Mass Mortality of the Invasive Sea Urchin Diadema setosum in Türkiye, Eastern Mediterranean Possibly Reveals Vibrio Bacteria Infection
Funding: The authors received no specific funding for this work.
ABSTRACT
The invasive Diadema setosum (Leske, 1778) long-spined sea urchin has been in the Mediterranean Sea since 2006, and then, it has been known that its population density has reached high values on the southern Aegean coasts of Türkiye. This study aimed to report the mass mortality event of D. setosum with the determined agent on the Aegean coast of Türkiye. In addition, it was targeted to provide information on the current status of its density based on seasonal SCUBA diving observations for the period between January 2023 and November 2023. The results showed that the mass mortality of D. setosum was determined in Muğla, Aegean Sea, in August 2023. Spine loss, the mucoid layer at the bottom of the appendages, and the outer body surface were observed as the clinical symptoms of the diseased samples. Bacterial growth was detected on Vibrio-selected TCBS agar plates, while no other parasitic agents were determined in the coelomic fluid of sea urchins. The results of the biochemical (API 20E) and molecular tests confirmed the isolated bacteria as Vibrio spp. The density of D. setosum showed statistically significant temporal changes, and the highest and lowest mean density values were recorded in autumn and spring, respectively. Environmental stressors, such as increasing sea temperature levels, affect the marine ecosystem and lead to opportunistic pathogens. Long-term monitoring of disease outbreaks is necessary to understand the interactions between species and the ecosystem.
1 Introduction
Echinoids are known as sea urchins, belonging to the class Echinoidea. Members of this class provide the structure and functionality of many shallow marine ecosystems; due to feeding habits, they control the excessive increase of macroalgae that are the main competitors of corals; thus, this grazing activity helps to sustain healthy rocky reefs, as well as echinoids, which are the prey of many economically important fish species (Steneck 2013; Dang et al. 2020; Vafidis et al. 2021; Hylkema et al. 2022; Roth et al. 2024).
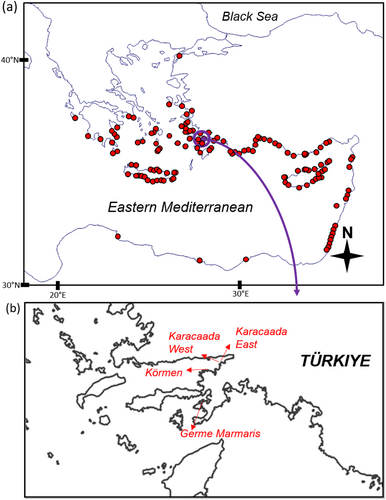
The genus Diadema is one of the most widespread and abundant sea urchins in tropical regions (Muthiga and McClanahan 2013). The long black spines are the characteristic patterns of the genus, and the extant species can be distinguished by spine morphology, test color pattern, and pedicellariae shape (Sweet 2020). Diadema setosum (Leske, 1778), a member of the Diadema genus, has been known to exist in the Mediterranean since 2006 (Yokes and Galil 2006). In recent years, D. setosum has successfully invaded the Mediterranean basin, especially the eastern Mediterranean (e.g., Vafidis et al. 2021; Öndes et al. 2022; Zirler et al. 2023) (Figure 1a). In Türkiye, this invasive species is widely distributed on the Mediterranean and Aegean coasts (Öndes et al. 2022) and the northernmost record was reported from the Sea of Marmara (Artüz and Artüz 2019). The mean population density of D. setosum was reported as 2.5 ± 1.48 individuals m−2 in Greece, the south Aegean Sea (eastern Mediterranean) (Vafidis et al. 2021). Furthermore, the density of D. setosum shows differences depending on habitat type. For instance, the mean density was reported as 30.6 ± 23.0 ind./100 m−2 in rocky habitats, 9.7 ± 9.5 ind./100 m−2 in vegetated habitats and 3.6 ± 5.6 ind./100 m−2 in sandy habitats in Türkiye, eastern Mediterranean (Öndes et al. 2022). It was highlighted that due to its grazing characteristics, D. setosum has a profound impact on shaping benthic communities across the Mediterranean (Vafidis et al. 2021).
Although mass mortality events (MMEs) have been observed in aquatic invertebrates for many years, it is obvious that there has been a noticeable increase in the number of cases reported in recent years, and mass mortality caused by diseases can affect the population size of sea urchins (Carpenter 1990). MME has threatened the populations of many echinoid species (e.g., Centrostephanus rodgersii (A. Agassiz, 1864), Lytechinus variegatus pallidus (H.L. Clark, 1925), Paracentrotus lividus (Lamarck, 1816), Strongylocentrotus droebachiensis (O.F. Müller, 1776), and Tripneustes ventricosus (Lamarck, 1816)) (Glynn 1968; Scheibling and Stephenson 1984; Andrew 1991; Girard et al. 2012). In particular, numerous studies in many different geographical locations of the world reported the MME in other species of the genus Diadema, Diadema antillarum (Lessios et al. 1984; Hughes et al. 1985; Liddell and Ohlhorst 1986; Carpenter 1990; Beck, Miller, and Ebersole 2014; Hewson et al. 2023; Hylkema et al. 2023). Furthermore, two other species of the same genus, Diadema africanum (Rodríguez, Hernández, Clemente and Coppard 2013) and Diadema mexicanum (A. Agassiz, 1863) were faced to MME (Clemente et al. 2014; Benítez-Villalobos, Díaz Martínez, and Martínez-García 2009). According to previous studies, bacteria, parasites, and fungi were presented as the fundamental causes of these mass deaths in echinoids and, more commonly, these aforementioned MMEs were relevant to bacteria including Vibrio, Flavobacterium, Pseudomonas, Aeromonas, Acinetobacter, and Alcaligenes (Wang, Chang, and Lawrence 2013).
Disease and MMEs in marine environments are commonly associated with increased sea surface temperature (SST) (Epstein 1996; Harvell et al. 1999; Clemente et al. 2014), and epidemics of marine organisms have increased in recent years (Beck, Miller, and Ebersole 2014; Dinçtürk et al. 2023). The predominant explanation of these disease cases was introduced as new pathogens and several stressors, including elevated sea temperature (Beck, Miller, and Ebersole 2014; Clemente et al. 2014). It was highlighted that some factors, such as ocean currents, changing water temperature values, predator density, and fishing pressure, can influence echinoderm diseases (Wang, Chang, and Lawrence 2013). However, according to the available literature, there are approximately 13 diseases that need to be highlighted. Among them, only three are named “paramoebiasis,” “bald sea urchin disease” and “vibriosis” (Sweet 2020). Future research on the dynamics associated with climate change and other related stressors in marine communities and ecosystems is required for the effective management of resources and marine ecosystems (Feehan and Scheibling 2014).
As opposed to other shallow-water benthic invertebrates, there are few reports of sea urchins outbreak diseases in the Mediterranean, and echinoderm infections are much more common in tropical waters (Clemente et al. 2014; Rivetti et al. 2014; Roth et al. 2024). Moreover, the disease events of sea urchin species were reported by several studies; however, the causative agent was not usually identified in the Mediterranean Sea. Mass death from D. setosum was reported very recently in Greece (Zirler et al. 2023). The present study reports the mass mortality of D. setosum on the Turkish Aegean coast and aims to determine the etiology of the disease. In addition, another objective of the study is to provide current information on the density of D. setosum based on diving surveys in different seasons.
2 Materials and Methods
2.1 Study Site, Sampling, and Determination of Disease Incidence
The SCUBA-diving surveys were carried out in four seasons (winter, spring, summer, and autumn) between January and November 2023. To provide information on the density and health status of sea urchins, data were collected at three sites of Muğla (Karacaada East, Karacaada West, and Körmen), Aegean Sea (Figure 1, Table 1) based on the transect method. At each sampling site, divers investigated three randomly determined transects, each with an area of 100 m2 (2 m wide × 50 m in length). All observations were performed between 1 and 20 m depth. The depth and water temperature of each dive were recorded by a diving computer (Sharewater Perdix 2), and the average depth was calculated. For each of the replicates, divers noted the sea urchin numbers. Throughout these observations, the health status (e.g., dead or individuals with lost or deformed spines) of D. setosum specimens was controlled.
| Site name | Coordinates | Mean depth (m) | Mean temperature (°C) |
|---|---|---|---|
| Karacaada East | 36.962972° N 28.199308° E | 10.8 | 23 |
| Karacaada West | 36.958257° N 28.188913° E | 14 | 20 |
| Körmen | 36.939609° N 28.123386° E | 13 | 20 |
Furthermore, based on our previous observations and local ecological knowledge of divers, we dived in January and August 2023 at another station (Germe—Marmaris, Muğla) where D. setosum population were common in shallow areas and exhibited mass mortality (Figure 1b). These dives were performed at depths of 3–10 m and both healthy and unhealthy individuals, which lost some spines (Figure 2), were collected (n = 35) for laboratory analyses. The aforementioned samples were immediately transferred to the Izmir Katip Çelebi University Faculty of Fisheries Fish Diseases and Biotechnology Laboratory in separated small containers that included seawater to prevent contamination risk.

2.2 Clinical Examination and Identification of the Disease Agent
The coelomic fluid was removed with an 18-gauge needle through the peristomal membrane into a sterile disposable syringe, and then the coelomic fluid sample was streaked onto TCBS (thiosulfate citrate bile salt sucrose) agar (Merck, Germany) and TSA (tryptic soy agar) supplemented with 1.5% NaCl (Merck, Germany) from suspected diseased (lost or deformed spines) and healthy individuals. The plates were incubated at 21°C ± 2 for 48 h, and bacterial growth was observed. The pure colonies were cultured on TSA supplemented with 1.5% NaCl for biochemical analyses. The motility of the bacteria was determined by the hanging-drop technique, and Gram staining, oxidase, catalase, and the sensitivity test to the Vibriostatic O/129 agent were carried out according to the standard procedures (Cappuccino and Welsh 2019). The API 20E biochemical tests were used for further biochemical tests (BioMerieux S.A., France). The sampled individuals were also examined for parasitic agents from the coelomic fluid and internal organs.
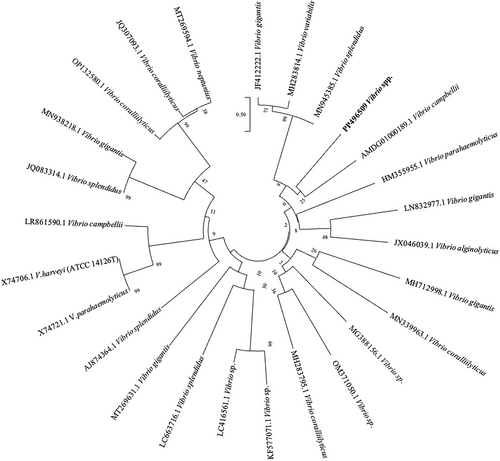
The EurX GeneMATRIX Bacterial & Yeast DNA Isolation Kit (Poland) was used for DNA isolation of the samples for identification of bacterial species. After DNA isolation, spectrophotometric measurement was performed on a Thermo Scientific Nanodrop 2000 (USA) to check the amount and purity of the DNA obtained. In the PCR study, the gene regions targeted for species identification were amplified with primers 27F-1492R as universal primers. The amplification results obtained by PCR (kyratec thermocycler) were electrophoresed in 1.5% agarose gel prepared with 1x TAE buffer at 100 V current for 90 min and visualized under UV light using ethidium bromide dye. A one-step PCR was performed to amplify a region of approximately 1470 bases. The PCR reaction was performed with Solis Biodyne (Estonia) FIREPol DNA polymerase Taq polymerase enzyme. After PCR for the samples, a single band was obtained on the agarose gel, indicating that the PCR process was successful. For the purification of the PCR product, the single-band samples were purified using the MAGBIO “High Prep TM PCR Clean-up System” purification kit and purified according to the kit's procedures. The ABI 3730XL Sanger sequencer (Applied Biosystems, Foster City, CA) and the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) were used for Sanger sequencing samples. The reads obtained with primers 27F and 1492R were contig-formed to generate a consensus sequence. This was performed using the CAP contig assembly algorithm in BioEdit software. The Vibrio sequences obtained from GenBank (NCBI) and the phylogenetic tree were generated with Mega 7 software with 1000 bootstrap replicates using the Maximum Composite Likelihood Method.
2.3 Statistical Analyses
To understand whether there are spatial and seasonal changes in the density of Diadema setosum, a Kruskal–Wallis test was performed. A Spearman's rank correlation was used to examine the correlation between seawater temperature and density of D. setosum. The R software was applied for statistical analyses (R Core Team 2022).
3 Results
3.1 Seawater Temperature Record
During the study period, the seawater temperature values varied between 16°C and 26°C. The lowest temperature value was recorded in March 2023 in the sampling site called Karacaada West, while the highest value was found in August 2023 in the sampling site called Karacaada East. Based on the combined data of the sampling sites, the mean values (±SD) of seawater temperature in different seasons were 18.7 ± 1°C in winter, 16.8 ± 0.5°C in spring, 21.6 ± 2.4°C in summer and 25 ± 0.1°C in autumn and the mean value of all seasons was 21.4 ± 3.2°C.
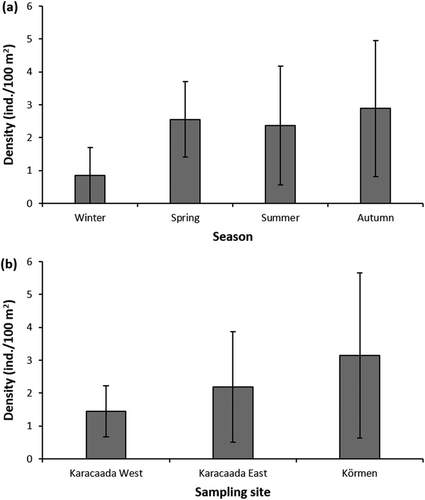
3.2 Seasonal and Spatial Variations in the Density of D. setosum
Throughout the sampling period, the mean density of Diadema setosum was observed as 2.3 ± 1.9 ind./100 m2 (mean ± SD). Concerning seasonal variations, the highest mean density was determined in autumn (3.1 ± 2.3 ind./100 m2), while the lowest mean value was found in winter (0.8 ± 0.8 ind./100 m2) (Figure 3a). There was a statistically significant difference in the density of D. setosum depending on the season (Kruskal–Wallis test, x2 = 13.100, df = 3, p = 0.004). Regarding the spatial distribution of density, the highest mean density was recorded in Körmen (3.2 ± 2.5 ind./100 m2), whereas the lowest mean density was at the sampling site called Karacaada West (1.4 ± 0.8 ind./100 m2) (Figure 3b). The results indicated that the density did not change significantly depending on the sampling sites (Kruskal–Wallis test, x2 = 2.614, df = 2, p = 0.271). Moreover, seawater temperature and urchin density are strongly correlated (Spearman's rank correlation, r = 0.40, p < 0.010). During the sampling period (between January and November 2023), we did not observe dead or unhealthy individuals of D. setosum at the sampling sites called Körmen, Karacaada West, and Karacaada East.
3.3 Infected Individuals and Mass Mortality Event
After communication with the local divers, we dived at another sampling site called Germe, Marmaris in January 2023 and August 2023 and for both sampling months, the unhealthy specimens of D. setosum were observed underwater, and the mass mortality event was photographed in August 2023 (Figure 2a,b) and our observations showed that dead individuals were consumed by fish species in a short time. A total of 20 and 15 individuals were sampled in January and August, respectively, for laboratory analyses. Among them, 8 infected and 12 healthy individuals were determined in January, while 12 infected and 3 healthy specimens were found in August. From sampled individuals, the infection rate is calculated as 80% based on microbiological analyses in the laboratory. The infected D. setosum samples exhibited extensive spine loss, surrounded by a mucoid layer on the outer body surface and at the bottom of the appendages (Figure 4b,c). No significant pathologic differences were identified in the dissection of sea urchin individuals.
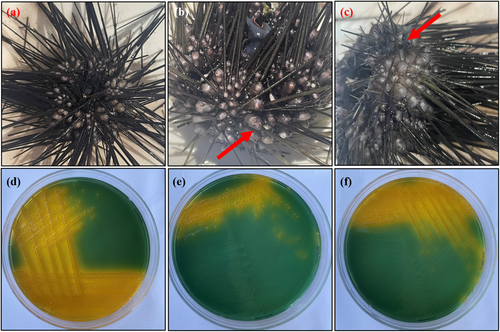
Pure colonies were isolated from each diseased D. setosum individuals, while pure yellow colonies were observed in the TCBS agar plates (Figure 4d–f) which indicates the presence of Vibrio spp. API 20E biochemical test profile, PCR amplification, and sequencing results of the isolated bacteria identified the pathogen as Vibrio spp. and registered in GenBank with accession number PP496509. Bacterial growth was not detected in healthy individuals. Furthermore, no parasitic agent was detected in the coelomic fluid or internal organs of the infected samples. The 16S rDNA sequences of Vibrio spp. that obtained from NCBI were compared in the phylogenetic tree in Figure 5.
4 Discussion
This study provided an analysis of the mass mortality events in Diadema setosum that occurred in the Aegean Sea, Türkiye and the current information on its density in this area. The first record of the invasive sea urchin D. setosum in the Mediterranean Sea was given by Yokes and Galil (2006) from Kaş, Levantine coast of Türkiye. Yapıcı (2018) observed this species for the first time on the Aegean coasts of Türkiye. Since then, the density of this invasive sea urchin has increased markedly on the Aegean and Levantine coasts of Türkiye (Öndes et al. 2022). The aforementioned study reported that from 2019 to 2020 there was a dramatic increase in the population of D. setosum in this region based on the ecological knowledge of divers, and its population density can reach up to 150 ind./100 m2. Öndes et al. (2022) compared to the density of D. setosum in different depth zones (0.0–5.0, 5.1–10.0, 10.1–15.0, 15.1–20.0 m), while in the present study a vertically larger depth zone (1–20 m) was investigated. However, although our methodology was not the completely same as Öndes et al. (2022), both observations in the studies were performed by the same researchers at the same sampling sites, and a certain decrease in the density of D. setosum from 2020 to 2023 had been clearly observed. It is considered that this decrease can be related to a MME. Similarly, Karakuş (2022) reported dead individuals of D. setosum at some sites (Gökova, Fethiye, and Kaş) around southern Türkiye; however, it has not been disclosed whether the deaths were due to disease or natural causes. Gökoğlu et al. (2023) notified that 98%–99% of the D. setosum population died in the Gulf of Antalya, Türkiye in February and March 2023. Zirler et al. (2023) published the mass mortality of D. setosum along the Greek coast and noted that dead individuals were found in July and September 2022. Zirler et al. (2023) also reported that MMEs were observed in shallow (0–5 m) and deeper (15–20 m) areas.
Sea urchin mass mortalities have had a significant impact on marine ecosystem structure, causing phase transitions from coral reefs and rocky barrens to communities dominated by macrophytes all over the world (Lessios 1988; Scheibling, Feehan, and Lauzon-Guay 2013; Feehan and Scheibling 2014; Karakuş 2022). There are several mass-mortality records reported from different locations and on different occasions. Of the seven Diadema spp., three have been found to have disease (Feehan and Scheibling 2014). Lessios et al. (1984) reported mass mortality of Diadema antillarum (black sea urchin) from the Caribbean waters, which started in January 1983 and extended to many other locations: Panama, Jamaica, and Çuraço (Bak, Carpay, and De Ruyter Van Steveninck 1984; Hughes et al. 1985; Lessios 1988). The causative agent of the disease remained undefined; however, waterborne pathogen transportation was assumed (Lessios et al. 1984). These outbreaks are frequently caused by changes in environmental factors such as temperature, salinity, or pH, which favor the spread of the infectious agent and affect how and how much a population is affected by the disease (Harvell et al. 1999; Girard et al. 2012; Gizzi et al. 2020). Actually, a wide range of opportunistic bacterial families have been linked to sea urchin disease outbreaks (Becker, Egea, and Eeckhaut 2008), and recent research has begun to show that complementing host microbes (the microbiome) may play a more global role in the causes of diseases (Sweet 2020). Sun et al. (2021) emphasized that sea urchin disease outbreaks are most common when the water temperature is below 20°C that could be related with the relationship between high temperature rates and immunity of sea urchins. Roth et al. (2024) recorded mass mortality events of several diadematoid species associated with a waterborne scuticociliate protozoan (Philaster apodigitiformes) and in this case, there is no relation with enhanced terrestrial nutrient existence or extreme water events, unlike this present study, which is related to water temperature. Similarly, previous studies also indicated that warm water could have affect on the boost of cauative disease agents' reproduction such as Flexibacter and Vibrio (Tajima et al. 1997, 1998; Wang et al. 2006, 2013; Li et al. 2020; Sun et al. 2021).
In several sea urchin species, bacterial infections are among the most common causes of mass mortality (Gizzi et al. 2020), and the temperature was mentioned to control the growth and spread of bacteria in sea urchins, and the highest values of disease outbreak frequently overlap with sea surface temperature (SST) peaks (Scheibling, Feehan, and Lauzon-Guay 2010; Feehan, Scheibling, and Lauzon-Guay 2012; Scheibling and Stephenson 1984; Miller and Colodey 1983; Jellett and Scheibling 1988; Gizzi et al. 2020). Due to ongoing changes in seawater conditions that play a role in the spread of infections, disease incidents appear more frequently (Scheibling, Feehan, and Lauzon-Guay 2010; Feehan, Scheibling, and Lauzon-Guay 2012; Clemente et al. 2014). There is still little information about the dynamics of disease in the marine environment, and hypotheses regarding how changes in climate could affect the frequency of disease are mainly unverified (Feehan and Scheibling 2014). However, the hypotheses were about bacterial disease or pollution. It is complex to identify the causative agents of mass mortality events; nevertheless, some research has established the mechanism between changes in seawater temperature and disease cases related to the propagation of pathogens (Scheibling, Feehan, and Lauzon-Guay 2010; Feehan, Scheibling, and Lauzon-Guay 2012; Clemente et al. 2014).
Typical signs of disease in sea urchins appear on their surface and vary in size (Grech et al. 2022). Clemente et al. (2014) reported epidermal necrosis associated with progressive deterioration of the epidermis, release of spines, and loss of tube foot function related to the presence of Vibrio alginolyticus infection. Similarly, Wang, Chang, and Lawrence (2013) reported several vibrio species such as Vibrio shilonii, Vibrio harveyi, Vibrio fortis, and Vibrio splendidus from another sea urchin species Strongylocentrotus intermedius, in North China they noticed that Vibrio and other bacterial groups (Shewanella, Pseudoalteromonas) were responsible for mass mortalities in this species. Li et al. (2020) identified Vibrio coralliilyticus as an agent of red spotting disease in S. intermedius. Hira and Stensvåg (2022) revealed a recent study on the effects of Vibrio echinoideorum on green sea urchin (Strongylocentrotus droebachiensis) as the causative agent of the “sea urchin lesion syndrome” with loss of external organs and formation of necrotic epidermal tissue. Dyková et al. (2011) reported Neoparamoeba branchiphila infections from moribund D.aff. antillarum in the Canary Islands (Spain), with the presence of these free-living trophozoites in their coelomic fluid. Feehan et al. (2013) reported Paramoeba invadens as the causative agent of green sea urchin mass mortality (S. droebachiensis), with clinical signs such as loss of attachment, non-functional tube feet, disheveled spines, and a gaping peristome. Another around Tenerife and La Palma Island in the eastern Atlantic reported a sea urchin outbreak (Diadema africanum) related to Paramoeba brachiphila, assuming the primary cause of mortality (Hernández, Sangil, and Lorenzo-Morales 2020). Hewson et al. (2023) determined Philaster apodigitiformis Fabre-Domergue, 1885 as the causative agent of the D. antillarum mass mortality event in the Caribbean Sea and confirmed the microorganism to fulfill Koch's postulate. In the present study, the disease symptoms were similar to those in previous cases mainly loss of spines. No amoeba species were detected in diseased sea urchin samples, only Vibrio spp. colonies were observed in TCBS media isolated from the unhealthy individuals of D. setosum, whereas there were none in healthy ones. The API 20E tests resulted in Vibrio spp. from isolated bacterial colonies. Certain marine pathogen species, such as marine Vibrio species, were in fact difficult to detect using biochemical tests; nevertheless, a more precise identification was subsequently ensured by biomolecular and genetic analysis (Grech et al. 2022). The isolated bacteria from the diseased samples showed 99% identity with Vibrio spp. in the sequencing results in the present study.
In conclusion, this study highlighted that not only sensitive endemic species, but also invasive species can exhibit a MME in the Mediterranean Sea. The present study indicated that the D. setosum showed a decreasing trend on the Aegean coasts of Türkiye compared with previous studies in the area (Öndes et al. 2022). This decrease is considered to be related to the MME that was detected at different times in 2022 and 2023 in the Aegean Sea, and the causative agent is considered Vibrio spp. The significant increase in seawater temperature in recent years has affected marine ecosystems and related bacterial communities. Diseases in benthic organisms have been spreading in the Mediterranean ecosystem, which is suffering from temperature changes, and monitoring the potential opportunistic pathogens is necessary for understanding mortality events.
Acknowledgments
The Republic of Turkey Ministry of Agriculture and Forestry provided the survey permission. Thanks to Murat Draman for providing one of the photographs. We thank anonymous reviewers for their comments and suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.




