Understanding marine biodiversity patterns and drivers: The fall of Icarus
Abstract
Biodiversity patterns are fundamental in our understanding of the distribution of life, ecosystem function, and conservation. In this concept analysis, A survey of the existing knowledge on marine biodiversity patterns and drivers across latitudes, longitudes, and depths indicates that none of the postulated patterns represent a rule. The paradigm of latitudinal gradients or bathymetric patterns of diversity vary across biogeographic regions or biodiversity components, kingdoms, or body sizes. The same holds true for the hypothesized longitudinal and cost-offshore patterns. Food availability and temperature influence all life forms and appear to be the most relevant factors shaping marine biodiversity. However, these drivers interact with many other variables such as spatial heterogeneity, ecological and physical processes creating a complex mosaic of shaping factors that limits any prediction. Climate change, with its implications for global primary productivity and temperature rise, can represent one of the major influences on future marine biodiversity. Understanding biodiversity emphasizes the need to complete the census of marine life in the next decade. The effort must use the most advanced technologies, develop holistic approaches and promote the integration of morphological- and genetic-based taxonomy to explore the biodiversity of organisms of all size classes, at large spatial scales and across habitat types, particularly open ocean and deep-sea ecosystems. Without this basic knowledge, coupled with identification of the drivers shaping the observed patterns, we will be unable to fill these knowledge gaps that are crucial for developing adequate conservation measures of marine biodiversity at global scale.
1 HOW WELL DO WE KNOW MARINE BIODIVERSITY?
Current estimates of global marine biodiversity indicate approximately a range between 0.5 and 3 million species (Appeltans et al., 2012; Costello et al., 2012; Mora et al., 2011). Yet current knowledge on marine biodiversity covers only an estimated 10%–30% of actual species number, with even lower percentages in the deep ocean (depths below 200 m; Danovaro et al., 2010). The World Register of Marine Species (WORMS), for example, records 33 animal phyla of which 32 in the ocean and only 17 phyla occur in terrestrial and freshwater ecosystems. Yet, the overall higher terrestrial biodiversity relative to marine biodiversity, likely reflects a higher speciation rate due to the presence of physical barriers to species dispersal (Wiens, 2015). Given the primary role of speciation in driving biodiversity patterns, the identification of the marine biodiversity hotspots can provide important insights into the drivers of speciation in marine ecosystems (Dalongeville et al., 2022). Yet we have so far identified only a (probably minor) part of marine habitats and hotspots. In addition, we started only very recently the investigation of marine biodiversity using integrated approaches able to couple molecular and classical tools to provide a comprehensive, reliable, and robust measure of biodiversity (Di Capua et al., 2023). At the same time, knowledge of marine biodiversity is much less than that of terrestrial ecosystems, and the presence of larger spaces without physical barriers in the global ocean makes the study of marine biodiversity crucially dependent on our ability to explore biodiversity across environmental gradients at multiple spatial scales. The results of the 10-years program Census of Marine Life (CoML; Snelgrove, 2010) suggested that most of the biogeographic regions of the world are largely unexplored with the most intensively investigated regions concentrated in the northern hemisphere and in proximity of developed countries (Gagné et al., 2020; Hughes et al., 2020; Tittensor et al., 2010). In terms of biodiversity, the least known marine regions are those offshore and at high latitudes (e.g. Webb et al., 2010). In general, farther we move away from the shore, and we extend offshore and into deeper waters, data availability quickly declines (Danovaro et al., 2014, 2020; Rex & Etter, 2011; Rogers et al., 2020; Snelgrove & Smith, 2002). Limited knowledge of the deep sea represents a major obstacle to a full comprehension of marine biodiversity: so far, we have investigated macro- megafaunal diversity of <0.001% of the enormous extension of bathyal and abyssal seafloor, which cover approximately 50% of the ocean surface (Danovaro et al., 2017). Our knowledge of microbial biodiversity is even more limited: we do not have a consistent or large-scale census of the prokaryotic diversity (Quéméneur et al., 2020), and other relevant microorganisms such as viruses and fungi remain based on a few samples worldwide (Costello & Chaudhary, 2017). We also lack any comprehensive assessment of biodiversity with an end-to-end approach (i.e., across the whole spectrum of kingdoms and sizes from viruses to large vertebrates) or even a single cubic meter of ocean habitat. The use of massive sequencing, metabarcoding and metagenomes, and environmental DNA (eDNA) can enhance current investigations but cannot fully address current gaps and requires confirmation through classical taxonomy (Dell'Anno et al., 2015). Thus, we must recognize that most of knowledge on biodiversity does not reflect the full spectrum of species, does not allow accurate prediction of the patterns at macroscale, and cannot adequately estimate the actual biodiversity of the ocean.
2 BIODIVERSITY PATTERNS: THE ROLE OF ENVIRONMENTAL GRADIENTS
Marine ecologists have long attempted to mimic terrestrial ecology in identifying the global patterns and drivers, with Rapoport's rule (Rapoport, 1982), but with very limited success (Costello et al., 2010; Fenton et al., 2023). At large spatial scales, following the terrestrial approach, the main apparent drivers proposed to date include latitudes, longitude, depth, productivity, and temperature. However, this list is far from comprehensive in identifying all factors potentially influencing marine biodiversity patterns (Lawton, 1999). Topographic and habitat diversity can help shape the patterns of species richness, but a comprehensive census of habitat heterogeneity remains a distant goal (Danovaro et al., 2024). Moreover, data are often very limited, unevenly distributed and concentrated in easily accessible habitats such as estuaries, mangrove forests, saltmarshes, seagrasses, coral reefs, and, macroalgal forests (Rogers et al., 2020), that collectively account for less than 1% of the ocean surface. In addition, biodiversity patterns generally focus on the (large) metazoans (Wilbur et al., 2023), whereas analyses of biodiversity patterns rarely include microbes, which largely dominate the biomass and functions in the ocean interior (Danovaro et al., 2017).
3 BIODIVERSITY PATTERNS AND LATITUDINAL GRADIENTS
At a global scale, the “latitudinal gradient” likely represents the most investigated biodiversity pattern, expressed as a tendency of biodiversity to decline from the low latitudes to the poles (Rex et al., 1993). Several causes and supporting evidence have been reported for different taxa (Menegotto & Rangel, 2018). The reasons hypothesized for such patterns include: (a) higher speciation rates at tropical latitudes (Clarke & Crame, 1997; Jablonski et al., 2006, 2013); (b) ecological drivers such as habitat variability (Zeppilli et al., 2016), (c) temperatures (Worm & Tittensor, 2018), and others, including life-cycle traits (Edgar et al., 2017; Hadiyanto et al., 2024; Pappalardo & Fernández, 2014; Roy et al., 1998). These patterns, however, vary among marine taxa. For example, micro- and macroalgae do not exhibit the same latitudinal gradient (Liuzzi et al., 2011). Marine mammal diversity shows a bimodal distribution, with total species richness lowest in polar regions, highest between 30° and 60° N or S, and intermediate in tropical waters (Kaschner et al., 2011). The pinnipeds peak at high latitudes, with a higher diversity at mid-latitudes for whales. These patterns, particularly those observed for several marine mammal and other large vertebrates (e.g., sharks and birds) appear to reflect evolutionary adaptations, more than the effect of environmental gradients (Jablonski et al., 2013). However, analyses focused on small-sized organisms, such as the nematodes, indicates different patterns than those previously reported for macro- and megafaunal species, with high biodiversity at all latitudes (Figure 1). Nematodes, the most abundant metazoans on Earth, account for more than 90% of the metazoans in all benthic habitats (Danovaro et al., 2023), thanks to their small size appear to be largely ubiquitous across latitudes and several nematode genera are even cosmopolite (Danovaro & Gambi, 2022). The absence of latitudinal patterns for small-size organisms such as marine nematodes could reflect their ability to spread over large areas when resuspended and transported by ocean currents. At the same time, current evidence on biodiversity patterns for other metazoan meiofauna, such as tardigrades and gastrotrichs (Fontaneto, 2011; Garraffoni & Balsamo, 2017), apparently contradicts the cosmopolitan distribution pattern in small-size metazoans. This example provides a warning against our propensity of infer biodiversity patterns from the analysis of a single taxon or ecological group (meio- macro- or megafauna). Moreover, the number of samples collected at different latitudes biases the abundance of species we encounter analyzing the latitudinal gradients. The same applies for the patterns of benthic Archaea that show an increase of the abundance and biodiversity moving from the equator to the high latitudes (Danovaro et al., 2016). These patterns deviate from the common equator-pole gradient, suggesting that different factors are involved (Snelgrove et al., 2016).
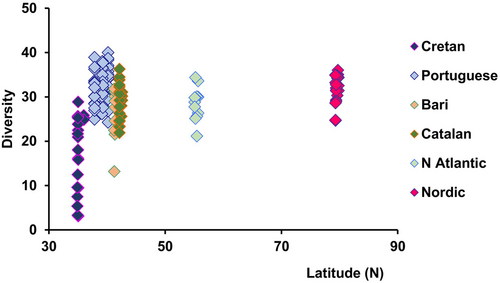
The shallow waters of the Nordic Seas (Greenland, Iceland and Norwegian Seas) and the Arctic Ocean may have similar diversity as lower latitudes, as shown in Figure 1. Changes in temperatures will evidently influence the shallow waters and the open sea in these waters, but the waters below around 700 m have a completely different pattern of diversity, with a low diversity seen for isopods (Svavarsson, 1997) and the ostracods (Jöst et al., 2022). The extensive Greenland-Iceland-Faeroe Ridge, with its lowest saddle depths of around 820 m, acts as a barrier for all migrations of species living below these depths. This region north of the ridge may be only several million years old as an arctic environment (the deep waters hold temperatures <0°C at depths below 700 m), having faced completely different evolution that the Atlantic Ocean south of the ridge. These data point out that latitudinal gradients are not informative when the evolutionary history of a region is not taken into account.
4 BIODIVERSITY AND LONGITUDINAL GRADIENTS
Some studies suggest steep longitudinal gradients in diversity, with an increase from both east and west towards Southeast Asia, and from east to west in the tropical Atlantic (Rogers et al., 2020). The hypothesis of longitudinal gradients reflects the presence of biodiversity hotspots around the lndo-Pacific Coral Triangle and to a lesser extent in the Caribbean (Asch et al., 2018; Reygondeau, 2019; Tittensor et al., 2010). At the same time, the central and western Indian Ocean, Red Sea, South-West Pacific Islands and Southeast Asia show similarly high levels of species richness (Rogers et al., 2020). This commonality could suggest that biodiversity patterns associated with longitudinal gradients might reflect more the presence of coral-reef areas than the gradient per se (Rogers et al., 2020). Moreover, while some groups reflect these patterns (such as reef-building corals, coastal fishes, shallow-water ophiuroids, snails, mangroves, coastal cephalopods, lobsters, and gastropods), other taxa do not (Rogers et al., 2020).
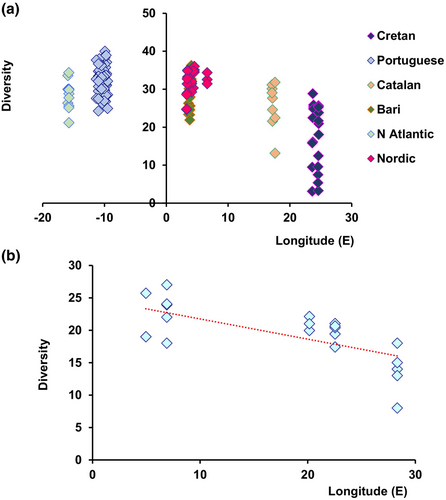
It is worth noticing that the hypothesis of a longitudinal biodiversity gradient lacks any robust scientific evidence or theoretical explanation. An attempt to test the hypothesis of longitudinal gradients in nematode biodiversity was carried out at single depths in the Atlantic and Mediterranean Sea (Figure 2a), by sampling deep-sea biodiversity at 3000-m thus eliminating the potential effect of depth as co-variate; Danovaro, Gambi, Lampadariou, & Tselepides, 2008; Figure 2b. These results indicate that nematode diversity decreases moving eastward following an apparent longitudinal gradient, generated by a parallel decrease in available energy (food availability measured as labile organic matter content of the sediments). Overall, we thus conclude that longitudinal gradients, when observed, reflect the decrease in energy availability or a combination of factors, other than longitude.
5 BIODIVERSITY PATTERNS AND BATHYMETRIC GRADIENTS
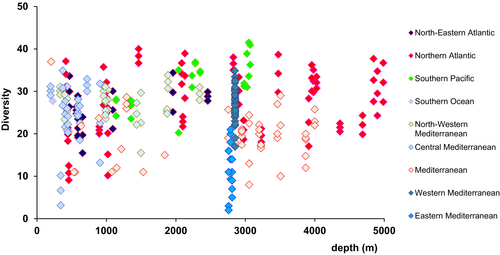
The best documented patterns in marine biodiversity refer to decreased species richness from shallow to deep water. This pattern arises from the historical “Azoic theory” proposed in the mid XIX century by E. Forbes who proposed that the harsh environmental conditions of the deep sea would reduce species persistence. However, studies conducted from the 1960 to 1990's reported higher biodiversity levels along the mid-continental slope (at depths ranging from 1000 to 2500 m). These diversity patterns have been proposed as “universal” (Rogers, 2015 and references therein). The observation of a mid-slope diversity peak has inspired considerable speculations. The unimodal (or parabolic) pattern of species richness and strong depth-differentiation of communities have invoked stability, ecophysiology, natural selection, disturbance, geometric constraints, metapopulation dynamics, and unrecognized resource heterogeneity as potential explanations (McClain & Etter, 2005). A unimodal diversity pattern with depth for fish species has been attributed to colonization of the deep sea by shallow-water organisms following multiple mass extinction events throughout the Phanerozoic (Brown & Thatje, 2014). Hydrostatic pressures and deep-sea temperatures have been suggested as a physiological bottleneck hampering the colonization of the deepest bathymetric ranges. It has been also hypothesized that speciation rates could be responsible for the diversity-depth pattern over time (Brown & Thatje, 2014). Adaptation that increases tolerance to high hydrostatic pressure and low temperature, allows colonization of abyssal depths. However, these patterns were not observed for some taxa or in some regions (Danovaro, Canals, et al., 2009). Pooling together data from multiple bathymetric gradients obliterates any hump shaped biodiversity patterns (Figure 3). Data reported for nematodes does not indicate any peak at intermediate depths (Gambi et al., 2014) and suggests that sometimes patterns observed for some macrofaunal taxa might require alternative explanations. In this regard, a multitude of concurring factors could indeed contribute to bathymetric patterns, including energy availability (food resources from shelf export, Levin et al., 2001; Danovaro, Gambi, Dell'Anno, et al., 2008) or habitat heterogeneity (Gambi et al., 2017; Gambi & Danovaro, 2006).
6 BIODIVERSITY AND DISTANCE FROM THE CONTINENTS
Habitat and species diversity have been hypothesized to be higher along the coasts and decrease towards the open ocean (Costello & Chaudhary, 2017). Like any general theory, the proposed explanations for most of these patterns are relatively simple and elegant. The decline in abundance and biomass relates to the decline in food quantity and quality with increasing depth and distance offshore, as both the export of primary productivity and lateral inputs from the shelf decrease (Wei et al., 2010). This gradient has been also assumed to reflect the variety of coastal and shelf ecosystems, and their interactions with the continents (Levin & Sibuet, 2012). However, these patterns vary across regions and might reflect the specific conditions of selected habitats investigated or biased by a different sampling effort in coastal areas versus offshore and deep sea (Rogers et al., 2020). Moreover, the enormous richness along mid slopes, or along the mid oceanic ridges does not support this simple and intuitive pattern of decreasing biodiversity with increasing distance from the continents. Different processes may control species richness among oceanic and coastal species (e.g., in terms of dispersal, mobility, or habitat structure).
7 BIODIVERSITY PATTERNS AND ENERGY (TROPHIC) GRADIENTS
Food availability dictates the flow of energy through marine ecosystems (Eddy et al., 2021). The abundance and productivity of primary producers influence the availability of energy for higher trophic levels, ultimately shaping the diversity and distribution of species throughout the food web. Food availability affects trophic interactions among species, because variations in the abundance and quality of food resources can influence feeding behaviors, foraging strategies, and competitive interactions among marine organisms (Gambi et al., 2017). Species may exhibit adaptations in response to specific food sources, enabling coexistence of diverse species within ecosystems. Food availability often correlates with habitat heterogeneity in marine environments (Danovaro, Bianchelli, et al., 2009; Zeppilli et al., 2016). Different habitats (hydrothermal vents, cold seeps, vegetated versus unvegetated habitats) provide different food resources, supporting diverse assemblages of species adapted to specific food resources. This is also the case for highly diverse and productive coral reefs, seagrass beds, algal, and animal forests that create complex physical structures that offer refuge and foraging opportunities for a large variety of organisms. Food availability fluctuates seasonally due to changes in primary productivity (nutrient inputs, temperature variations, etc.,). These fluctuations influence temporal changes in biodiversity of both coastal and deep-sea species (Rowe, 2013). A reduction in primary production can influence the biodiversity and the metabolic rates of benthic communities (Woolley et al., 2016), including the microbial components (i.e., prokaryotes and viruses; Danovaro et al., 2011). Marine assemblages can cope with fluctuations in food availability and previous studies reported that some taxa increase their efficiency in exploiting food sources up to 300% in extremely food-limited conditions, such as the deep sea (Gambi et al., 2017). This means that deep-sea benthos can provide a responsive adaptation to decreasing food (Jones et al., 2014). Thus, alteration in primary production will certainly result in, possibly not linear, changes observed due to the adaptation of marine organisms to ecosystem “oligotrophication” (Jones et al., 2014). Changes (i.e., decrease) in food availability can also influence biodiversity by selecting smaller size taxa, and altering the species composition by favoring the taxa with higher ability to adapt to more oligotrophic conditions (Danovaro et al., 2014).
8 BIODIVERSITY AND TEMPERATURE GRADIENTS
Temperature is one of the main environmental factors determining the distribution and diversity of life in the oceans (Antão et al., 2020; Yasuhara & Danovaro, 2014). Different species have specific temperature ranges. Temperature variation across latitudinal gradients and across depths in the ocean confines species within their thermal tolerance limits and in regions with temperatures close to their preferences (Burrows et al., 2019). Temperature gradients can influence species interactions and ecological processes including predator–prey dynamics, competition for resources, and symbiotic relationships. Temperature gradients drive seasonal migrations and reproductive cycles of many marine species, including whales that migrate to warmer waters for reproduction (Von Hammerstein et al., 2022) or species that migrate southward during winter, or poleward in cooler waters during summer to exploit seasonal food resources. Temperature cues also trigger spawning events and larval development in many marine organisms, influencing recruitment and population dynamics (Burrows et al., 2019). Midlatitude locations with intermediate temperature regimes from summer to winter, often support high biodiversity because of the wide range of temperature conditions (Antão et al., 2020). Strong coupling between biodiversity and temperature has been reported for marine systems (Ibarbalz et al., 2019), where species richness typically increases with increasing seawater temperature (Tittensor et al., 2010), with temperature as the only environmental predictor strongly related to diversity across 13 marine taxa. These results indicate a fundamental role for temperature in structuring marine biodiversity and indicate that changes in ocean temperature may ultimately rearrange the global distribution of life in the ocean. At the same time, biodiversity responses to temperature gradients depend on the baseline conditions, and temperature increase in temperate locations can increase species richness, while leading to species decrease in the tropics (Antão et al., 2020). Although the role of temperature remains crucial for many species living at high latitudes, for several taxa and regions this is not the case (Chaudhary et al., 2021). Moreover, global-scale patterns of diversity in the deep ocean remain largely unknown (Rex et al., 1993), as is the response of deep-sea species to temperature increase (Danovaro et al., 2004; Yasuhara & Danovaro, 2014). In addition, bias in our knowledge of diversity and its spatial distribution biases towards large size species, and the response of microbial diversity to increasing temperatures is still unknown (Danovaro et al., 2016).
9 BIODIVERSITY PATTERNS ACROSS MULTIPLE DRIVERS/GRADIENTS AND THE ROLE OF CLIMATE CHANGE
Disentangling the effect of different drivers or environmental gradients remains the most daunting task we face. Coastal regions, where warm and cold-water masses create multiple temperature and salinity gradients also show productivity gradients and support rich biodiversity (Coll et al., 2010). Similar conditions are reported for upwelling areas, where nutrient-rich deep waters rise to the surface, support high primary productivity and consequently diverse communities of marine organisms (Blanchette et al., 2009). Global climate change is increasing ocean temperatures to rise along with enhance water column stratification and decreased primary productivity (Li et al., 2020). These changes profoundly alter marine biodiversity as species may shift their ranges poleward or to deeper waters in response to warming temperatures, changing community composition, and biodiversity patterns. Faunal movements towards polar latitudes, which will likely further increase in the future, could also lead to a biodiversity increase poleward through the invasion/penetration of warmer water species (Worm & Lotze, 2021), with potential negative consequences on native biodiversity (Hillebrand et al., 2018). An increasing number of studies demonstrates that effects of climate change on marine biodiversity are already apparent from local to global scales (Rhoades et al., 2023). Long-term monitoring of pelagic taxa (fish and plankton) has provided evidence of climate-driven changes in species distribution and diversity across latitudes (Brierley & Kingsford, 2009). The expected decrease in ocean primary productivity, which will decrease carbon export to the deep sea, can potentially result in decreased biodiversity in food-deprived regions (Moore et al., 2018). Moreover, sessile organisms such as corals and seaweeds, that cannot move easily, will experience excessive temperature increases (Katao et al., 2015). Episodic heatwaves can disrupt critical ecological processes determining the coral bleaching, mass mortality events in coastal waters and altering the entire ecosystem functioning (Schoepf et al., 2015; Tkachenko & Hoang, 2022). Species loss resulting from elevated heat stress has been reported in both tropical and temperate regions (Wernberg et al., 2012). Synergistic interactions might arise by a combination of ocean warming and decrease in primary production that remains difficult to assess (Gao et al., 2012). The effects of temperature and food availability might interact with others, including changes in circulation and current speed, oxygen decrease, and other aspects related to global change (Bridges et al., 2022; Sousa et al., 2021). The effects of climate change will likely become increasingly evident in the future, representing the major driver of macroscale patterns in marine biodiversity, particularly for large-sized species and the higher trophic levels (Boavida-Portugal et al., 2022).
10 CONCLUSIONS
The analyses presented here do not lend support to the broad-scale patterns proposed so far. These patterns indeed differ extensively between taxa and much more data are needed to provide predictions of biodiversity patterns. Based on present knowledge, none of the postulated biodiversity patterns (e.g., latitudinal, longitudinal and bathymetric) holds true. The drivers and patterns identified for some taxa do not apply to other taxa or to all biogeographic regions and even appear across different biological components, or body sizes. Attempts to predict biodiversity patterns without census of the global marine biodiversity or understanding of biodiversity drivers recalls the attempt of Icarus in Greek Mythology, who fell to earth when the sun melted the wax holding together his wings. We are trying to fly too high (i.e., making predictions) without the data to do so, and, in the meantime, we risk to loss of an important fraction of biodiversity and related ecosystem functions. More than 99% of the available information on marine biodiversity from shallow to coastal waters represents less than 5% of ocean surface and <2% of ocean volume. The limited knowledge of the open and deep ocean precludes any assessment of biodiversity patterns and drivers at a global scale (Danovaro et al., 2001). Multiple evidence supports the conclusion that food availability and temperature influence marine biodiversity and its patterns. Climate change, and its implications in global primary productivity and temperature rise, can thus represent one of the major factors altering marine biodiversity patterns in the future. However, these factors interact with a multitude of additional variables, including habitat heterogeneity/complexity, ecological, and physical processes, often with contrasting effects that currently defy any prediction. However, detection of changes in biodiversity patterns and assemblage structure can increase our understanding of the sensitivity of marine assemblages to different drivers and environmental gradients. Shifts in biodiversity and the geographical expansion of some species can provide insights into the actual combined effects of the environmental and climate change on marine ecosystems. Comprehending responses of marine biodiversity to a changing marine world is a task increasingly complicated by the interactions between biodiversity and multiple direct human impacts. Filling the knowledge gaps and understanding the effects of multiple gradients and climate change on marine biodiversity remains one of the major priorities in marine sciences, biodiversity conservation, and policymakers.
ACKNOWLEDGMENTS
This study was conducted in the framework of the National Recovery and Resilience Plan (NRRP) of the Italian Ministry of University and Research funded by the European Union – NextGenerationEU; Project code CN_00000033 – National Biodiversity Future Center – NBFC. LAL acknowledges support from NSF OCE-2048720. The work was carried out in the framework of Project REDRESS (n. 101135492) funded by the EU. Thanks Cristina Gambi, Stefano Varrella and Cinzia Corinaldesi for the useful critical reading of a draft of the manuscript.
CONFLICT OF INTEREST STATEMENT
The author declares no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
Data are available from the original sources of the papers cited in the text.




