Coral recruitment in the Toliara region of southwest Madagascar: Spatio-temporal variability and implications for reef conservation
Abstract
Investigating coral recruitment is critical to better understand replenishment and resilience capacities of coral reef ecosystems and to improve their conservation. Here, we examined the spatio-temporal patterns of coral recruitment and the influence of confamilial adult coral cover in the region of Toliara, southwest Madagascar. Terracotta tiles were immersed from October to late January over a 3-year period (2018–2021) at 10 stations located on major reef habitats. Overall recruitment rates were relatively high compared to those of other reefs in the Southwestern Indian Ocean, ranging from 219.20 recruits.m−2 in 2018–2019 to 156.30 recruits.m−2 in 2020–2021. Recruit assemblages were dominated by Acroporidae (45.5%) and Pocilloporidae (45.0%), whereas Poritidae (1.9%) and “other” recruits (3.6%) were rarely recorded. Recruitment patterns varied among stations and habitats, with higher rates in patch reef (187.06 recruits.m−2) and outer slope stations (156.99 recruits.m−2) compared to inner slope stations (108.04 recruits.m−2). With the exception of “other” recruits, recruitment rates decreased between 2018 and 2019 and 2019 and 2020, followed by an increase in 2020–2021 that reached or even exceeded initial values at some stations. The abundance of Pocilloporidae recruits was positively correlated with the cover of confamilial adult corals, highlighting potential stock–recruitment or recruitment–limitation relationships, or an aggregative settlement of young stages near the established adult colonies, whereas no such relationships were recorded for other coral family categories. This study identified sites on the outer slope and patch reefs to consider prioritizing for protection as recruitment hotspots, as well as degraded inner slope sites that could benefit from restoration, with the important caveat that any measures should be accompanied by alternative income-generating activities through local involvement that suits the Malagasy context, such as locally marine managed areas.
1 INTRODUCTION
World coral reefs have suffered in recent decades due to significant impacts of various natural and anthropogenic disturbances (Bellwood et al., 2004; Hoegh-Guldberg & Bruno, 2010; Hughes et al., 2017; Obura et al., 2022). Degradation of coral reefs during the Anthropocene is mostly associated with mass bleaching events caused by thermal anomalies, exacerbated by other threats such as overharvesting, sedimentation, predator outbreaks, and cyclones (Hoegh-Guldberg et al., 2007; Hughes et al., 2018; Mumby et al., 2006). These threats may induce mass mortalities of scleractinian corals, the major reef-building taxa and key components of reef health and biodiversity, which in turn cause habitat degradation and decline in reef organism diversity and abundance (Hongo & Yamano, 2013; Pratchett et al., 2011). In some cases, major disturbances may cause phase shifts, which involve the replacement of corals by macroalgae or other non-reef-building benthic organisms and lead to fewer goods and services to human populations (McManus & Polsenberg, 2004). One of the challenges in overcoming these coral-reef crises is for scientists and policymakers to determine the resilience of coral reefs, defined by their ability to maintain or return to their original state after being impacted by disturbances (Hughes et al., 2010; Lam et al., 2020). Two key components of resilience are resistance, the ability of an ecosystem to absorb disturbances while retaining its function and provision of services, and recovery, the capacity to return to its initial state after being transformed (Carpenter et al., 2001; Lam et al., 2020; McClanahan et al., 2012). Recovery of coral populations depends on coral recruitment, the arrival of newly settling larval recruits to adult populations (Caley et al., 1996; Edmunds, 2023), as well as on the growth and propagation of surviving coral colonies (Adjeroud et al., 2018; Gilmour et al., 2013; Hughes & Tanner, 2000; Lukoschek et al., 2013; McClanahan et al., 2012; Mumby et al., 2016). Coral recruitment is critical to maintain and renew coral assemblages (Doropoulos et al., 2015; Doropoulos, Evensen, et al., 2017; Gilmour, 1999; Jouval et al., 2019; Luter et al., 2016; Mwachireya et al., 2015) and is also a key indicator of resilience (Adjeroud et al., 2017; Edmunds, 2018; Gouezo et al., 2019, 2020; Guerrini et al., 2020; Hughes et al., 2019; McClanahan et al., 2012; Sutthacheep et al., 2019).
Coral recruitment often shows a marked variability across spatial scales (Adjeroud et al., 2007; Davidson et al., 2019; Edmunds, 2023; Hughes et al., 2002; Jouval et al., 2019; Sola et al., 2015) due to the heterogeneity of environmental conditions. For example, local-scale variation in coral recruitment may be influenced by physico-chemical factors such as hydrodynamics and wave exposure, sedimentation, substrate characteristics, or water quality (Edmunds, 2022; Edmunds et al., 2010; Ritson-Williams et al., 2009). Coral recruitment is also controlled by biotic factors such as predation, allelopathy, and competition with algae through space pre-emption, overgrowth, and chemical cues (Dixson et al., 2014; Kuffner et al., 2006). Recruitment patterns vary among coral taxa (Gouezo et al., 2020; Kayal et al., 2015; Richmond et al., 2018; Ritson-Williams et al., 2009; Thomson et al., 2021) in relation to contrasting life history traits such as reproductive strategies, larval preferences, and selective mortality (Baird et al., 2003; Doropoulos et al., 2020; Harper et al., 2023; Mundy & Babcock, 1998; Shlesinger & Loya, 2021). Temporal variability also characterizes coral recruitment patterns; a marked seasonal variability in recruitment rates and composition is often related to the single annual reproductive cycle for most broadcast spawning species (Baird et al., 2009; Harrison & Wallace, 1990; Richmond et al., 2018). Interannual variability in recruitment rates is documented for most coral reefs worldwide, linked to variation in climatic and oceanographic conditions and the occurrence of large-scale perturbations such as bleaching events or cyclones, all of which affect coral fecundity, dispersal, and pre- and post-settlement mortality (Doropoulos et al., 2014; Edmunds, 2021b; Harper et al., 2023; Hughes et al., 2019; Lukoschek et al., 2013; Mallela & Crabbe, 2009; Mumby, 1999). The temporal pattern is thus often characterized by the succession of ‘good’ and ‘bad’ recruitment years with important implications for the structure and dynamics of adult populations (Connell et al., 1997; Edmunds, 2017; Harper et al., 2023; Hughes, 1990, 1996; Hughes et al., 2000). However, the increasing frequency and severity of large-scale disturbances and local stressors in the last five decades have resulted in a variation of coral recruitment worldwide, with a general decline in recruitment rates and a shift in recruit assemblage composition for several reefs (Edmunds, 2023; Edmunds & Riegl, 2020; Guerrini et al., 2020; Hughes et al., 2019; Price et al., 2019), with other reefs still showing consistent, high recruitment rates (Adjeroud et al., 2022).
In Madagascar, coral reefs have confronted severe degradation since the 1980s. Major bleaching episodes associated with El Niño events in 1998 and 2015–2016 caused high mortality within coral assemblages, particularly in the northwest and southwest coasts (Obura, 2005). Other threats such as overfishing, occasional use of destructive fishing methods, gleaning activities, and water pollution have also affected several coral reefs of the island. To mitigate the impacts of local stressors and large-scale disturbances on reefs and to promote their resilience, several marine protected areas (MPAs) have been implemented by Malagasy authorities and NGOs, most often with the strong involvement of local populations through locally marine managed areas (LMMAs; Ratsimbazafy et al., 2019). However, most of these MPAs have been designed to protect fishery resources and rarely target coral assemblages (McClanahan & Jadot, 2017; Randrianarivo et al., 2022; Ratsimbazafy et al., 2019).
While spatial patterns and the community structure of coral assemblages around Madagascar have been documented, with recent progress on the effects of environmental factors and MPAs at local (Botosoamananto et al., 2021; Carter et al., 2022) and regional (Randrianarivo et al., 2022) scales, studies have not examined coral recruitment processes, with the exception of a preliminary study by Todinanahary et al. (2021). This lack of knowledge on coral recruitment at Madagascar contrasts with several studies conducted in the South Western Indian Ocean (SWIO) region and investigations in Kenya (Mangubhai et al., 2007), Seychelles (Chong-Seng et al., 2014), Mozambique (Sola et al., 2015), Mascarene Islands (Jouval et al., 2019), and South Africa (Glassom et al., 2006). However, information on coral recruitment processes is crucial to implement and improve upon appropriate conservation and restoration measures for degraded reefs at Madagascar.
In this context, the present study examined the spatial and temporal patterns of coral recruitment in the region of Toliara, southwest Madagascar. Variability of recruitment rates and taxonomic composition was assessed at 10 stations located on the three major reef habitats (patch reefs, the inner slope, and the outer slope), during three consecutive years (2018 to 2021). To better understand the underlying factors of recruitment variability, we examined the potential influence of confamilial adult corals on recruitment at the station scale. We also discuss the study's implications for reef conservation and management, such as the potential prioritization of sites with identified higher recruitment rates.
2 MATERIALS AND METHODS
2.1 Study area and sampling stations
This study was conducted in the region of Toliara, with a focus on the Great Reef of Toliara (GRT), one of the most developed barrier reefs of the southwest coast of Madagascar (Figure 1). The human population living in Toliara City has considerably increased in recent years, with strong impacts on coral reefs through fishing and gleaning activities (Harris, 2007; Laroche & Ramananarivo, 1995). High sedimentation resulting from the poor management of mining and agricultural activities of the two main upstream rivers, Fiherenana in the north and Onilahy in the south, also affects coral assemblages of the GRT (Maina et al., 2013; Sheridan et al., 2014). The major large-scale natural disturbances that have impacted coral reefs of the GRT are thermally induced coral bleachings, with major events reported in 1998, 1999, and 2016 (Gudka et al., 2020; McClanahan et al., 2007; Obura et al., 2018) and a minor event in 2020 (Botosoamananto, personal observation; Figure S1). Thermal stress in 2001, 2006, 2009, and 2021 (Figure S1) has likely caused mild effects on coral assemblages of the GRT, but no mass bleaching events were reported. Cyclones are less frequent in the southwest coast of Madagascar compared to the eastern coast or to other regions in the SWIO, and though Cyclone Haruna passed 120 km north of Toliara in February 2013 (Carter et al., 2022), no significant impacts on the GRT were reported. Similarly, outbreaks of the coral predator Acanthaster spp. have not been reported in recent years on the GRT.
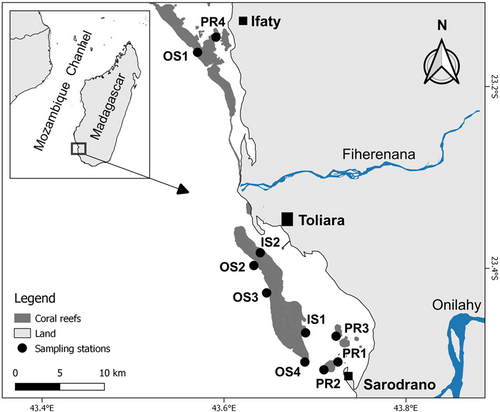
A nested sampling design, with 10 stations disposed on the three major reef habitats, was selected to assess the spatial and temporal variation of coral recruitment rate and composition. Four stations were established on patch reefs (PR1, PR2, PR3, and PR4), two on the inner slope (IS1 and IS2), and four on the outer slope (OS1, OS2, OS3, and OS4; Figure 1). Station codes are abbreviated as follows: the first two letters represent the habitats (PR: patch reefs, IS: inner slope, and OS: outer slope) and the numbers specify each station. All stations were located between 7 and 12 m depth, consistent with those already established to survey the diversity, abundance, and cover of adult coral assemblages (Botosoamananto et al., 2021).
2.2 Deployment and analysis of recruitment tiles
At each station, coral recruitment was characterized using 20 unglazed terracotta tiles (11 × 11 × 1 cm) directly attached to the substrata (Mundy, 2000). Tiles were immersed for 4 months (October to late January) over a 3-year period (October 2018–January 2019, October 2019–January 2020, and October 2020–January 2021; hereafter referred to as 2018–2019, 2019–2020, and 2020–2021 recruitment events, respectively). At the end of each immersion period, tiles were retrieved and bleached to expose and identify coral recruits under a stereomicroscope. For each surface of the tile (upper, lower, and sides), recruits were counted and identified. At this stage of development, three families (Acroporidae, Pocilloporidae, and Poritidae) were distinguished, and all other coral families were compiled into a category named “other” recruits (Babcock et al., 2003). Broken recruits (i.e., too damaged to be identified with certainty), which represent <4% of the overall recruits (all stations and years pooled), were not analyzed but were considered in the overall recruitment counts (all categories pooled).
2.3 Characterization of living substrate composition
Using the station as a sampling unit, we analyzed the influence of confamilial adult corals on the distribution and abundance of coral recruits. At each station, the percent cover of scleractinian corals was assessed in 2018, 2019, and 2020 using 30 photoquadrats of 0.25 m2 (50 × 50 cm) taken along three transects of 10 m length with an underwater camera (Nikon W300; Botosoamananto et al., 2021). Percent cover was estimated using the Coral Point Count with Excel extension software (CPCE 4.1; Kohler & Gill, 2006). On each photograph, 100 random points were used to quantify the percent cover of hard corals (Botosoamananto et al., 2021).
2.4 Statistical analysis
Spatial and temporal variations of recruit abundance among the three habitat types, 10 stations, and three time periods were tested for goodness of fit of a negative binomial distribution. We used generalized linear mixed-effect models (GLMM) using the Lme4 (Bates et al., 2015) package in R (R Core Team, 2021) with three-factor levels (years, habitats, and stations), as this approach takes into consideration unbalanced sampling and does not require the verification of parametric test assumptions (Bolker et al., 2009). Analyses were performed for the overall coral recruitment (all taxa pooled) and each of the dominant coral family categories: Acroporidae, Pocilloporidae, Poritidae, and “other” recruits. A separate GLMM was performed to compare the abundance of recruits on the three different surfaces (lower, upper, and sides) of recruitment tiles. To assess the effects of confamilial adult corals on recruit abundance, we conducted a third negative binomial error structured GLMM with percent cover (mean of the 3 years) of the four main coral family categories (Acroporidae, Pocilloporidae, Poritidae, and “other taxa”) as a predictor and habitats nested within year as a random factor. For model selection, the Akaike Information Criterion corrected for small size (AICc) was used. Best models of AICc <4 were selected. The model averaging approach was performed to explore the spatial and temporal variation of recruit abundance. The relative importance of each predictor in the averaged model was calculated by summing the Akaike weights for the predictor over the best models. This index ranges from 0 to 1, with a higher value for the higher relative importance. Statistical analyses were performed using R 4.1.0 (R Core Team, 2021).
3 RESULTS
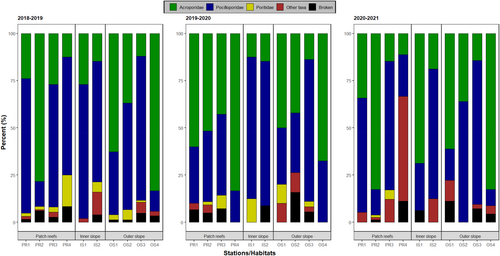
Over the 3 years of the study, recruit assemblages (all habitats/stations pooled) were dominated by Acroporidae (45.5%) and Pocilloporidae (45.0%), whereas “other” recruits (3.6%) and Poritidae (1.9%) were greatly less abundant. However, while the relative contribution of Acroporidae and Pocilloporidae was similar during the first 2 years, it changed in the last year of the study (42.6% in 2018–2019 and 41.5% in 2019–2020 for Acroporidae and 48.2% in 2018–2019 and 47.6% in 2019–2020 for Pocilloporidae; 55.7% of Acroporidae and 35.3% of Pocilloporidae in 2020–2021, Figure 2). Acroporidae dominated the recruit assemblage on patch reefs (representing 55.5% of the total recruits), had a slightly lower relative abundance on the outer slope (41.8%), and further reduction on the inner slope (22.4%), except at station IS1 in the final year (2020–2021). Pocilloporidae dominated the recruit assemblage on the inner slope (65.7%) as well as the outer slope (49.3%), but on patch reefs, it was 35.4%, with a reduced contribution at stations PR2 and PR4, depending on the year. The relative abundance of Poritidae and “other” recruits was low at all three habitats (2.3% and 3.2% on patch reefs, 2.5% and 6.0% on the inner slope, and 1.6% and 3.4% on the outer slope, respectively), although station PR4 was characterized by a high contribution of “other” recruits during the last year of the study (2020–2021).
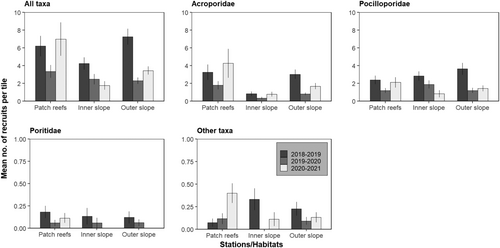
The overall recruitment rate (all taxa and habitats/stations pooled) was variable among the 3 years (Tables S1 and S2), with a higher value in 2018–2019 (6.27 ± 0.59 recruits.tile−1, mean ± SE, representing 219.20 recruits.m−2), compared to both 2019–2020 (2.71 ± 0.33 recruits.tile−1, representing 94.75 recruits.m−2) and 2020–2021 (4.47 ± 0.77 recruits.tile−1, representing 156.30 recruits.m−2). Slightly higher recruitment rates (all taxa and years pooled) were recorded at patch reefs (5.35 ± 0.70 recruits.tile−1, representing 187.06 recruits.m−2), compared to the outer slope (4.49 ± 0.42 recruits.tile−1, representing 156.99 recruits.m−2) and the inner slope (3.09 ± 0.39 recruits.tile−1, representing 108.04 recruits.m−2; Tables S1 and S2). During the study period, a decreasing trend was observed on the inner slope (4.23 ± 0.68 in 2018–2019 to 1.77 ± 2.01 recruits.tile−1 in 2020–2021; Figure 3). At both patch reefs and the outer slope, recruitment rates decreased from 2018–2019 to 2019–2020, and, in 2020–2021 continued to decrease on the outer slope (7.25 ± 0.89 in 2018–2019 compared to 3.44 ± 0.35 recruits. tile−1 in 2020–2021; Figure 3, Tables S1 and S2). However, at patch reefs, values in 2020–2021 increased and exceeded initial values (7.71 ± 1.97 in 2020–2021 compared to 6.20 ± 1.14 recruits.tile−1 in 2018–2019).
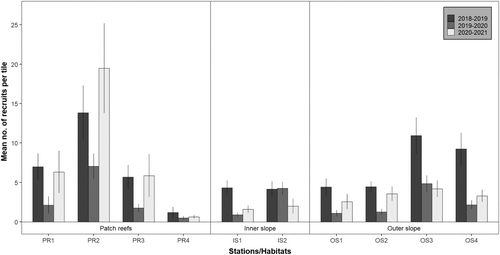
At the station scale, stations on patch reefs showed a decrease in recruitment rates between 2018 and 2019 and 2019 and 2020, but a return to initial values in 2020–2021 at stations PR1 and PR3, and even a higher value at station PR2 (Figure 4). Station PR4 was characterized by lower recruitment rates compared to other patch reef stations, with no significant temporal trend (Tables S1 and S2). The outer slope stations and one of the inner slope stations (IS1) also showed a decrease in recruitment rates between 2018 and 2019 and 2019 and 2020, followed by a slight increase in 2020–2021, except at OS3, which never exceeded initial values of 2018–2019 (Figure 4, Tables S1 and S2).
A marked spatial and temporal heterogeneity of recruit abundance was observed for each of the four coral categories (Figure 5, Tables S1 and S2). For Acroporidae, overall recruit abundance (all years pooled) was higher on patch reefs (2.97 ± 0.55 recruits.tile−1) than on the outer slope (1.87 ± 0.23 recruits.tile−1) and inner slope (0.69 ± 0.04 recruits.tile−1; Figure 3, Tables S1 and S2). This pattern was mainly driven by the high abundance recorded at station PR2 (Figure 5). At all three habitats and at most stations, abundance of Acroporidae recruits decreased from 2018–2019 to 2019–2020, but was followed by an increase in 2020–2021 that exceeded initial values at stations PR1 and PR2 on patch reefs (Figure 5, Tables S1 and S2).
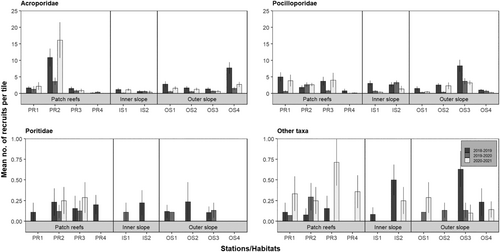
For Pocilloporidae among the habitats, overall recruit abundance (all years pooled) was slightly higher on the outer slope (2.21 ± 0.30 recruits.tile−1) than on the inner slope (2.03 ± 0.30 recruits.tile−1) and patch reefs (1.39 ± 0.07 recruits.tile−1; Figure 3, Tables S1 and S2). This pattern was mainly driven by the higher abundance recorded at station OS3 during the 3 years (Figure 5). A decrease in the abundance of Pocilloporidae recruits between 2018 and 2019 and 2019 and 2020 was observed across all stations, except PR2 and IS2; in 2020–2021, this was followed by an increase to, or approaching, initial values of patch reefs and OS2 (Figure 5, Tables S1 and S2).
For Poritidae, overall recruit abundance was low but slightly higher at patch reefs (0.12 ± 0.03 recruits.tile−1) compared to the inner slope (0.07 ± 0.02 recruits.tile−1) and the outer slope (0.07 ± 0.04 recruits.tile−1; Figure 3, Tables S1 and S2). Abundance of Poritidae recruits decreased from 2018–2019 to 2019–2020 at several stations of all three habitats and further decreased in 2020–2021 with no recruits recorded at any sites other than PR2 and PR3, which returned to values similar to the first year (Figure 5, Tables S1 and S2).
For “other” recruits, overall abundance (all years pooled) was low and similar between the habitats (0.17 ± 0.04 recruits.tile−1 on patch reefs, 0.18 ± 0.06 recruits.tile−1 on the inner slope, and 0.15 ± 0.03 recruits.tile−1on the outer slope; Figure 3, Tables S1 and S2). At all patch reef stations, abundance of “other” recruits increased over the 3 years, notably at stations PR3 and PR4, while a decreasing trend was observed on the inner and outer slopes, except OS1 (Figure 5, Tables S1 and S2).
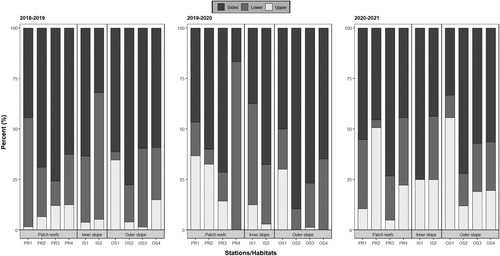
The proportion of recruits recorded on the different surfaces of the tiles varied among years, habitats, and stations (Tables S3 and S4). In general (all taxa and all years and habitats/stations pooled), recruits were more abundant on the sides (60.0%), compared to the lower (24.5%) and upper (15.5%) surfaces. This pattern was consistent for each of the four main recruit categories (Acroporidae, Pocilloporidae, Poritidae, and “other” recruits). In 2018–2019, the vast majority of recruits (>80%) were recorded on the sides and the lower surface at most stations, except OS1 on the outer slope where 30% of recruits were found on the upper surface (Figure 6, Tables S3 and S4). In 2019–2020, an increase in the proportion of recruits on the upper surface was recorded at stations PR1 and PR2, whereas this proportion decreased at stations PR4, OS2, and OS4. In 2020–2021, the proportion of recruits on the upper surface was higher at most stations compared to those in previous years, particularly at one patch reef (PR2) and one outer slope (OS1) station, where ~50% of the recruits were recorded on this surface (Figure 6, Tables S3 and S4).
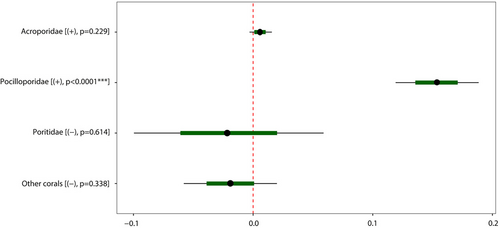
Our results from the GLMM highlighted that the relationships between confamilial adult coral cover and recruit abundance varied among categories of recruits (Figure 7, Tables S5–S8). A significant (p < .05) positive correlation between abundance of recruits and cover of confamilial adult colonies was only found for Pocilloporidae, whereas no such relationship was recorded for any other coral family categories.
4 DISCUSSION
4.1 Spatio-temporal patterns
Our results clearly demonstrate the high degree of spatial and temporal heterogeneity in coral recruitment in the Toliara region. This is consistent with patterns recorded at several reefs worldwide, from the east African coast (Sola et al., 2015), Mascarene islands (Jouval et al., 2019; Nzali et al., 1998), and the Seychelles (Chong-Seng et al., 2014) in the SWIO, to the Great Barrier Reef (Davidson et al., 2019; Doropoulos et al., 2015; Dunstan & Johnson, 1998), New Caledonia (Adjeroud et al., 2022), and French Polynesia (Adjeroud et al., 2007; Edmunds et al., 2010; Penin & Adjeroud, 2013) in the Pacific, as well as the Red Sea (Abelson et al., 2005; Glassom et al., 2004; Guerrini et al., 2020) and the Caribbean (Edmunds, 2021a; Green & Edmunds, 2011; Harper et al., 2023; Moulding, 2005). Overall (all taxa pooled) recruitment rates documented in the present study ranged from 17.48 ± 8.04 to 681.81 ± 198.60 recruits.m−2 across stations and years, with highest values (>200 recruits.tile−1) recorded at patch reefs and outer slope stations, notably during the first (2018–2019) and last (2020–2021) year of this study. Although comparisons among surveys with different methodologies should be taken with caution, the highest values recorded at Toliara are high compared to those from other SWIO reefs, such as Kenya (Mangubhai et al., 2007), Mozambique (Sola et al., 2015), South Africa (Glassom et al., 2006), Seychelles (Chong-Seng et al., 2014), and Reunion and Rodrigues islands (Jouval et al., 2019), and reefs in the Central Pacific such as French Polynesia (Adjeroud et al., 2007), where recruitment rates are generally less than 150 recruits.m−2. However, highest recruitment rates at Toliara remain greatly lower than those recently recorded in the southwestern lagoon of New Caledonia, with up to 13,572 recruits.m−2 during particularly ‘good’ recruitment years (Adjeroud et al., 2022).
Recruit assemblages at Toliara were characterized by the similarly high (~45%) contribution of both Acroporidae and Pocilloporidae recruits, though with slight variations in contribution across sites and years, and the constantly low abundance of Poritidae and “other” recruit families. This characteristic contrasts with that of other SWIO reefs, where recruit assemblages are generally dominated by either Acroporidae, such as in Mozambique (Sola et al., 2015), or Pocilloporidae, such as in Reunion Island (Jouval et al., 2019), with even Poritidae dominate reefs recorded at Rodrigues (Jouval et al., 2019). The high contribution of Acroporidae in the present study may be attributable to the relatively high abundance and cover in adult assemblages (Botosoamananto et al., 2021) and to the synchronized spawning of Acropora corals in the region (Gress et al., 2014), potentially inducing a high concentration of larvae over a short period of time that settle on recruitment tiles. Similarly, the high contribution of Pocilloporidae recruits could be related to the high abundance of Pocillopora and Stylophora adult colonies at our study sites (Botosoamananto et al., 2021). In contrast, this suggested relationship between abundance of adults and recruits is not valid for Poritidae since a low contribution of recruits was recorded despite abundant adult colonies of Porites in the region of Toliara (Botosoamananto et al., 2021). The extended period of reproduction and spawning of Porites recorded at some reefs in the region (Gress et al., 2014; Mangubhai et al., 2007), which dilutes the larval pool over long periods of the year, may explain the low abundance of Poritidae recruits on our recruitment tiles that are immersed for 4 months each year.
As documented for several coral reefs worldwide (Babcock & Davies, 1991; Bauman et al., 2013; Fisk & Harriott, 1990; Ho & Dai, 2014; Maida et al., 1995, among others), we recorded a higher proportion of recruits on the lower surface and sides of recruitment tiles. The lower abundance on the upper surface likely results from either post-settlement mortality induced by high grazing levels by herbivorous organisms or by colonization of fleshy algae that compete for space with recruits and trap sediments that smother young colonies (Babcock & Davies, 1991; Bauman et al., 2013; Edmunds, 2023; Fisk & Harriott, 1990; Ho & Dai, 2014; Maida et al., 1995).
A marked spatial variability was also found among habitats and stations, with highest recruitment rates consistently recorded at patch reefs and outer slope stations, though several stations in these habitats also showed low values. In contrast, recruitment rates were consistently lower at inner slope stations during the 3 years of the study. This pattern may be linked to the degraded environmental conditions generally found on the inner slope, with higher sedimentation and fishing pressure and lower abundance of several adult coral taxa, notably Acropora and Pocillopora, compared to patch reefs and the outer slope (Botosoamananto et al., 2021). However, additional studies are necessary to examine more precisely the environmental characteristics of these habitats.
We recorded a significant interannual variability of recruitment rates for all coral taxa and for each of the four recruit categories, consistent with studies on many other coral reefs (Adjeroud et al., 2007, 2022; Dunstan & Johnson, 1998; among others). A large part of this temporal variability results from variation in the fecundity of coral populations (Hughes et al., 2000), which is driven by changes in climatic and oceanographic conditions. Sea surface temperature (SST) is a major determinant of variation in coral fecundity, and thermally induced bleaching events are expected to reduce coral fecundity and recruitment in the upcoming years (Edmunds, 2023; Hughes et al., 2000, 2019; Ward et al., 2002). However, our results do not strictly follow this hypothesis. In fact, a minor bleaching event was observed from February 2020, resulting from elevated SST (~31°C) in January and February, but did not cause a major reduction in recruitment the following year. Instead, we recorded an increase in recruitment rates at several sites in October 2020–January 2021, sometimes reaching or even exceeding the values in 2018–2019. Moreover, the decrease in recruitment rates that we recorded in October 2019–January 2020 was not related to an increase in SST the previous years. Thus, other environmental factors such as sedimentation loads and wave energy exposure (Edmunds et al., 2010), for which we do not yet have rigorous data, are probably more related to the interannual changes in recruitment rates recorded at Toliara.
The marked variability among the 3 years of this study emphasizes the need for long-term interannual monitoring of coral recruitment to identify trends and potential sporadic peaks in recruitment such as reported in New Caledonia (Adjeroud et al., 2022) and to determine the drivers of variability (Edmunds, 2023).
Several studies have highlighted the importance of substrate composition on coral recruitment patterns (Dunstan & Johnson, 1998; Harrington et al., 2004; Jorissen et al., 2020; Kuffner et al., 2006). Our results underscore that the relationships between confamilial adult coral cover and recruit abundance were highly variable, with significant relationships for Pocilloporidae, but not for any other coral family categories. This variability is additional to the variable influence of spatial heterogeneity on recruitment rates among major coral families and underlines the strong variation in recruitment patterns among taxa, in relation to contrasting life history traits (Harper et al., 2023). The positive correlation between the cover of confamilial adult colonies and Pocilloporidae recruits may indicate the likelihood of a stock–recruitment model for population regulation, where adults drive the number of recruits, or a recruitment–limitation model, where recruits drive the number of adults (Adjeroud et al., 2017; Baird et al., 2003; Caley et al., 1996; Penin et al., 2007). This relationship may also be the result of an aggregate settlement of recruits near the established adult colonies (Adjeroud et al., 2017; Edmunds, 2000; Sims et al., 2021), as documented in other coral reefs for brooding coral taxa with short dispersal and self-seeding (Harper et al., 2023).
The present study only examined the potential influence of confamilial adult colonies on coral recruitment, but a more precise survey on other biological and physico-chemical factors, including larval connectivity between adjacent and distant reefs, is necessary to better understand coral recruitment processes and to improve conservation measures (Edmunds, 2023).
4.2 Implications for conservation
The relatively high recruitment rates recorded in the Toliara region compared to other sites in the SWIO, together with the high contribution of keystone taxa such as Acroporidae and Pocilloporidae, are encouraging results for coral conservation. These positive outcomes for recruitment processes demand implementation of management and conservation measures focused on recruitment to support the capacity of coral assemblages to maintain a healthy state (Botosoamananto et al., 2021).
In the context of resilience-based management, actions should be taken to reduce the negative effects of nutrients and sediment loads from the Fiherenana and Onilahy rivers that have increased due to unmanaged mining and agricultural and deforestation activities (Bruggemann et al., 2012; Sheridan et al., 2014). These actions are critical as coral recruits are particularly sensitive to increasing levels of sedimentation (Brunner et al., 2021; Wakwella et al., 2020) and nutrients (D'Angelo & Wiedenmann, 2014; Evensen et al., 2021). Moreover, privileging better land and watershed management actions will also improve water quality and coral reef health to the benefit of other reef communities.
Management of fishing activities should also be reinforced, with specific actions to protect the biomass and diversity of herbivorous fishes and invertebrates that control algal biomass to support coral recruitment processes and coral assemblage resilience. Destructive fishing methods are also part of the main cause of habitat degradation in the region of Toliara (Bruggemann et al., 2012; Harris et al., 2010; Sheridan et al., 2014). This threat is particularly important to address as we showed that coral recruitment of Pocilloporidae, one of the dominant taxa, is highly dependent on the cover of adult colonies.
The marked spatial variability of recruitment rates and composition that we recorded also has implications in terms of area-based management actions. We identified sites on the outer slope (station OS3) and on patch reefs (station PR2) that consistently showed higher recruitment rates throughout the 3 years of this survey, as well as high abundance and cover of adult colonies (Botosoamananto et al., 2021). These recruitment hotspots for these habitats, and additional ones to be identified through a complementary, larger-scale study, should be protected in priority and with drastic measures, such as no-take zones. These sites could act as a reservoir of coral colonies that could sustain a larval pool to replenish nearby reefs. Our results also identified sites, mainly on the inner slope, where recruitment rates are lower, and with less diverse and abundant adult assemblages (Botosoamananto et al., 2021). These sites, where the habitats are particularly degraded and unsuitable for corals, must be the subject of measures to restore the quality of environmental conditions and make them conducive to coral settlement and growth. These sites could also be candidates for restoration measures such as coral transplantation. However, as local populations are highly dependent on reef resources, it is imperative that these conservation measures be accompanied by alternative income-generating activities, such as aquaculture, with the strong involvement of local populations through locally marine managed areas (LMMAs; Ratsimbazafy et al., 2019), which seem well-suited to the Malagasy context (Todinanahary et al., 2016).
ACKNOWLEDGMENTS
We would like to thank the JEAI-ACOM and the LMI Mikaroka that have funded this research. Radonirina Lebely Botosoamananto benefited from an IRD PhD Thesis Grant (ARTS). We thank Dr. Jamal Mahafina, Director of the IHSM, for facilitating this research, and all the staff of IHSM for their logistical support. We gratefully acknowledge critical comments made by Jane Ballard. We also thank Monja Theodore, Noelson, Tovo, and all the volunteers who helped us during the sampling period.
FUNDING INFORMATION
Funding was provided by IRD (French National Research Institute for Sustainable Development) Grant to RLB (ARTS).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
CONSENT TO PARTICIPATE
All authors consented to participate in this research.
CONSENT TO PUBLICATION
All authors consented to publish this research.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are presented in this manuscript and given as Appendix S1. There are no data to deposit in the repository.




