Marine biodegradation of natural potential carrier substrates for seagrass restoration
Abstract
Seagrass meadows provide essential ecosystem services but have been strongly declining over the past. Due to their incapability to recover effectively naturally, assisted restoration is used. This study aimed to test textile fabrics from natural derivatives to serve as carrier substrates for seagrass transplantation. The use of biotextile fabrics should enable seagrasses to better withstand hydrodynamic forces, especially in high-energy areas and during autumn and winter storms in the initial phase of restoration, thereby increasing restoration success. Here, the biodegradation behavior of three natural textiles was assessed in different configurations. Coir, sisal, and jute meshes were fixed on the top and bottom of a coir nonwoven mat, forming a so-called “sandwich structure.” Specimens were buried in the Ria Formosa Lagoon, Portugal, and retrieved weekly within the first months of burial and subsequently monthly over a total period of 3 months. Weight, tensile strength, and oxygen consumption rate were used as descriptors for biodegradation and tested after each retrieval. The results obtained in this study were discussed in the context of the application of the tested materials on Zostera marina transplants. Due to experimental errors, these results are solely used for discussion purposes in a conservative manner. Based on the three descriptors, coir mesh was the least degraded by the end of the experiment. Yet, it is vital to analyze the microbiome in a study site to understand the biodegradation process and based on that select a textile material. Coir fibers appear to be a good choice in highly biologically active areas to prolong the degradation process, whereas in areas with less activity sisal could be sufficient and even beneficial through the release of compounds that foster vegetations induced by degradation.
1 INTRODUCTION
Nature is declining worldwide at an unprecedented rate (IPBES, 2019). Especially, marine environments suffer from anthropogenic exploitation. The majority of human activities operate in the nearshore, intertidal, and infralittoral zones populated by marshes, mangroves, sand beaches, dunes, seagrass beds, and coral and oyster reefs, pressuring these ecosystems to a higher extent than the offshore regions (Barbier, 2017; Halpern et al., 2008). These marine ecosystems are pivotal to human welfare as they provide so-called ecosystem services (ES). A vast number of essential ES are provided by seagrass meadows, which are one of the most productive ecosystems in the world (Descamp et al., 2017; Duarte, 2000; Reynolds, 2013). Seagrasses are angiosperms (flowering plants) that inhabit coastal areas from the intertidal up to depths excess of 50 m (Duarte, 2000; Reynolds, 2013) from the Southern Hemisphere to tropical regions up to the Arctic (Reynolds, 2013). Seagrass meadows play an important role in primary production, beach protection from erosion, and nursery homes for other species and act as blue carbon sinks (Descamp et al., 2017; Unsworth et al., 2019). Due to continuously imposed stressors on seagrass meadows, a constant decline since the preindustrial times has been recorded (Eriander, 2017). One-third of the world's seagrass meadows disappeared since they first were recorded in 1879 (Waycott et al., 2009). The diminishing of seagrass meadows can be primarily attributed to anthropogenic stressors. These include the input of chemical loads into the system, physical damage (dredging, mooring, and propeller scars), the input of increased nutrient loads, and the cutting of shoots for restoration purposes (Descamp et al., 2017; Fonseca et al., 1998; IPBES, 2019). Restoration efforts have been made worldwide since the late 1930s (Tan et al., 2020). Especially, the United States and Australia are well experienced in seagrass restoration and were among the first nations to give attention to these ecosystems (Fonseca et al., 1998; UNEP-Nairobi Convention/WIOMSA, 2020). Nevertheless, due to various reasons such as the slow recovery and establishment rate of seagrasses and their seeds, or logistical difficulties and high costs of operation (e.g., divers), large-scale and long-term restoration of meadows has turned out to be a difficult task and success rates are therefore considerably low (average 37% success rate) (Eriander, 2017; Fonseca et al., 1998; Paulo et al., 2019; Xu et al., 2016).
Currently, a wide number of innovative methodologies and approaches are under development and tested globally on different seagrass species at different latitudes (Govers et al., 2022; Tan et al., 2020; Unsworth et al., 2019). The main issue, arising with the application of traditional transplanting methods, is the adverse effect on the donor meadows. Adult plants are used for transplanting efforts; therefore, the population of the donor meadow declines for restoration efforts. Especially, in large-scale projects, donor seagrass meadows suffer from the exploitation of sods and shoots from their meadows. Most often, the donor meadows cannot recover from the loss due to their slow recovery rate (Fonseca et al., 1998; Xu et al., 2016, Basconi et al., 2020). Furthermore, premature meadows suffer from hydrological pressures such as waves and storm events and often cannot withstand disturbing forces (Paulo et al., 2019).
1.1 Textiles in seagrass restoration
The use of textiles has been adopted widely for seagrass restoration (Tan et al., 2020). A variety of textile carrier substrates for either shoots or seeds have been tested. Advantages associated with textiles are, for example, the protection from predation (Tan et al., 2020), the stabilization of shoots (Ferretto et al., 2019), and the protection of meadows from bioturbation, therewith increasing chances of survival (Wendländer et al., 2019). Furthermore, textiles are simple to deploy in the marine environment. In consecutive research in Adelaide, Australia, sprigs of Amphibolis antarctica (Labillardière, Sonder & Ascherson ex Ascherson, 1868) were sewed on hessian bags, and seedlings were placed into sand-filled hessian bags (Irving et al., 2010, 2014; Tanner et al., 2014). After 8 months of monitoring, the hessian bags were degraded and dislodged due to intense storms and excessive wave energy. In the United Kingdom, seeds of Zostera marina (Linnaeus, 1753) were sown on hessian bags and in another series of experiments placed inside small hessian bags together with sand. In both cases, the hessian bags were degraded after 8–9 months and only a few rhizomes rooted into the sediment below (Unsworth et al., 2019). It is evident that in the majority of restoration studies with textile carrier substrates, the textile degraded too rapidly for the roots to incorporate into the sea bed. Therefore, long-term success could not be achieved. This underpins that appropriate material selection and design are pivotal for the success of restoration with carrier substrates and must be further studied to get deeper insight into the biodegradation rate of these fabrics. Information on the biodegradation rate of natural fibers in the natural marine environment is lacking. Contrarily, multiple studies have been conducted on the terrestrial biodegradation pattern of natural fibers, in laboratory conditions as well as in the natural environment. A widely used standardized test procedure is the so-called soil burial test (DIN EN ISO 11721-1:2001) applied to natural and synthetic fibers (Arshad & Mujahid, 2011; Sülar & Devrim, 2019) along with the standard test procedure on biodegradation via composting (DIN EN 13432:2000-12; FITR, 2008). Nevertheless, data on material degradation rate vary strongly within studies and cannot be directly compared due to modifications of the test procedures and differences in reporting (Table 1). Yet, these data can be used as a rough guideline for the assessment of these materials in the marine domain and similar methods can be applied. This work adds valuable data to fill the knowledge gap on the degradation rates of naturally derived materials under aquatic conditions. Based on the findings of this study, the development of feasible and large-scale seagrass restoration methods can be improved and thus save time and costs. In this study, suitable materials and textile structures for seagrass restoration purposes were identified by investigating their physical performance in regard to durability and degradation as a function of time exposed to the marine environment.
| Material | Environment | Degradation time | Source |
|---|---|---|---|
| Coir | N/A | 6–36 months | Daria et al. (2020) |
| Coir | Compost (50°C) | 215 days | FITR (2008) |
| Coir | Soil | 36–48 months | Greenfix |
| Jute | N/A | 6–18 months | Daria et al. (2020) |
| Jute | Soil | 40% weight loss after 3 months | Arshad and Mujahid (2011) |
| Sisal | N/A | 12 months | Daria et al. (2020) |
| Sisal | Compost (50°C) | 41 days | FITR (2008) |
| Sisal | Soil | 24–36 months | The East Africa Sisal Company Ltd |
2 MATERIALS AND METHODS
2.1 Study site
The study was conducted in the south of Portugal in the Ria Formosa at Ramalhete Marine Station of CCMAR (Center of Marine Sciences; 37.0059 N, −7.9682 E) in May–July 2021. The Ria Formosa is a barrier island system, one of the most vital systems for seagrass populations in Portugal, and has been used as a donor site for national restoration programs (Cunha et al., 2009). The intertidal and subtidal domains of the Ria Formosa Lagoon are colonized by the seagrass species Cymodocea nodosa (0.913 km2; Cunha et al., 2013), Zostera marina (0.05 km2; Cunha et al., 2013), and Zostera noltei (14.49 km2; Guimarães et al., 2012). Water temperature in the lagoon ranges from 12°C in the winter to 27°C in the summer and salinity accounts for 13–36.5 ppt, depending on the fluvial or oceanic influx at a given point (Newton & Mudge, 2003).
2.2 Material selection
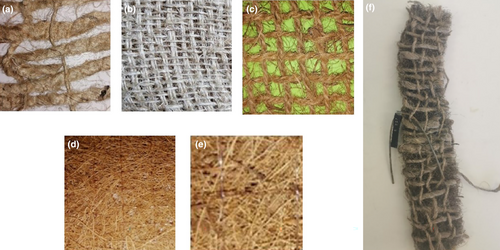
A vast number of synthetic and naturally derived fibers from different sources are available on the market. Despite their high durability and low costs, synthetic fibers are major pollutants and deplete nonrenewable resources during production, making them unsuitable for sustainable marine applications (Daria et al., 2020). Natural fibers can derive from different types of plant fibers such as cotton, jute, sisal, coconut, and nettle, or animal fibers such as wool (Daria et al., 2020). Plant fibers differ in the weight proportion of cellulose, hemicellulose, and lignin, which determines the physical properties of the fibers (Table 2; Wu et al., 2020). Based on a greater performance of coir and sisal fibers in the marine environment and the economic advantage of jute fibers, this fiber selection appeared to be suitable for this study. The increased lignin percentage in the coir fiber (Cocos nucifera, Linnaeus 1753) results in low water absorption and thus increases resistance toward microbial attack and seawater (Daria et al., 2020; Satyanarayana et al., 1981; Sumi et al., 2018, Rajan et al, 2005). Despite the rather low resistance of jute fibers (Corchorus capsularis/Corchorus olitorius, Linnaeus 1753) against moisture, acid, and UV light (Singh et al., 2018), they perform sufficiently in geotechnical applications such as drainage, stabilization, and erosion control at low costs (Chattopadhyay & Chakravarty, 2009; Daria et al., 2020; Datta, 2007). Sisal fibers (Agave sisalana, Haworth 1802) withstand deterioration in saltwater and therefore are commonly utilized for ropes, twines, and chords in the marine sector (Haque et al., 2015; Li et al., 2000; Ramamoorthy et al., 2015). Three meshes with varying grid sizes were chosen for this study. These included a coarse coir mesh, a dense sisal mesh, and a remarkably wide-gridded jute mesh along with two nonwoven coir mats, differing in weight and density (Figure 1; Table 3).
| Type of fiber | Cellulose (wt%) | Lignin (wt%) | Hemicellulose (wt%) | Density (g/m3) | Strain at break (%) | Tensile strength (Mpa) | Young's modulus (Mpa) |
|---|---|---|---|---|---|---|---|
| Coir | 36–43 | 41–45 | 0.15–0.25 | 1.2 | 15–30 | 175–220 | 4–6 |
| Jute | 45–71.5 | 12–26 | 13.6–21 | 1.3–1.46 | 1.5–1.8 | 393–800 | 10–30 |
| Sisal | 47–78 | 7–11 | 10–24 | 1.33–1.5 | 2–14 | 400–700 | 9–38 |
| Product | Material | Matrix | Weight (g/m2) | Tensile strength (kN/m) |
|---|---|---|---|---|
| Coconet 400 | Coir | Mesh | 400 | 11.2 |
| Geo-Sisal Peatsock | Sisal | Mesh | 1000 | 1.2 |
| Geojuta | Jute | Mesh | 500 | 15.0–20.0 |
| Cocomat | Coir | Nonwoven | 450 | 0.5 |
| Type 7 | Coir | Nonwoven | 762 | 2.1 |
2.3 Sample preparation
Each mesh was combined with each nonwoven mat, resulting in six different textile configurations (Figure 1, Table 4). For each textile configuration, five replicates were prepared measuring 50 × 300 mm each per sampling round (number of sampling rounds = 7). Replicates were prepared as so-called “sandwich structures” by placing each nonwoven mat in between a top and bottom layer of a mesh, sewn together with a sisal thread (mesh–mat–mesh; Figure 1f). Textile configurations were labeled as follows: CC (coir mesh–coir nonwoven), CT7 (coir mesh–coir nonwoven Type 7), JC (jute mesh–coir nonwoven), JT7 (jute mesh–coir nonwoven Type 7), SC (sisal mesh–coir nonwoven), and ST7 (jute mesh–coir nonwoven Type 7). A total number of 210 specimens were prepared for this research.
| Mesh | Nonwoven | Layout identifier |
|---|---|---|
| Coconet 400 | Cocomat | CC |
| Coconet 400 | Type 7 | CT7 |
| Geo-Sisal Peatsock | Cocomat | SC |
| Geo-Sisal Peatsock | Type 7 | ST7 |
| Geojuta | Cocomat | JC |
| Geojuta | Type 7 | JT7 |
2.4 Textile burial
Prior to burial, textile specimens were oven-dried for 24 h at 60°C and the initial weight (wi) was measured. Per textile configuration, five replicates were buried 5 cm underground during low tide in the intertidal of the Ria Formosa Lagoon for each sampling round over a spatial scale of approximately 300 m−2. Sampling was conducted at intervals of 7 days in the first month (five sampling rounds). The 7-day interval was chosen based on literature, in which the offset of natural fiber degradation is identified after approximately 5–7 days (FITR, 2008). After 4 weeks, specimens were collected on a four-weekly basis up until the twelfth week, as it was expected that degradation would slow down after the first month. Subsequently, the final specimens were left for another 12 weeks and recovered after a total burial time of 26 weeks. Specimens of the same time interval were buried in clusters to facilitate recognition of specimens in case of weathering or removal of the labels due to environmental conditions. Within a cluster, specimens were buried randomly; thus, comparable environmental conditions were assured within each testing round. The average temperature in the sediment was 22.5°C (±3.83 SD). Granulometry analysis of the sediments from the study site was conducted and categorized as “medium sand” with some inclusions of muddy and gravely sediments (Wentworth, 1922). Specimens were rinsed with freshwater after retrieval at the given sampling round. The water was collected during the process and filtered through a nylon sieve with a mesh size of 80 μm to retain fibers that were washed out during rinsing. The retained fibers were labeled according to the matching specimen, and together, they were oven-dried at 60°C for 72 h (including residues of sediments inside the specimens). Subsequently, the dried retained fibers were separated via sieving (1 mm) from the sediments and weighted to record the textile weight loss during rinsing.
2.5 Biodegradation descriptors
Three descriptors for the assessment of the biodegradation pattern of the specimens were selected: weight loss, tensile strength loss, and oxygen consumption rate. These proxies were chosen on the basis of terrestrial degradation protocols (Arshad & Mujahid, 2011; DIN EN ISO 11721-1:2001; n.d.; Sülar & Devrim, 2019). The final weight (wf) of the retrieved, rinsed, and dried specimens was taken to determine the weight loss over time. The average weight of the retained fibers during washing ww per specimen was added to the final weight of the corresponding specimen. The relative weight loss was calculated from the arithmetic means of the initial weight wi and the final weight (including retained fiber weight) wf + ww for each textile configuration in percentage (adapted from Chakraborty et al., 2014). Tensile strength loss over time was examined using the INSTRON 5565, Illinois Tool Works Inc., at Reutlingen University, Reutlingen, Germany. Instrument settings were set to 50 mm ± 0.5 width, 200 mm + clamp lengths, 100 mm min−1 extension rate, and 0.5 N pretension (International Organization for Standardizationn, 1999). Force was exclusively applied to the meshes of the specimens and not to the incorporated nonwoven mats. Thus, the degradation of the mats was not included in this strength examination. Five specimens per textile configuration were tested along with five controls (specimens without treatment nor burial), thereby providing a set of data for comparison, indicating the initial maximum force tsi of each textile configuration before burial. The arithmetic mean was calculated for all textile configurations and controls, and the relative tensile strength loss over time was computed. As a proxy for microbial textile degradation, respiration on the surface of the textile pre-burial (controls) was determined directly after specimen retrieval with field luminescent DO sensors of the Hach Oxygen HQ40D Portable Dissolved Oxygen Meter. Measurements were taken every 30 s for 5 min and converted into oxygen consumption rate (OCR) by fitting a linear regression of the decreasing concentration and quantifying the negative slope in μmol m−3 min−1 (Dietz et al., 2019). After 12 weeks, solely weight loss analysis was conducted, prompted by limitations in equipment and time constraints.
2.6 Statistical analysis
The significance level was defined at α = .05. To identify outliers in weight loss, tensile strength, and OCR, Grubb's test was applied and outliers were substituted with the mean (Newman, 2020-2023). PERMANOVA was executed in PRIMER 6 to detect differences among textiles along with PERMDISP analysis in case of heterogeneous variances among factors. Factors used in PERMANOVA were layout and time interval applied on the parameters weight loss, tensile strength loss, and respiration rate. The time interval was reduced to 12 weeks because all three descriptors were measured until that point. Due to the low number of permutations, p-values were determined using the Monte Carlo randomization (number of permutations: 999). Boxplots and line graphs were generated in the program MATLAB. Boxplot charts were generated from five replicates per layout and time interval. Spearman's correlation, 5% critical value (two-tailed) = .207 for n = 30, was used to determine the relationship among the biodegradation descriptors.
3 RESULTS
3.1 Weight loss
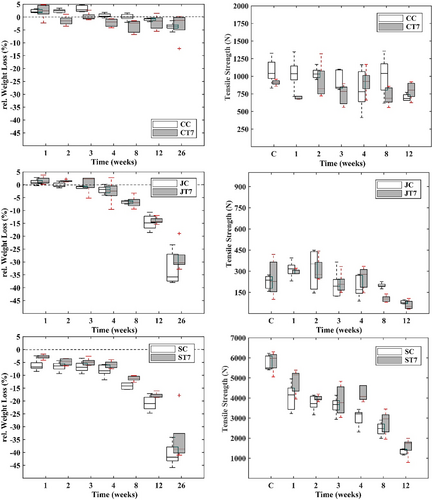
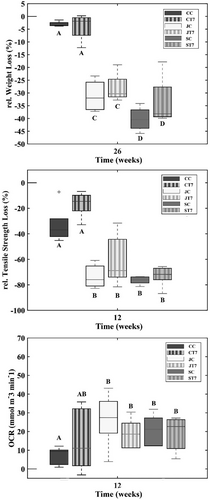
Textile configurations composed of coir nets showed an overall constant weight until the final phase of the experiment, in which they experienced a marginal drop (Figure 2). The average weight of CC specimens increased by approximately 3% in the first 3 weeks (P(M) < 0.05) and dropped to the initial weight in the fourth week, indicating no weight loss (P(M) < 0.05). After 12 weeks, weight was reduced by 0.66% (P(M) < 0.05), and after 26 weeks, weight was reduced by 4% (P(M) < 0.05). CT7 specimens exhibited a similar and comparable pattern to the CC configuration, with weight fluctuating between increasing and decreasing trends in the first 3 weeks. In week 8, the weight reduced by 2% (P(M) < 0.05) and stayed constant from thereon for the following month (P(M) > 0.05) until after 26 weeks the weight experienced a final loss of 3% (P(M) > 0.05). No weight loss was recorded for the JC configuration within the first 4 weeks, but a sudden drop was recorded after 8 weeks with a final weight loss of 33% (P(M) < 0.05). JT7 specimens lowered an overall 7% in weight after 8 weeks, which was doubled after 12 weeks (P(M) < 0.05) and 28% at the end of the experiment (P(M) < 0.05). Weight of SC specimens reduced in the first week of burial by 7% (P(M) < 0.05) and stayed constant for the following 3 weeks until it dropped by 21% in week 12 (P(M) < 0.05) and 41% in week 26 (P(M) < 0.05), respectively. A similar weight loss pattern was observed for ST7 specimens, which lost in total 35% of their initial weight (P(M) < 0.05). Among textile configurations, specimens composed of coir meshes showed the lowest weight loss, as opposed to the ~10 times higher weight loss of sisal textile configurations for CC and CT7 (P(M) < 0.05) (Figure 4, top). Final weight loss of jute mesh configurations was on average ~ 7.5 times higher than specimens of the coir configurations (P(M) < 0.05) and in the similar range of the sisal specimens (Figure 4; P(M) < 0.05).
3.2 Tensile strength loss
The tensile strength of CC specimens did not decrease significantly up until 3 months of burial, after which a decrease of 34% was recorded (P(M) < 0.05; Figure 2, right). CT7 specimens showed high durability throughout the whole experiment, and tensile strength only reduced after 2 months by 25% (P(M) < 0.05), with some fluctuations (±12%) of increase and decrease beforehand. Tensile strength of JC specimens stayed constant until the final period of the experiment, in which strength was 3 times lower, compared to the controls (P(M) < 0.05). Loss of tensile strength was initiated a month earlier for JT7 specimens than for JC. The samples experienced a reduction in strength after 8 weeks of 55% (P(M) < 0.05) and after 12 weeks of 78% (P(M) < 0.05) in total. Sisal specimens showed low resistance against degradation regarding the preservation of tensile strength. A first drop of 25% in tensile strength was observed for SC specimens after 7 days of burial (P(M) < 0.05). Subsequently, strength loss stagnated and did not lower up until the 2-month mark, where tensile strength lost 58% of its original strength. This was followed by another drop at the 3-month mark, resulting in a total strength loss of 74% (P(M) < 0.05). ST7 specimens revealed a similar pattern as SC specimens with a 4% lower final tensile strength loss of 70% compared to the SC (P(M) < 0.05). Absolute tensile strength remained the highest in sisal specimens before and after incubation; nevertheless, the relative reduction was most pronounced in sisal and jute textile configurations.
3.3 Microbial degradation
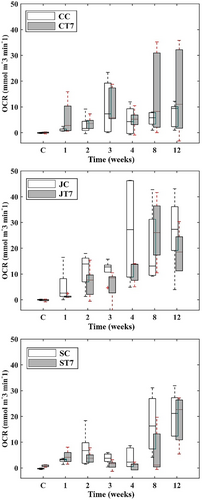
Microbial degradation, approximated by oxygen consumption rate (OCR), showed that dry unburied controls (no burial nor other treatments) featured low to absent aerobic microbial activity, with OCR values revolving around zero (Figure 3). Among controls, ST7 respiration differed from all configurations, featuring slightly higher microbial activity (P(M) < 0.05). In the first week after burial, OCR within CC replicates was initiated, indicating the start of aerobic microbial activity within the fabric, resulting in a final OCR of 9.38 μmol m−3 min−1 (±4.81 SD). OCR within CT7 was 104 times higher at the end than at the start of the experiment ranging around 11.07 μmol m−3 min−1 (±17.09 SD). In JC specimens, OCR increased 415 times over the course of the experiment (P(M) < 0.05), reaching an OCR of 27.39 μmol m−3 min−1 (±14.53 SD), whereas JT7 had an overall increase of 207 times (18.57 μmol m−3 min−1 (±8.09 SD); p ≤ .016). Differences in OCR for SC specimens were recorded after 2 months, accounting for 63 times higher OCR than the controls (P(M) < 0.05) and an absolute OCR of 21.20 μmol m−3 min−1 (±8.05 SD). A significant increase for ST7 specimens was observed after 12 weeks (P(M) < 0.05), with OCR reaching around 22.70 μmol m−3 min−1 (±9.04 SD). Up until the second week, OCR did not differ among configurations. After the third week, some differences were noted between sisal specimens and JC, sisal being slightly lower (P(M) < 0.05), as well as in between ST7 and CT7 specimens (P(M) < 0.05), which, however, disappeared toward the end of the experiment. A comparison among all layouts at the final stage of the experiment (12 weeks) shows that CC specimens were the least by aerobic organisms colonized configuration (P(M) < 0.05), followed by the CT7 (Figure 4, bottom). Despite the visual differences (Figure 5) among the other textile configurations (sisal and jute) and CT7, no statistical difference was detected due to the increased variance in all textile configurations. OCR in sisal and jute configurations appears alike after 12 weeks, and no differentiation can be made. PERMANOVA results (Table 5) demonstrate the significant influence of time and textile configuration on all three biodegradation descriptors.
| Parameter | Factor | DF | Pseudo-F | P (MC) | Significance |
|---|---|---|---|---|---|
| Weight loss | |||||
| Layout | 5 | 124.12 | 0.001 | * | |
| Time interval | 6 | 173.13 | 0.001 | * | |
| Layout * Time interval | 30 | 15.22 | 0.001 | * | |
| Residuals | 168 | ||||
| Tensile strength | |||||
| Layout | 5 | 1066.80 | 0.001 | * | |
| Time interval | 6 | 84.79 | 0.001 | * | |
| Layout * Time interval | 30 | 26.55 | 0.001 | * | |
| Residuals | 168 | ||||
| OCR | |||||
| Layout | 5 | 7.10 | 0.001 | * | |
| Time interval | 6 | 11.37 | 0.001 | * | |
| Layout * Time interval | 30 | 1.97 | 0.002 | * | |
| Residuals | 168 |

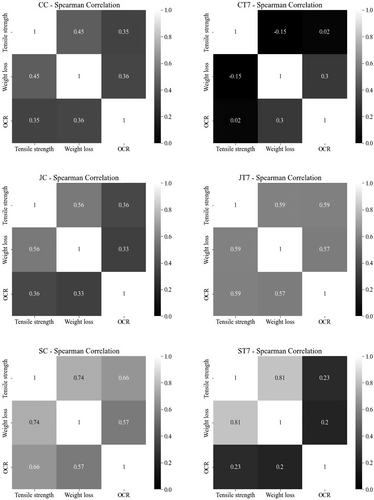
3.4 Correlation between biodegradation descriptors of textile carrier substrate
The correlation between biodegradation (Figure 6) descriptors reveals an overall trend among the configurations albeit with minor variations. CC specimens exhibit a moderate positive relation between the weight and tensile strength loss (0.45; p < .05), whereas the relation between OCR and the other two descriptors is rather low (0.35; p < .05). The correlation for the CT7 textile configuration is not statistically significant for any of the descriptors (p > .05) and will not be further discussed. JC and JT7 specimens show a higher moderate positive correlation among weight loss and tensile strength with correlation coefficients of .56 and .59, respectively (p < .05). Furthermore, the correlation coefficient of JT7 specimens between weight loss and OCR (.57; p < .05) indicates a moderate positive relationship. The weak relationship among these two descriptors for JC specimens is statistically insignificant (.33, p > .05). SC and SCT7 specimens depict a similar pattern with respect to the weight loss and tensile strength loss relation as the other configurations (CC, JC, and JT7) with even more pronounced positive correlation coefficients of 0.74 and 0.81, respectively (p < .05). The relationship between OCR and other descriptors is not statistically significant in ST7 replicates (p > .05); however, SC values show a moderate positive relation among OCR and weight loss (.57; p < .05) and tensile strength (.66; p < .05).
4 DISCUSSION
This series of experiments examined the biodegradation rate of naturally derived textiles in the marine environment, which has only been little studied. Yet, textile substrates are attractive low-cost carrier substrates for restoration purposes, which many studies anticipate to benefit from. Nevertheless, a lack of understanding of the substrates' characteristics and degradation patterns in the marine environment leads to faulty experimental design and premature failure of restoration studies. Hence, this study aspires to create ground knowledge on natural carrier substrates and their biodegradation rate in the marine environment by identifying pivotal parameters that must be considered when selecting materials for seagrass restoration purposes. Weight loss, textile strength loss, and respiration rate were selected as proxies for biodegradation for this study. Alongside biodegradation experiments, another series of mesocosm experiments was conducted, in which shoots of Zostera marina were sewn into each textile configuration and out-planted into independent mesocosms. Due to increased shoot mortality, potentially caused by lack of acclimatization after sampling, this experiment will not be presented in further detail. Few key observations of these experiments were used for the discussion in a conservative and careful matter, exclusively. The authors emphasize that observations should not be viewed as scientific findings; hence, experimental design and method were not covered in this manuscript.
The physical properties of the individual fibers and their processing into woven textiles influenced the outcome of the biodegradation experiments. Of the tested textiles, coir fibers are considered the most durable fiber due to their high lignin content and thus increased hydrophobicity and resistance against microorganisms (Lekha, 2004; Prambauer et al., 2019; Ramamoorthy et al., 2015), complying with the marginal weight loss of both coir mesh layouts from this study. The initial increase in weight in coir and jute layouts is expected to be associated with the very porous structure of the mesh and mats, making it easily accessible for microorganisms or sediment accumulation, obscuring weight loss by degradation (Di Franco et al., 2004), especially at the initial phase. This heightened ballast could prove beneficial in dynamically active hydrological regions, yet increased accumulation could lead to the burial of the shoots. This initial increase was not observed in sisal specimens leading to the assumption that the dense grid did not allow any accumulation of biomass or sediments and resulting in an initially measurable weight loss. In the mesocosm experiment, it was observed that the wide-gridded weft in the coir configurations proved to be unsuitable for anchoring shoots. Shoots detached prematurely from the coir configuration due to lack of stability. Contrary shoots intertwined into the dense sisal meshes experienced lower shoot and leave loss than the other configurations. In other restoration efforts, it was found that the rough surface of coarse weave meshes facilitates root anchoring (Irving et al., 2010; O'Brien, 2019; Tanner et al., 2014). This implies that a dense woven coir mesh could offer sufficient support for shoot recruits. The mechanical properties of textile fabrics are influenced by the individual fiber properties and subsequently modified by the conversion into yarns and the final fabric (Saiman et al., 2014). The original higher performance of the sisal mesh can be attributed to its high tensile strength of the individual fibers (Haque et al., 2015) in combination with the high weft yarn density of the mesh, which increases strength in addition (Nassif, 2012). Higher tensile strength reduces the risk of failure during transport and out-planting of seagrasses into the marine environment. Coir fibers possess lower tensile strength than jute fibers (Wu et al., 2020). Yet, the tensile strength of the coir mesh was far better than that of the jute mesh due to the low density of weft yarn within the jute mesh, leading to inferior capability of withstanding the applied load. Despite the highest initial strength of sisal specimens, these specimens were subject to the most significant strength loss over time. Sisal fibers are more prone to microbial degradation than coir fibers, which directly impacts tensile strength (see chapter 3.4). The correlation between aerobic microbial activity and the loss of tensile strength was reported in most configurations, explaining the more profound loss in strength for sisal and jute compared to coir specimens, which suffered less from aerobic microbial attack. This finding is congruent with the so-called “cotton strip assay” (CSA), which is a technique to assess soil microbial activity based on the tensile strength loss of a cotton strip, buried in soil (Harrison et al., 1988). Biodegradation in sisal layouts was induced prior than in coir configurations due to the prior explained reasons. In mesocosm experiments, shoot survival count was higher in shoots planted into sisal configurations. Early-induced degradation can affect shoot evolution via the provision of compounds that foster vegetation (Marczak et al., 2020). The authors hypothesize that there was a release of vegetation supporting compounds into the mesocosm, supporting shoots in the production of new leaves and maintaining their health, supported by the fixation into the dense sisal mesh. Coir fibers are subject to slow degradation; thus, nutrients might be released in lower concentrations compared to the sisal layouts. Deteriorated shoot integrity was observed in shoots planted onto coir configurations. Lack of support and possible limited nutrient release from the fiber are potential causes for this inferior performance. Therefore, it is crucial to have a basic understanding of the microbial community at the restoration site to achieve an equilibrium between a degradation period that is long enough to offer sufficient support but short enough for the shoots to benefit from the released natural compounds during degradation. A restoration site with an abundant microbial community, for example, within an estuary, requires different material selection along the littoral or infralittoral zone with lower microbial abundancy (Anas et al., 2021). Sisal fibers possibly degrade too rapid within a highly microbial dominated area as seen in the biodegradation experiment in the Ria Formosa Lagoon, in which rapid weight loss and strength loss were recorded. Yet, they might offer a suitable solution in open ocean applications in which degradation decelerates (lower microbial activity) but natural compounds are still released due to the high susceptibility of sisal to microbial activity even in lower microbic active areas. Coir fibers in the contrary offer significant resistance toward microbial degradation and could withstand longer in a microbial active environment while still releasing fostering compounds (due to high microbial activity), which, if selected as material in the, for example, littoral zone, might be too little release to have a positive impact on shoot integrity due to little degradation (lower abundance in microbial activity). Alongside potential advantages, early-induced degradation bears some risk, as identified by Tanner et al. (2014). It was found that parts of the textile carrier become loose, disturbing the shoots and therewith putting them under physical pressure, emphasizing the importance of selecting materials that undergo a certain degradation pace to avoid that the carrier substrates prolong for an overly extended time period and therewith reduce the risk of physical harm. Another crucial aspect to consider is the impact of biofouling of the submerged textile substrates on the ecosystem and nearby community along with changes in the biogeochemical composition of the surrounding water (Bullerie & Chapman, 2010; Malerba et al., 2019). Further studies must be undertaken to address the adverse impacts of introducing terrestrial structures into the aquatic environment to generate a clearer picture on the (dis-)advantages of this method for seagrass restoration.
Finally, this work showcased that textile substrates degrade more rapidly submerged in the marine environment than in the terrestrial environment. Sisal fibers degrade within 24–36 months in contact with soil (The East Africa Sisal Company Ltd.). This study revealed that if sisal would degrade at the same rate as during the 3 months of the experiment, a full weight loss of less than 12 months would be achieved. Weight loss in coir textiles proceeded at a slower rate than the given 36–48-month rate by Greenfix. Nonetheless, tensile strength reduction was more pronounced in the marine environment, with an average loss of 30% after 3 months, double the pace compared to findings from Sumi et al. (2018), in which coir was submerged in sand.
5 CONCLUSION
This study underpins the value of taking three main components into consideration when selecting a textile carrier substrate for seagrass restoration purposes: material with respect to surface roughness and degradation pattern, configuration (high grid density), and study site with emphasis on hydrodynamics and the microbiome. For transplantation into a marine environment with little microbial abundancy, it appears that sisal fibers, with an intermediate degradation span, configurated as a dense mesh, offer sufficient support and anchoring for the shoots, whereas coir fibers in that case, with overly slow degradation, might result in a high risk of the fabric eventually detaching from the sea bed and destroying the newly transplanted meadow. However, in another scenario, in which shoots are transplanted into a highly microbial active ecosystem coir, fibers with their slow degradation rate woven into a dense mesh might just be the right choice. Further study is needed on the ecological response of seagrasses toward the material and configurations as well as on the influence of the microbiome at the study site and the effects of biofouling of submerged artificial structures on the surrounding ecosystem. Nevertheless, this study offers a worthwhile starting point to fine-tune material selection and reduce the risk of restoration failure due to improved experimental design.
AUTHOR CONTRIBUTIONS
S. Rautenbach carried out the experiment and analyzed the data. R. Pieraccini, A. Hillebrand, and K. Nebel supported experimental design and data analysis. K. Nebel supervised and assisted in textile strength testing. S. Rautenbach wrote the manuscript with support from R. Pieraccini, A. Hillebrand, and K. Nebel. A. Hillebrand and R. Pieraccini supervised the project.
ACKNOWLEDGMENTS
This study received funding from the Agentschap Innoveren & Ondernemen (VLAIO; grant number: HBC2020.2260). The authors would like to thank the DEME Group and the Jan De Nul Group for their financial support, as well as the Center of Marine Science (CCMAR), Portugal, and the Department of Marine Biology at Ghent University, Belgium. The authors further thank the Reutlingen Research Institute for providing testing equipment and technical support during tensile strength testing. This study received Portuguese national funds from the Foundation for Science and Technology (FCT) through projects UIDB/04326/2020, UIDP/04326/2020, and LA/P/0101/2020, contract CEECINST/00114/2018 to AHE and the PlantAMillion project. Supported by Iceland, Liechtenstein and Norway through the EEA Grants for the Blueforest project, under the scope of the blue growth program.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest to disclose. All authors declare that this study is not in conflict with any interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon request from the authors.




