaining net was then molded into the base and the edges were bound with nylon cable ties producing a crater-like structure with an opening at the top. The purpose of the plastic mesh was to help reduce predation but provide juvenile fishes access and prevent the non-fixed substrates from washing out. Thus, no tests were performed on potential effects of plastic mesh, which was consistent among all treatments (detailed below). The SMURFs were deployed at eight different locations around the perimeter of the PS-1047 reef (Figure 1). All sites (hereafter treatment stations) were randomly selected based on images produced by Humminbird 1198s SI side scan sonar (455 kHz, Johnson Outdoors Marine Electronics, Inc., Eufaula, Alabama) of the PS-1047 reef site to ensure a minimum distance of 160 m from any existing structure, and from one another to reduce possible movements of individuals among SMURFs (Topping and Szedlmayer 2011; Zeller, Stoute, and Russ 2003). At each location, four units along with three 18.9 L (5-gal.) buckets filled with three different types of substrates by volume, concrete rubble diameter of either ~10 or ~20 cm, and ~10 cm oyster shells were deployed. Noteworthy, the latter may provide a more suitable substrate for the fouling community to develop, and hence, provide more food for fishes (Anderson, DeMartini, and Roberts 1989; Svane and Petersen 2001). A 1.8 m fence post and two cinder blocks were also deployed at each treatment station to serve as the center point to facilitate diver orientation given the characteristic poor visibility conditions in this area of the Gulf of Mexico.
The experimental set up was based on a randomized complete block design with four distinct treatments: the two non-overlapping categories of concrete rubble diameter (~10 and ~20 cm), oyster shell and a bare substrate treatment as a control consisted of only the cement block with plastic netting. At each site, the 10 m circumference circle was divided into four quadrants to account for any variation so that the observed differences were due to true differences among treatments. The treatments were then assigned at random to a quadrant and allocated within a 360° circumference with the use of a diving compass. The four treatments were set up randomly in June 2017 at the edge of 10 m circumference circles (blocks) in barren areas around the PS-1047 reef (Figure 2).
2.3 Sampling Regime
Treatment stations were originally planned to be surveyed quarterly; however, due to unfavorable conditions this was not possible. Sampling was conducted from July 2017 to July 2019, resulting in a total of nine surveys. Field research was suspended after the first survey (July 2017) due to Hurricane Harvey, which crossed the Caribbean Sea, and into the Gulf of Mexico in August 2017 where it intensified into a category 4 strength bringing heavy rainfall (van Oldenborgh et al. 2017), and unfavorable diving conditions to the study site. The study site was visited approximately 1 month and again 3 months after the storm to determine the condition of the SMURFs, which were found to be intact; however, visibility was too low to conduct appropriate visual surveys. Surveys resumed June 2018 as weather conditions allowed to account for known recruitment cycles of commercially important species such as Lutjanus campechanus (June–August) (Froehlich, Lee et al. 2021; Glenn, Cowan, and Powers 2017). By the third survey (July 2018), the concrete base of most SMURFs had sunk below the plane of the ocean floor. After being surveyed, sunken SMURFs were pulled to the surface, cleaned, and reset. Standard monitoring units for the recruitment of reef fish whose base did not sink were cleaned in the same manner during the reset process to avoid confounding factors. A five-week period was allotted before surveys resumed. Five more surveys, weather, vessel, and divers' availability permitting, were completed in 2018; and one more in July 2019 to allow for a comparison of this month among years. Because of these adjustments, inherent modifications to data analyses were needed (detailed in Section 2.4).
Surveys were conducted by SCUBA using visual and video documentation, where two divers entered the water. The first diver (surveyor) was equipped with a slate, pencil, and a data sheet to conduct a visual SCUBA fishes survey following the Bohnsack and Bannerot (1986) stationary sampling method with some modifications. The second diver carried a GoPRO HD Hero 3+ camera to conduct the video documentation and reels to attach to the center post and conduct a 10 m sweep to locate each SMURF at each treatment station. Upon encountering each SMURF, both divers recorded adult and juvenile fishes surrounding the unit coming from opposite positions and rotating clockwise as they approached the SMURF. The survey lasted around 2–3 min depending on the time needed to count all fishes present. The surveyor would then investigate the SMURF through the opening at the top to record hidden fishes and remove excess algal growth that impairs visibility within the unit. All fishes observed were included in the analyses. Video documentation was then analyzed to confirm fish counts by SCUBA surveys. Additionally, Lutjanus campechanus approximate age and size (total length: TL) were recorded from the videos using the mesh surrounding the SMURF as a scale. Age/size life stages were assigned based upon data from Gallaway, Szedlmayer, and Gazey (2009) as follows: (a) settlement stage: age 0 (26–30 days old, < 20 mm TL), (b) post-settlement stage: age 0 (30–66 days old, 20–50 mm TL), (c) post-recruit stage: age 0–1 (> 67–365 days old, 50–200 mm TL), and (d) adult stage: age 2 (2 years old, > 200 mm TL).
2.4 Statistical Analyses
Species abundance, richness, evenness, and diversity were estimated using the Shannon-Wiener Diversity and Pielou's Indices generated by the diversity routine in PRIMER-E (v7). Data were tested for normality using Kolmogorov–Smirnov test and for homoscedasticity using Levene's test to meet parametric analyses assumptions (Sokal and Rohlf 2012). A square root transformation was applied to data that failed the normality test which did not offset the distribution. Nonetheless, species indices were analyzed independently using a one-way analysis of variance (ANOVA) followed by Tukey post hoc test to identify where any difference(s) among substrate treatments lie with untransformed data because ANOVAs are robust to violations of normality (Underwood 1997).
For further analyses (nonparametric tests), a square root transformation was applied to all the data to increase the influence of less abundant species and stabilize abundant species, and a dummy variable was added to include samples with no species observed (Clarke et al. 2014; Verdiell-Cubedo et al. 2012). Bray–Curtis similarity index was constructed from the transformed data, and a non-metric multidimensional scaling (NMDS) using 100 iterations of bootstrap averaging with 95% confidence ellipses was applied to this matrix to visualize differences in reef community assemblages among dates and treatments. The default in PRIMER-E (v7) is 50 iterations, but according to Clarke et al. (2014) and Clarke and Gorley (2015) doubling that number ensures a near-optimal solution. It also mentions that if the same (lowest) stress value is obtained over 50% of the time, which was the case, it is very unlikely to be improved by further iterations. Furthermore, three independent two-way analysis of similarity (ANOSIM) were performed on the Bray–Curtis similarity matrices to compare reef community assemblages from surveys done before, during and after resetting the SMURFs, surveys done within the year of 2018; and the surveys done in the month of July across the years with an a priori null hypothesis of no differences among dates or treatments. The ANOSIM generates an R statistic that quantifies the extent of segregation between groups and a p value indicating the significance of the difference observed. The R statistic ranges from zero to one, where the closer the value to one indicates groups are similar and the closer to zero indicates groups are different (Clarke and Warwick 2001). The similarity percentages test (SIMPER) was also applied to determine which species drove dissimilarities among treatments and dates (Clarke and Warwick 2001; Clarke et al. 2014).
Lutjanus campechanus abundances and size (life stage) comparisons among treatments and dates were evaluated separately using one-way ANOVAs. If significant differences occurred, Tukey post hoc test results were performed. All statistical analyses were performed at an α level of 0.05 using the IBM SPSS statistical package (v25.0) and the PRIMER-E (v7) (Clarke and Gorley 2015; Clarke and Warwick 2001; Clarke et al. 2014).
3 Results
A total of 5238 individual fishes consisting of 39 juvenile reef species across 23 families were surveyed across all sites (Table 1). Out of the 23 families, those from the family Lutjanidae dominated (48.85%) followed by fishes from Serranidae (16.51%), Sciaenidae (12.49%), and Haemulidae (11.76%), contributing to almost 90% of the taxa. The dominant species in each of these families were Lutjanus campechanus (45.67%), Diplectrum bivittatum (6.05%), Pareques umbrosus (12.49%), and Haemulon aurolineatum (10.35%), respectively (Table 1). Out of the 39 species, nine were only observed thrice or less. These rare species included Myripristis jacobus, Ocyurus chrysurus, Cantherhines pullus, Stegastes partitus, Synodus foetens, Anisotremus virginicus, Holocentrus adscensionis, Brotula barbata, and Rachycentron canadum (Table 1).
| Family | Species | Authority | Common name | B (n) | O (n) | S (n) | L (n) | N | % |
|---|---|---|---|---|---|---|---|---|---|
| Apogonidae | Apogon maculatus | (Poey, 1860) | Flamefish | 4 | 0 | 0 | 0 | 4 | 0.08 |
| Balistidae | Balistes capriscus | Gmelin, 1789 | Gray Triggerfish | 7 | 33 | 68 | 62 | 170 | 3.25 |
| Batrachoididae | Opsanus beta | (Goode & Bean, 1880) | Gulf Toadfish | 7 | 12 | 14 | 12 | 45 | 0.86 |
| Blenniidae | Ophioblennius macclurei | (Silvester, 1915) | Redlip Blenny | 1 | 2 | 2 | 1 | 6 | 0.11 |
| Parablennius marmoreus | (Poey, 1876) | Seaweed Blenny | 2 | 7 | 2 | 1 | 12 | 0.23 | |
| Carangidae | Seriola dumerili | (Risso, 1810) | Greater Amberjack | 0 | 0 | 0 | 8 | 8 | 0.15 |
| Seriola lalandi | Valenciennes, 1833 | Yellowtail Jack | 10 | 1 | 0 | 1 | 12 | 0.23 | |
| Chaetodontidae | Chaetodon sedentarius | Poey, 1860 | Reef Butterflyfish | 0 | 1 | 2 | 5 | 8 | 0.15 |
| Ephippidae | Chaetodipterus faber | (Broussonet, 1782) | Atlantic Spadefish | 15 | 1 | 25 | 1 | 42 | 0.80 |
| Gobiidae | Gobiosoma robustum | Ginsburg, 1933 | Code Goby | 0 | 1 | 3 | 0 | 4 | 0.08 |
| Haemulidae | Anisotremus virginicus | (Linnaeus, 1758) | Porkfish | 0 | 2 | 0 | 1 | 3 | 0.06 |
| Haemulon aurolineatum | Cuvier, 1830 | Tomtate | 77 | 90 | 236 | 139 | 542 | 10.35 | |
| Orthopristis chrysoptera | (Linnaeus, 1766) | Pigfish | 10 | 24 | 26 | 11 | 71 | 1.36 | |
| Holocentridae | Holocentrus adscensionis | (Osbeck, 1765) | Squirrelfish | 0 | 0 | 2 | 1 | 3 | 0.06 |
| Myripristis jacobus | Cuvier, 1829 | Blackbar Soldierfish | 0 | 0 | 0 | 1 | 1 | 0.02 | |
| Labridae | Halichoeres bivittatus | (Bloch, 1791) | Slippery Dick | 25 | 10 | 11 | 2 | 48 | 0.92 |
| Lutjanidae | Lutjanus campechanus | (Poey, 1860) | Red Snapper | 479 | 583 | 689 | 641 | 2392 | 45.67 |
| Lutjanus griseus | (Linnaeus, 1758) | Gray Snapper | 0 | 13 | 0 | 0 | 13 | 0.25 | |
| Lutjanus synagris | (Linnaeus, 1758) | Lane Snapper | 23 | 56 | 45 | 29 | 153 | 2.92 | |
| Ocyurus chrysurus | (Bloch, 1791) | Yellowtail Snapper | 0 | 1 | 0 | 0 | 1 | 0.02 | |
| Monacanthidae | Cantherhines pullus | (Ranzani, 1842) | Orangespotted Filefish | 1 | 0 | 0 | 0 | 1 | 0.02 |
| Stephanolepis hispida | (Linnaeus, 1766) | Planehead Filefish | 2 | 2 | 1 | 4 | 9 | 0.17 | |
| Ophidiidae | Brotula barbata | (Bloch & Schneider, 1801) | Bearded Brotula | 0 | 0 | 1 | 2 | 3 | 0.06 |
| Ostraciidae | Lactophrys trigonus | (Linnaeus, 1758) | Buffalo Trunkfish | 3 | 3 | 1 | 0 | 7 | 0.13 |
| Pomacanthidae | Holacanthus bermudensis | Goode, 1876 | Blue Angelfish | 1 | 3 | 7 | 5 | 16 | 0.31 |
| Pomacanthus paru | (Bloch, 1787) | French Angelfish | 3 | 2 | 8 | 12 | 25 | 0.48 | |
| Pomacentridae | Stegastes partitus | (Poey, 1868) | Bicolor Damselfish | 1 | 0 | 0 | 0 | 1 | 0.02 |
| Stegastes variabilis | (Castelnau, 1855) | Cocoa Damselfish | 0 | 5 | 1 | 0 | 6 | 0.11 | |
| Priacanthidae | Pristigenys alta | (Gill, 1862) | Short Bigeye | 4 | 2 | 2 | 0 | 8 | 0.15 |
| Rachycentridae | Rachycentron canadum | (Linnaeus, 1766) | Cobia | 0 | 0 | 0 | 3 | 3 | 0.06 |
| Sciaenidae | Pareques umbrous | (Jordan & Eigenmann, 1889) | Cubbyu | 130 | 155 | 166 | 203 | 654 | 12.49 |
| Scorpaenidae | Scorpaena plumieri | Bloch, 1789 | Spotted Scorpionfish | 22 | 38 | 16 | 14 | 90 | 1.72 |
| Serranidae | Centropristis philadelphica | (Linnaeus, 1758) | Rock Sea Bass | 53 | 49 | 91 | 65 | 258 | 4.93 |
| Diplectrum bivittatum | (Valenciennes, 1828) | Dwarf Sand Perch | 106 | 53 | 99 | 59 | 317 | 6.05 | |
| Hyporthodus nigritus | (Holbrook, 1855) | Warsaw Grouper | 1 | 0 | 1 | 2 | 4 | 0.08 | |
| Rypticus maculatus | Holbrook, 1855 | Whitespotted Soapfish | 11 | 24 | 22 | 19 | 76 | 1.45 | |
| Serranus subligarius | (Cope, 1870) | Belted Sandfish | 27 | 90 | 59 | 34 | 210 | 4.01 | |
| Synodontidae | Synodus foetens | (Linnaeus, 1766) | Inshore Lizardfish | 0 | 0 | 1 | 0 | 1 | 0.02 |
| Tetraodontidae | Sphoeroides spengleri | (Bloch, 1785) | Bandtail Puffer | 3 | 2 | 4 | 2 | 11 | 0.21 |
| Total | 1028 | 1265 | 1605 | 1340 | 5238 |
- Note: Number of fish observed (n) across the different treatments: B = bare or no substrate, O = oyster shells (~10 cm), S = small concrete rubble (~10 cm), L = large concrete rubble (~20 cm). Total number of fish (N) and percentage (%).
3.1 Community Indices
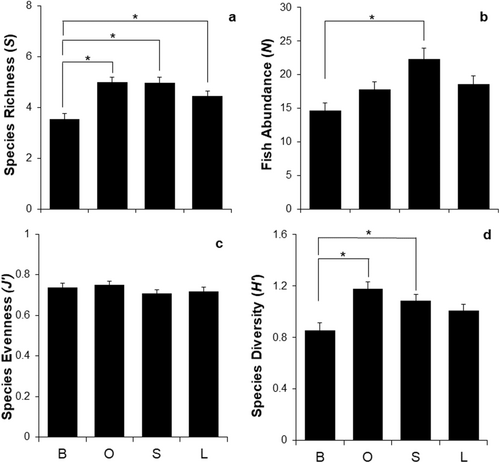
The average species richness (± standard error (SE)) was significantly different across the different substrate treatments (ANOVA: F3,281 = 10.014, p < 0.001). Species richness increased from bare (3.54 ± 0.2), to large (4.46 ± 0.2), to small (4.96 ± 0.2), and to oyster (4.99 ± 0.2). On two occasions SMURFs with bare substrate and once SMURF with oyster shells substrate could not be found thereby affecting sample size. Tukey post hoc analysis revealed that the mean increase from the bare to oyster substrate (1.45, 95% Confidence Intervals (CI) [0.66, 2.22]) was statistically significant (p = 0.001), as well as the increase from bare to small (1.42, 95% CI [0.64, 2.19], p = 0.001), and even from bare to large (0.92, 95% CI [0.14, 1.69], p = 0.013, Figure 3a). One-way ANOVA of average fish abundance showed significant differences among substrates (F3,281 = 5.680, p < 0.001, Figure 3b). Species abundance increased from bare (14.69 ± 1.1), to oyster (17.82 ± 1.1), to large (18.61 ± 1.2), to small (22.29 ± 1.7). Tukey post hoc analysis revealed that the mean increase from the bare to small rubble substrate (7.60, 95% CI [2.81, 12.40]) was the only significantly different treatment (p = 0.001; Figure 3b). No significant differences in species evenness (F3,266 = 0.944, p = 0.420, Figure 3c) were found across substrate treatments; however, highest species evenness was observed at the SMURFs with the oyster treatment (0.75 ± 0.02) and the lowest at the SMURFs containing small rubble (0.71 ± 0.02). The change in degrees of freedom from 281 to 266 was due to the omission of samples that did not yield an evenness value because of species richness values equating to zero or one. Average species diversity also showed significant differences among treatments (F3,281 = 6.606, p < 0.001, Figure 3d). There was a significant mean increase from bare to small rubble (0.23, 95% CI [0.04, 0.43], p = 0.012), and even a greater increase to the oyster treatment (0.33, 95% CI [0.13, 0.52], p = 0.001).
3.2 Reset Event and 2018 Temporal Variation Among SMURF Substrates
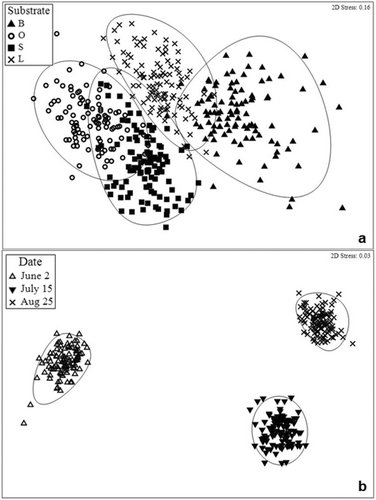
The NMDS ordination comparing fish assemblages before, during and after the SMURF reset event revealed clustering of samples with a stress value of 0.03 indicating fish assemblages present among these dates were different; however, overlapping clusters were evident among treatments indicating fish assemblages were quite similar, stress value of 0.16 (Figure 4). The two-way crossed ANOSIM revealed no significant differences in fish assemblages among substrate treatments (R = 0.037, p = 0.077). However, significant differences among reset event dates (p = 0.0001) were apparent, a low R value (R = 0.336) indicated date did not have a great effect on the fish assemblages overall. Despite the low R value for the significant differences present among reset event dates as per the ANOSIM, the visualization separation (Figure 4b) was further investigated with the pairwise tests indicating that before (June 2nd) and after (August 25th) the reset event showed somewhat different fish assemblages (R = 0.485, p = 0.0001). The SIMPER analysis also indicated the greatest dissimilarity between these two dates (68.91%) and identified the top three species that were most responsible for this distinction: Diplectrum bivittatum (20.06%), Lutjanus campechanus (17.97%), and Pareques umbrosus (14.90%). Although no significant differences are present among substrates as per the ANOSIM, pairwise test indicated that the bare and small rubble also displayed the greatest dissimilarity (59.80%) among treatments sampled throughout the reset event. The species responsible for this difference were L. campechanus (23.88%), D. bivittatum (16.31%), and Centropristis philadelphica (8.95%). The bare differed from the large rubble treatment by 59.25% and from the oyster shells by 57.82%. The top two species contributing the most to these dissimilarities were L. campechanus and D. bivittatum.
The ordination through the NMDS revealed groupings of samples according to the type of substrate treatment, and the date sampled on fish assemblages for the year 2018 yielding stress values of 0.14 and 0.06, respectively (Figure 5). The two-way crossed ANOSIM test based on Bray–Curtis similarity matrix revealed no significant differences in fish assemblages among substrate (R = 0.023, p = 0.103). However, significant differences among dates (p = 0.0001) were evident, but a low R value (R = 0.313) indicated that dates did not have a great effect on the fish assemblages overall. The SIMPER analysis identified which substrate treatment and dates differed the most and identified the species that caused those discrepancies. Although no significant differences were indicated by ANOSIM values, the bare and small rubble treatment displayed the greatest dissimilarity (55.39%) among treatments sampled within the year 2018. The species that contributed the most to this difference were Lutjanus campechanus (21.20%), Haemulon aurolineatum (9.46%), and Pareques umbrosus (9.15%). The bare treatment differed from the oyster shell by 54.96% and from the large rubble treatment by 54.03%. The greatest dissimilarity among dates occurred between June 2nd and September 9th (71.55%) with Diplectrum bivittatum, L. campechanus, and P. umbrosus contributing the most to this difference (17.93%, 16.17%, and 12.81%, respectively).
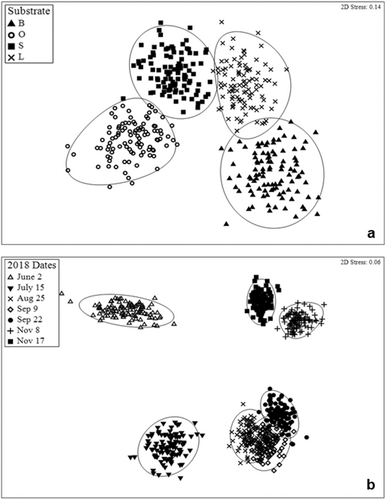
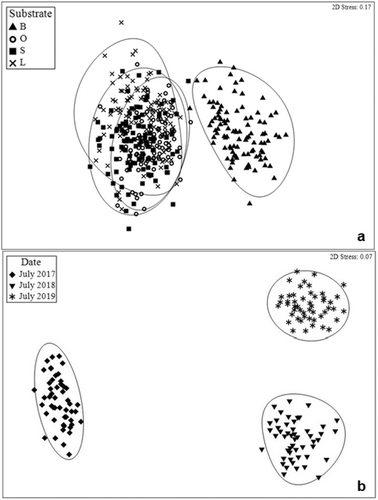
3.3 SMURF Treatments Sampled in July Over Three Consecutive Years
The NMDS ordination comparing fish assemblages in July across the 3 years sampled (i.e., 2017, 2018, and 2019) revealed distinct fish assemblages with no overlaps across the years, but among substrate it showed overlapping groupings meaning no differences among substrate treatments, except for bare (Figure 6). Two-way crossed ANOSIM confirmed separations among fish assemblages throughout the years for the month of July (R = 0.537, p = 0.0001) and that no differences in fish assemblages were evident among substrate treatments (R = 0.033, p = 0.101). Pairwise tests showed fish assemblages in the years 2017 and 2019 differed most significantly (R = 0.753, p = 0.0001), 2017 and 2018 followed (R = 0.666, p = 0.0001). Even though fish assemblages between 2018 and 2019 were significantly different (p = 0.0001), the R value was low (R = 0.212) indicating that the factor (year) had a small effect on the fish assemblages. The SIMPER analysis identified the species that typified each group and those that distinguished between factor levels. The average dissimilarity between 2017 and 2018 was 75.55% with Lutjanus campechanus contributing most to the dissimilarity (15.93%) followed by Lutjanus synagris (11.79%), and Centropristis philadelphica (11.54%). The average dissimilarity between 2017 and 2019 was 76.54% with greatest dissimilarity contribution from Pareques umbrosus (19.13%), followed by L. campechanus (16.20%), and L. synagris (12.57%). The average dissimilarity between 2018 and 2019 was 58.42% because of P. umbrosus (18.70%), L. campechanus (18.10%), and C. philadelphica (14.12%).
3.4 Lutjanus campechanus Abundances and Size Comparisons
A total of 2392 Lutjanus campechanus were observed throughout the SMURFs (Table 1). Lutjanus campechanus abundances did not significantly differ among the substrate treatments (ANOVA: F3,281 = 1.788, p = 0.2, Figure 7a); however, the highest abundance was observed at the SMURFs with the small rubble treatment (9.57 ± 0.87) followed by the large rubble treatment (8.90 ± 0.94). The SMURFs containing the bare treatment yielded the lowest abundances of L. campechanus (6.84 ± 0.76).
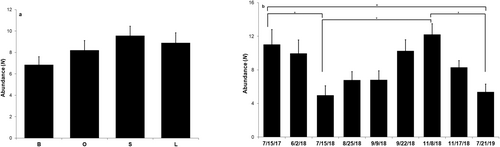
The average Lutjanus campechanus abundances showed significant differences across the different sampling dates (ANOVA: F8,276 = 4.262, p < 0.001, Figure 7b). The change in degrees of freedom from 281 to 276 was due to a change in number of parameters from four substrate treatments to the nine different sampling dates. Tukey post hoc analysis revealed that the average L. campechanus abundance decreased significantly from July 2017 (n = 32, 11.03 ± 1.78) to July 2018 (n = 32, 4.97 ± 1.14) by a mean difference of 6.06 (95% CI [0.55, 11.57], p = 0.019) and to July 2019 (n = 32, 5.38 ± 0.92) by 5.65 (95% CI [0.14, 11.17], p = 0.039). A significant L. campechanus abundance increase was evident from July 2018 to November 8th of the same year (n = 31, 12.23 ± 1.27) by a mean difference of 7.26 (95% CI [1.70, 12.81], p = 0.002, Figure 7b), and an abundance decrease from November 8th to July 2019 (6.85, 95% CI [1.29, 12.41], p = 0.004).
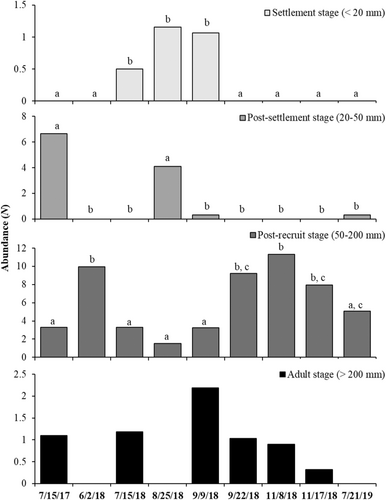
Total lengths of Lutjanus campechanus ranged from approximately 15 mm up to 300 mm. No significant differences among life stages could be discerned across substrate treatments (ANOVAs: F3,281 = 2.139, p = 0.095, F3,281 = 0.582, p = 0.628, F3,281 = 0.669, p = 0.572, F3,281 = 0.860, p = 0.462, for < 20 mm, 20–50 mm, 50–200 mm, and > 200 mm, respectively, Figure 8). Lutjanus campechanus < 20 mm abundances increased from large (0.15 ± 0.09), to bare (0.17 ± 0.09), to small rubble (0.21 ± 0.12), and to oyster (0.69 ± 0.31). The highest abundance for individuals between 20 and 50 mm TL was observed at the SMURFs with the large rubble treatment (1.51 ± 0.50). Individuals between 50 and 200 mm and > 200 mm had the highest abundances in the small rubble treatment (6.67 ± 0.75, 1.19 ± 0.50, respectively).
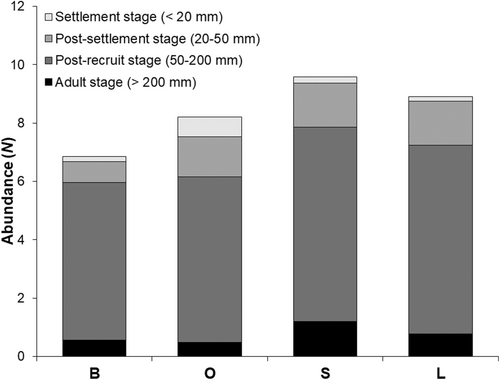
Individuals < 20 mm TL showed significant differences across the sampling dates (ANOVA: F8,276 = 3.639, p = 0.001) as they were only seen from July through the beginning of September of 2018 (Figure 9). The highest abundances were found on August 25th (1.16 ± 0.64). Individuals from 20 to 50 mm TL also showed significant differences across the sampling dates (F8,276 = 15.069, p = 0.001). July 2017 (6.66 ± 1.57) and August 2018 (4.09 ± 0.97) displayed the highest mean abundances that differed from the rest of the dates but not between them (Figure 9). Individuals 50–200 mm TL were found across all sampling dates and differed among them (F8,276 = 13.726, p = 0.001). November 8th of 2018 yielded the highest mean abundance of this size range (11.32 ± 1.10) followed by June 2nd of the same year (9.97 ± 1.60) (Figure 9). No significant differences were found across sampling dates for individuals > 200 mm TL even though Lutjanus campechanus were not observed on three dates (F8,276 = 2.090, p = 0.067; Figure 9).
4 Discussion
4.1 Species Differences Among Substrates
As expected, the largest differences in both recruitment and species composition occurred in SMURFs with a type of substrate and those without it (bare). Species richness, abundance and diversity differed significantly among the variety of substrates; the oyster shell treatment yielded higher species richness and diversity and the small rubble treatment yielded the highest species abundance (Figure 3). Various studies such as Luckhurst and Luckhurst (1978) suggest that reefs with different crevice sizes offer shelter to numerous body shapes and sizes, increasing species richness which would be the case for the oyster shell and small rubble treatment when compared to the large rubble. Although all the SMURFs were surrounded by a plastic mesh, the way the large rubble would lay on top of each other did not offer much protection for small, cryptic species such as those from the family Blenniidae (Ophioblennius macclurei, Parablennius marmoreus) and Gobiidae (Gobiosoma robustum). These species were most abundant in the oyster shell and small rubble treatment (Table 1), and most divers described seeing them peeking out from within small crevices in between the substrate. In fact, most were often missed by survey divers but were fortunately caught on video. Even Lutjanus campechanus < 20 mm also had the highest abundance in the oyster shell and small rubble treatments (Figure 8) further confirming the idea that smaller fish preferred these types of substrates due to the variety of hole sizes. This concurs with studies done by Gratwicke and Speight (2005) which constructed several ARs in the British Virgin Islands to investigate the effects of habitat complexity on fish assemblages and found out that hard substrate and refuge crevices were key factors influencing species richness, and that increasing the number of small reef holes increased fish abundance on reefs. Moreover, Arney, Froehlich, and Kline (2017) reported SMURFs had higher diversity indices of juvenile fishes than those gathered by visual SCUBA surveys on culvert reefs in PS-1047 because of the cryptic nature of the studied fishes. While low-relief ARs in a neighboring and more recent AR had higher species richness, frequencies, and abundances of juvenile fishes than unconsolidated sediment (Dance et al. 2021).
Other species, such as Opsanus beta, Anisotremus virginicus, Haemulon aurolineatum, Holocentrus adscensionis, Lutjanus griseus, Lutjanus synagris, Lactophrys trigonus, Stegastes variabilis, Centropristis philadelphica, Rypticus maculatus, and Serranus subligarius, were mostly seen as juveniles, and as a result were shorter in height and most commonly observed in the oyster and small rubble treatment as well. Those who were vertically longer (height) such as the Pareques umbrosus, which have a long sailfin like dorsal fin as juveniles, were found across all treatments but most abundantly in the large rubble treatment given the crevices were much larger and accommodated to their anatomy. Balistes capriscus, also with a deep highly compressed body shape, was most commonly found in the small rubble treatment followed by the large rubble (Table 1). Species from the family Pomacanthidae (Holacanthus bermudensis and Pomacanthus paru) were found across all substrate treatments but were most abundant in either the small or large rubble treatment (Table 1), possibly showing preference to the type of substrate rather than size of the crevices. Chaetodon sedentarius, sister taxon to Pomacanthidae (Bellwood, van Herwerden, and Konow 2004) (juveniles < 50 mm), were also found more abundant in the large rubble treatment. These findings confirm the need to use different substrates to aid in the recruitment of species that benefit from the provided habitat by ARs, particularly in areas like the northwestern Gulf of Mexico, and could be used by TPWD in the continued and further establishment of ARs in the Texas coast.
Overall, the fish assemblages captured in SMURFs consisted mainly of juveniles compared to those usually found in intermediate to high relief structures indicating that either predation protection, structural complexity, sites lacking any structure in a 160 m radius is a driving factor for these species to settle on these SMURFs. Because enhancing fisheries is one of the goals of the TPWD Artificial Reef Program, it is here recommended to continue developing other designs of low-relief to be allocated in between existing medium-and high-relief (i.e., > 1 m) ARs that should serve as stepping stones for the survival of species of fisheries interest (e.g., Lutjanus campechanus). The distance from low-relief ARs to higher ones is a significant factor that must be accounted for and further studied. As Arney, Froehlich, and Kline (2017) observed and was found here, Warsaw Grouper (Hyporthodus nigritus) and Yellowedge Grouper (Hyporthodus flavolimbatus (Poey, 1865)) exhibited a preference for areas within a 30-m radius away from any culvert reef with significant structure. Additionally, it is important to bear in mind that the designs of purpose-built ARs should be tailored to the specific target species, as highlighted by Blount et al. (2021), and as indicated here by the possible preferential use fishes had for different AR substrates. For instance, Holacanthus bermudensis, Pomacanthus paru, and Chaetodon sedentarius might favor either small or large rubble ARs potentially due to the difference in concrete texture as compared to the oyster shells, which were preferentially used by another suite of fishes. Alternatively, these substrates hosted different fouling assemblages yielding different interactions among its users; however, that is subject for a different study, and not discussed any further.
As previously mentioned, predators play an important role in structuring ecological communities and have consistently shown negative effects of predators on abundance, species richness and recruitment (Beukers and Jones 1998; Webster 2002). For example, Caley (1993) periodically removed predatory species on small, ARs and found that species richness and total abundance were generally greater than those in which predators were not removed. Carr and Hixon (1995) also removed piscivore species from isolated coral patch reefs and found that Azurina cyanea (Poey, 1860) and Halichoeres pictus (Poey, 1860) were significantly greater on reefs where resident predators had been removed. While in Portugal a higher natural mortality of demersal young of the year or juvenile fishes may have been the cause for an increase in predator–prey interactions at the Algarve ARs (Leitão et al. 2008). In this study, the presence of predators might have affected the abundance for the rest of the species. Arney, Froehlich, and Kline (2017) noted that increments in structural density measured by a variable number of culverts were inversely related to community indices (i.e., species richness, evenness, diversity, and abundance) of juvenile fishes but recognized that some of that difference could be attributed to higher abundance of predators, in alignment with the denser culvert locations of Froehlich and Kline (2015). In the present study, Scorpaena plumieri was found across all substrate treatments being most abundantly at SMURFs with the oyster shell substrate and the least at the small and large rubble (Table 1). Abundance of the remaining species was highest for the small and large rubble treatments and the least at the bare and oyster treatments suggesting S. plumieri could possibly be a potential cause for these differences, but this hypothesis requires further examination.
4.2 Differences Among July Over Three Consecutive Years
No significant month-to-month variations regarding fish assemblages in the year 2018 were recorded, regardless of the SMURF reset event, seasonal patterns due to water temperature, or spawning events that as per Gallaway, Szedlmayer, and Gazey (2009) could influence and affect species composition. The only significant differences regarding fish assemblages occurred across the various years of the month of July, most likely due to the large-scale climate event, Hurricane Harvey, that happened not too long after the first survey (July 2017). From July 2017 to July 2018, Lutjanus campechanus decreased from an average abundance of 11.03 to 4.97, having the greatest mean difference between these 2 years (Figure 7b). Lutjanus synagris also decreased between the years from 3.19 to 0.09 average abundance, whereas Centropristis philadelphica increased from 0.03 to 3.03. Both Lutjanidae species, L. campechanus and L. synagris have very similar spawning periods from April to September and peaking during June to August (Froehlich, Lee et al. 2021; Gallaway, Szedlmayer, and Gazey 2009; Luckhurst, Dean, and Reichert 2000). Centropristis philadelphica, on the other hand, spawns from January to April showing preference to spawn in colder water temperatures (Herrema et al. 1985). A drop in water temperature did occur from July 2017 (25.5°C) to July 2018 (23.4°C) but perhaps not a sufficient amount to explain why both Lutjanidae species decreased and C. philadelphica increased in abundance. It is more reasonable to suggest Hurricane Harvey could have caused Lutjanidae species to disperse/move from the unit and/or interannual variations of recruitment, spawning success, and changes in water currents. Something similar happened to Watterson et al. (1998) in their mark and recapture study on L. campechanus; a few months after the start of their study, the eye of Hurricane Opal passed 40 km within the AR sites located 20–32 km south of Mobile Bay, Alabama and fish demonstrated greater movement during the hurricane covering distances over 100 to 200 km. Hurricane Opal affected not only the movement of L. campechanus but the site fidelity of the fish which is generally high even to ARs (e.g., Froehlich, Garcia, and Kline 2018; Strelcheck, Cowan, and Patterson 2007). An even greater difference in mean abundance was recorded between July 2017 and July 2019 when Pareques umbrosus counts increased from 0.16 to 6.81, L. campechanus decreased by 5.66 mean difference (Figure 7b), and L. synagris numbers decreased to zero. During this window of time, the spawning success of P. umbrosus could have increased, which are known for their high spawning frequency and high relative fecundity (Holt and Riley 1999).
4.3 Lutjanus campechanus Abundances and Size Comparisons
In the present study, Lutjanus campechanus abundance did not vary significantly among substrate treatments despite length sizes as these were found across all treatments. This coincides with the notion that L. campechanus quickly settle over any hard substrates displaying an attraction to low-relief habitat that provides adequate shelter and protection (Gallaway, Szedlmayer, and Gazey 2009; Watterson et al. 1998; Wells and Cowan 2007). The small and large rubble treatments reported the highest abundance values and as expected, the bare treatment yielded the least, which provided little protection compared to the others (Figure 7a). Similarly, excluding their control, Dance et al. (2021) found that juvenile L. campechanus abundances were significantly lower in unconsolidated sediments (i.e., control) than in either oyster shell, 7–13 cm limestone rubble, or composite (consisting of a concrete base with a mixture of oyster shells and limestone) treatments, which were not different from one another.
Treatments separated by age/size did provide some insight as to how L. campechanus utilized them throughout its life cycle. Individuals < 20 mm TL appeared in higher abundances at the oyster shell treatment and were only seen throughout the months of July through September coinciding with peak spawning months of L. campechanus (June–August) (Froehlich, Lee et al. 2021; Glenn, Cowan, and Powers 2017). Newly settled Lutjanus campechanus abundances were ca. thrice in oyster than in limestone experimental reefs and were higher in July than August 2017 within the then recently established AR area near PS-1047 (Dance et al. 2021). Various studies have demonstrated relic-shell habitat such as oyster shells offer primary nursery habitat for new settlers (juveniles) due to the smaller hole sizes provided by these shells (Gallaway, Szedlmayer, and Gazey 2009; Lingo and Szedlmayer 2006). Highest abundance of L. campechanus of increased size/age (> 20–300 mm TL) settled at the small and large rubble treatment given that crevices were much larger in size. The most abundant individuals throughout the treatments and sampling dates were those age 0–1 indicating that these SMURFs were essential habitat at this stage in their lives. Most individuals sized 2 > 200 mm TL, better characterized as subadults because none were greater than 300 mm TL, were found roaming around the SMURFs rather than entering and exiting the structure despite that the plastic netting was open at the top. These subadults had the lowest abundances at the oyster shell treatment coinciding with the concept that as fish size increases the fish require and seek structured habitat with larger hole size (Szedlmayer and Lee 2004). Moreover, these subadults might have been foraging for food from their actual permanent residence given that studies have reported adults from age 2–7 to feed on smaller fishes, crabs, and shrimps from the surrounding areas of their habitat (Gallaway, Szedlmayer, and Gazey 2009). In this study, divers reported the presence of shrimps, crabs, sea urchins and other organisms in or around the SMURFs across all substrates which was not the case in the barren ocean floor. Given that age 2 L. campechanus, at least smaller than 300 mm TL, did not show significant differences among treatments nor dates (Figures 8 and 9), suggests their presence was more stochastic. Perhaps these individuals were traveling from nearby sites, such as the culverts or natural patch reefs present at the PS-1047 reef, in search of food as there might have been excessive competition with older individuals as well as added pressures from predatory species at those sites.
5 Conclusions
In summary, all treatments recruited reef-associated species. Moreover, fish assemblage captured in SMURFs consisted mainly of juveniles compared to those usually found in intermediate to high relief structures indicating that either predation protection, structural complexity, and/or sites lacking any structure in a 160 m radius and/or a combinations of these factors is a driving factor for these species to settle on these SMURFs. Species richness and diversity were highest at SMURFs with the oyster shell substrate followed by the small and large rubble. In terms of fish abundance, the small rubble substrate yielded the highest value. As expected, SMURFs with no substrate (bare treatment) had the lowest values indicating that plastic netting was not optimal to recruit and provide suitable habitat for most juvenile species. There were no effects on fish assemblages after resetting the SMURFs regardless of the disturbance. Seasonal fish assemblage variations were not seen across 2018 dates. However, reef fish assemblages were different among the summers, and this could be attributed to Hurricane Harvey and/or perhaps variations in recruitment and spawning success in subsequent years as well as changes in water currents. Lutjanus campechanus was the most abundant species across all treatments and as expected lowest at the bare treatment. Most L. campechanus individuals were between 50 and 200 mm TL suggesting SMURFs were more suitable for smaller individuals to thrive. This species was also observed across all treatments but depending on their current life stage generally preferred the oyster shell substrate as they began to settle from the water column, and as they grew older, they preferred small or large rubble substrate. These findings suggest that oyster shells and small rubble substrate could be the most effective types of substrates to deploy especially during the peak spawning months of L. campechanus to provide essential habitat for these individuals to settle and use as protection as they grow before moving to much larger reefs. If these substrates are deployed using SMURFs, it would be necessary to include some mechanism to avoid SMURFs from sinking; perhaps adding friction pilings which rely on the friction between the sediment and the pole, like those used for construction when the ground is soft, could prevent SMURFs from sinking below the plane of the seafloor (Hansbo 1984).
Predators such as Scorpaena plumieri were observed at SMURFs in some cases even inside the SMURFs, lying on top of the substrate. A study monitoring the effects of caging effects on fish recruitment at One Tree Island in the Great Barrier Reef indicated that plots with completely closed off cages, rather than partial cages or open plots, always harbored more recruits (Doherty and Sale 1986). Completely closing the plastic netting on SMURFs could have added protection to juveniles from S. plumieri or other piscivorous species. However, Arney, Froehlich, and Kline (2017) used completely enclosed SMURFs and reported similar results. Additionally, assessing the effects the invertebrate community present including crabs, sea urchins, octopuses (which were occasionally found inside or under the SMURFs), sponges or even algal growth have on the recruitment of fishes could provide more insights.
Nevertheless, finding commercially important species as juveniles at sites lacking significant structure suggests that fisheries management may benefit from producing ARs with small, nontoxic, and inexpensive substrates distant from larger structures where juveniles can recruit and grow, and thus enhance desired fish stocks. Implementing the results from this study may also help increase abundance and potentially contribute to the establishment and maintenance of recreational fisheries and diving activities. Moreover, monitoring information regarding the performance of the study overall and its constituent features will be highly useful to individuals designing current and future AR projects with similar goals.
Acknowledgments
This study was funded by the Texas Parks and Wildlife Department (TPWD) Artificial Reef Program (contract # 475342) for which we are very thankful. The first author also acknowledges the University of Texas Rio Grande Valley for their financial support throughout graduate studies. The thesis version of this manuscript benefited from the constructive comments of D. Provenzano and R. J. Kline. Feedback from two anonymous reviewers allowed improvements to the manuscript. Authors also acknowledge the valuable input, guidance and support of D. Shively and B. Shipley (TPWD). Finally, thank you to the vessels' captains, dive team, and crew members for their assistance in the field without whom this project would not have been possible.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data from this study are provided within the article.




