Individual and population-level variation in susceptibility to temperature in early life history stages of giant kelp
Abstract
Because foundation species create structure in a community, understanding their ecological and evolutionary responses to global change is critical for predicting the ecological and economic management of species and communities that rely on them. Giant kelp (Macrocystis pyrifera) is a globally distributed foundation species with seasonal fluctuations in abundance in response to local nutrient levels, storm intensity, and ocean temperatures. Here we examine genetic variation in individual and population-level responses of early life history stages (zoospore settlement, survival, and gametogenesis) to increased temperatures to determine the potential for natural selection on temperature-tolerant individuals that would allow adaptation to a changing climate. We collected fertile M. pyrifera sporophyll blades from three sites along the California coast (Los Angeles, Santa Barbara, Monterey Bay) and induced zoospore release in the lab. Spores settled on microscope slides at three treatment temperatures (16, 20, and 22°C), matured for 21 days, and were imaged weekly to determine settlement, survival, and maturation success. On average, individuals from all sites showed lower rates of settlement and maturation in response to increasing temperature. However, the magnitude of the responses to temperature varied among populations. Survival tended to increase with temperature in Los Angeles and Santa Barbara populations but decreased with increasing temperature for the Monterey Bay population. We observed little genetic variation in temperature responses among individuals within sites, suggesting little scope for evolution within populations to increase the resilience of M. pyrifera populations to warming ocean temperatures and predicted declines in kelp abundance. Yet sufficient dispersal among populations could allow for adaptation of early life history traits among populations via evolutionary rescue of declining populations.
1 INTRODUCTION
Global change is a fast-acting and imminent threat to the world's ecosystems, which are already being affected by extreme weather, changes in precipitation patterns, ocean acidification, and warming (IPCC, 2021). Many organisms and ecosystems show negative responses to anthropogenic effects, including ocean acidification (Cripps et al., 2011; Doney et al., 2009; Hoegh-Guldberg & Bruno, 2010), increased frequency and strength of natural disasters (Adger, 2005; Gunderson, 2010; Van Aalst, 2006), and warming temperatures (Botkin et al., 2007; Colwell et al., 2008; Malcolm et al., 2006). Predicted global temperature increases suggest that the thermal limits of many species will be pressed by the end of the century (Parmesan & Yohe, 2003; Thomas et al., 2004). Species that are not able to acclimate to these temperatures or migrate to cooler habitats must either adapt or face extinction.
Adaptation via evolutionary changes in traits was historically thought to occur over long time scales (hundreds to millions of years), but a large body of work now demonstrates that evolution can occur on time scales rapid enough to affect the outcome of ecological interactions (Hairston et al., 2005; Schoener, 2011; Thompson, 1998). For example, coral holobionts that can evolve on the scale of weeks to months may affect the bleaching response of corals to increasing temperature (Maire & van Oppen, 2022). Climate change that results in declines in population abundances also imposes selection pressure on those populations, which can result in evolutionary rescue if declining populations can adapt to the changing environment (Carlson et al., 2014; Gomulkiewicz & Holt, 1995; Gonzalez et al., 2012). Evolutionary rescue occurs when adaptive changes in gene frequencies increase the abundance of a previously declining population. A prerequisite for such evolutionary rescue is that populations contain sufficient genetic variation in traits that affect fitness in response to stressful conditions in order for natural selection to act (Gonzalez et al., 2012; Hiltunen et al., 2017).
Genetic variation can drive ecological dynamics and thus is studied with increasing frequency. The average ecological response of a species to biotic and abiotic interactions reflects the result of often widely variable individual responses (Bolnick et al., 2011). Individuals with different genotypes in a population may fill different niches and increase ecosystem function. Just as species diversity increases ecosystem function (Hooper et al., 2005), an increase in genetic diversity in a population can increase seagrass resistance to disturbance and herbivory (Hughes & Stachowicz, 2004, 2009), oyster settlement and survival (Hanley et al., 2016; Hughes et al., 2019), and litter decomposition and nutrient levels under cottonwood trees (Schweitzer et al., 2005). In fact, intraspecific variability can be as or more consequential for determining ecological outcomes as variation among species (Des Roches et al., 2018). Rather than serving as distracting outliers, variation among individuals within a population, or among populations, may be critical for predicting ecological outcomes (Bolnick et al., 2011; Forsman, 2014).
Foundation species, such as corals or kelp, create habitat for other organisms and have large effects on species diversity in communities (Bruno & Bertness, 2001; Dayton, 1972; Stachowicz, 2001). For foundation species under threat of local extinction due to climate change, genetic diversity and the potential for evolutionary rescue of foundation species, can have cascading consequences for other species in the community. The loss of foundation species can transform ecosystems, resulting in local species extinctions and economic costs, such as when the loss of bull kelp led to less diverse urchin barrens and the collapse of a significant abalone fishery (Rogers-Bennett & Catton, 2019). Further, if different genotypes of a foundation species vary in their response to environmental change, but also in their effect on other species in the community, then community structure will depend on which genotypes of the foundation species persist in the face of climate change (Bailey et al., 2009, 2014; Whitham et al., 2006).
Kelp (Laminariales) are brown algae and globally-distributed foundation species that are among the most productive ecosystems in the world (Mann, 1973; Steneck et al., 2002). Covering roughly a quarter of the earth's coast lines, kelp forests are home to fishes, invertebrates, other species of algae, and marine mammals (Steneck et al., 2002). Additionally, kelp beds provide many ecosystem services to humans, including food production, medical uses, and beauty products, and are estimated to contribute billions of US dollars to the world economy (Beaumont et al., 2008). In addition to their ecological benefit in providing habitat to other species, in the near future, kelp may play an important role in carbon sequestration and minimizing ocean acidification (Arnold, 2016; Hirsh et al., 2020; Krause-Jensen & Duarte, 2016).
Giant kelp, Macrocystis pyrifera (Laminariales, Phaeophyceae), is the dominant marine foundation species along the west coast of North America (Steneck et al., 2002). Giant kelp abundance in California fluctuates seasonally, declining in late summer and fall when waters are warm, upwelling and nutrients are low, and large storms are more common (Dayton & Tegner, 1984; Seymour et al., 1989; Tegner & Dayton, 1987). Regular oscillations in ocean temperatures, such as those due to ENSO warming events, also affect kelp abundance (Edwards, 2004; Hollarsmith et al., 2020). On longer time scales, kelp abundance in the Santa Barbara Channel has consistently declined over the past two decades (McPherson et al., 2021; Reed et al., 2016). One mechanism that allows M. pyrifera to avoid local extinction is zoospore release during stressful periods in which large adult sporophytes decline (Rothäusler et al., 2011). Zoospores mature into microscopic gametophytes on the benthos and eventually sexually reproduce to form zygotes that develop into sporophytes. Although these early-life history stages of M. pyrifera (zoospores, gametes, and zygotes) also respond negatively to warming temperatures, low pH, and other environmental stressors (Gaitán-Espitia et al., 2014; Reed et al., 1991; Shukla & Edwards, 2017), zoospores may form gametophyte banks that persist for long periods of time or offer a mechanism for long-distance dispersal by rafting to new populations (Carney & Edwards, 2010; Dayton & Tegner, 1984; Ladah & Zertuche-González, 2007; Reed et al., 2016). Variation in temperature responses of early-life history stages may be a strong driver of adaptation to temperature within and among populations (Hollarsmith et al., 2020; Postma & Ågren, 2016).
Settlement of motile M. pyrifera spores produced by adult sporophytes, and the subsequent maturation into gametophytes, are key life-history stages for successful reproduction. Reproduction begins when a fertile diploid sporophyll blade near the bottom of an adult sporophyte releases haploid motile zoospores. The zoospores settle on hard substrates and develop into male or female gametophytes. Soon after gametogenesis, the female gametophyte releases eggs, and pheromones prompt a nearby male gametophyte to release sperm. The flagellated sperm locate and fertilize eggs, creating a diploid zygote. The zygote divides, and half of this structure forms the holdfast, which is responsible for anchoring the resulting sporophyte to the substratum for the entirety of its life, while the other half continues to grow vertically to form the bulk of the sporophyte (North, 1987). Here we examine the responses of early-life history stages of M. pyrifera to warming ocean temperatures, and the potential for individual and population-level variation to enable population persistence via evolutionary rescue. Specifically, we studied variation in spore settlement, survival, and gametophyte maturation in response to temperature, within and among three California populations (Los Angeles, Santa Barbara, and Monterey Bay). These three populations vary in the annual average temperatures they experience and belong to three of the five independent genetic demes identified along the California coast (Johansson et al., 2015). We hypothesized that giant kelp populations would differ in the responses of these early life history stages to increasing temperature.
2 METHODS
We collected fertile Macrocystis pyrifera sporophyll blades from three sites along the California coast. From south to north, we collected immediately offshore from Cabrillo Beach in Los Angeles, California (33.7110° N, 118.2833° W), Arroyo Burro Beach in Santa Barbara, California (34.4028° N, 119.7432° W), and Lover's Point, in Monterey Bay, California (36.6269° N, 121.9170° W). All three sites hosted visually healthy kelp forests at the time of collection. These three sites belong to distinct genetic demes with varying levels of diversity within demes (Johansson et al., 2015). Allelic richness is highest in Los Angeles (AR = 8.29), intermediate in Arroyo Burro (AR = 6.24), and lowest in Monterey (AR = 5.11) (Johansson et al., 2015). These sites vary in their mean and range of temperature, with Los Angeles experiencing the warmest temperatures, and Monterey Bay experiencing the coolest (Table 1). We recorded temperature at the time of collection on dive computers and confirmed temperatures against the nearest NOAA buoy temperature data (Wright et al., 2016).
| Region | Temperature at collection (°C) | Avg. annual low (°C) | Average (°C) | Avg. annual high (°C) |
|---|---|---|---|---|
| LAa | 16 | 14.5 ± 1.2 | 16.8 ± 2.0 | 18.4 ± 1.7 |
| SBb | 16 | 12.4 ± 2.6 | 14.5 ± 3.0 | 17.3 ± 2.6 |
| MBc | 11 | 11.8 ± 1.1 | 13.3 ± 1.8 | 14.8 ± 0.7 |
- Note: Collection data recorded from dive computers. Data presented as average ± SD from NOAA buoys between 2009 and 2016.
- a Station OHBC1.
- b Station NTBC1.
- c Station 46,240.
We collected multiple fertile sporophyll blades from the base of M. pyrifera individuals at each site (Los Angeles [LA] n = 16, Santa Barbara [SB] n = 20, Monterey [MB] n = 20] in September and October 2016. All blades were collected between 5 and 10 m depth and we chose individuals haphazardly but assured they were never nearest neighbors to one another (~2–5 m apart) and that the adults were similar heights. We collected individuals from a site on the same day using SCUBA. We separated the blades from the stipe by hand, taking care not to tear the blades. We immediately placed up to 10 fertile sporophyll blades from each individual in a sealed plastic bag underwater to maintain both separation and survival of individuals. We then placed the collections in a cooler on a thin layer of ice to keep cool during transport immediately to California State University, Northridge.
We induced zoospore release in the laboratory following previously established methods (Deysher & Dean, 1984). We rinsed all individuals with filtered seawater to reduce potential bacteria and mucus released in transit. We wrapped the rinsed sporophylls in damp towels and placed them back into the sealed plastic bag and stored them overnight at 15°C in a temperature-controlled room. Such desiccation promotes the release of spores from sporophylls. The next morning, we removed the sporophylls from storage and placed four sporophylls from each individual into 1 L containers of 15°C filtered seawater. We completed this procedure for all collections and kept sporophylls from different individuals separate at all times to prevent cross-contamination. We removed sporophylls from the seawater after 30 min and discarded them. After removing the sporophylls, we took a 1.5 mL sample from each well-mixed spore solution. We quantified the spore density in each sample using a hemocytometer.
We established three target temperature treatments in this experiment: 16, 20, and 22°C. The lowest temperature represents the average high temperature among the three sites (Table 1), while 20 and 22°C fall within the IPCC predictions for end of the century temperature increase (IPCC, 2021). The highest treatment is beyond at least 1 standard deviation unit of the average upper temperature currently experienced by the sites (Table 1). Although the SB and MB sites do not currently experience 20 or 22°C, they likely will experience these temperatures within this century. We performed all work in a temperature-controlled room maintained at 15.4 ± 1.05°C (mean ± SD) and used heating pads placed below petri dishes to establish the target treatment temperatures (RootRadiance, DL Wholesale, Livermore, CA, USA). Lights were set to a 12:12 h day:night cycle, with 8.56 ± 0.266 μmol photons m−2 s−1 during the day cycle.
We placed three glass microscope slides in a square 10 × 10 cm plastic petri dish to cover the bottom of the dish and added 50 mL of the spore solution to each dish. We established three replicate dishes per individual at each temperature (3 temperatures × 56 individuals × 3 replicates = 504 dishes). Spores were allowed to settle on microscope slides at treatment temperatures for 24–36 h in the dark. Preliminary work showed uniform settlement both within and among slides in the same petri dish, so we haphazardly chose one of the three slides in each petri dish to quantify settlement. Actual settlement was 53.7 ± 2.1 (mean ± SE) spores per field of view. Using a compound microscope, we took digital images of one randomly selected field of view at 400x total magnification on each slide. We used ImageJ (NIH version 1.50i) with the Cell Counter plugin (https://imagej.nih.gov/ij/plugins/cell-counter.html) to count the number of successfully settled spores in each image, identified by an extended germ tube (Figure 1).
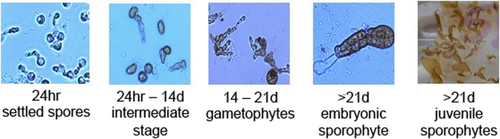
After imaging, we returned the slides to the petri dishes and replaced the spore solution with 50 mL of Provasoli-enriched seawater (NCMA/Bigelow Laboratory for Ocean Sciences; East Boothbay, ME). We imaged slides at the same location on the slide weekly for 21 days to monitor gametogenesis and/or spore death. We replenished the media at least once each week and disposed of slides that no longer contained spores. To evaluate successful gametogenesis, we analyzed the last image of each individual (21 days, or day of disposal) and classified spores as mature, immature, or dead. We classified individuals that fully developed from spore to gametophyte as mature and identified mature gametophytes by their distinct shapes (Figure 1). Immature gametophytes were identified by stunted maturation; for example, if the settled spores never matured further than their initial state, but still contained visible pigment (Roleda et al., 2004). We calculated the total number of dead spores by subtracting the sum of the mature and immature spores from the initial number of settled spores.
2.1 Data analyses
All analyses were conducted in R (version 4.0.2). We constructed three separate Generalized Linear Mixed Models (GLMM) to examine the fixed effects of temperature, site, and their interaction on (1) settlement, (2) proportion of settlers that survived to day 21, and (3) proportion of settlers that survived and matured to gametophytes by day 21. We included individuals nested within site as a random factor. In the model examining settlement, we included the density of spores in the stock solution as a covariate to account for any initial differences in spore availability, and in the other two models, we included Settlement as a covariate to account for any density effects. Data were transformed to meet model assumptions when necessary. We tested significance of fixed factors using Type III SS and Satterthwaite approximation of degrees of freedom. To test random effects, we constructed separate models with only temperature, individuals, and their interaction as factors. Early in the experiment, we lost one replicate of every individual from MB at 22 degrees due to a malfunction in the heating pads, and those individuals were excluded from the analysis.
3 RESULTS
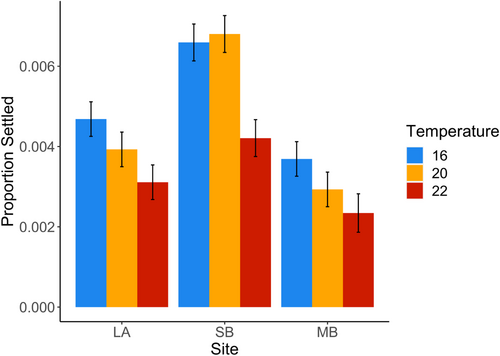
Increasing temperature decreased spore settlement at all sites, but how individuals from different sites responded to temperature varied among sites (Figure 2 and Table 2). Spores from SB individuals had the highest settlement at all temperatures. Although individuals from LA and MB showed a steady decline in settlement with increasing temperature, SB individuals showed no difference in settlement between 16 and 20 degrees, but a steep decline in settlement at 22 degrees. Individuals within sites had very different settlement rates (Table 2 and Figure S1). However, there was no significant difference in how individuals within sites responded to temperature (Table 2).
| Factor | Settlement | Proportion survived | Proportion matured | |||
|---|---|---|---|---|---|---|
| F df | p | F df | p | F df | p | |
| Temperature | 79.0 2,422 | <.001 | 5.23 2,443 | .006 | 51.5 2,441 | <.001 |
| Site | 2.492,52 | .092 | 4.54 2,452 | .011 | 1.772,467 | .171 |
| Temp*Site | 4.18 4,422 | .002 | 18.1 4,424 | <.001 | 9.32 4,421 | <.001 |
| Individual | 13.1 19,423 | <.001 | 3.29 19,423 | <.001 | 1.6019,423 | .053 |
| Individual*Temp | 0.43138,423 | .999 | 0.64738,423 | .950 | 0.95138,423 | .555 |
- Note: Significant values (p < .05) are shown in bold.
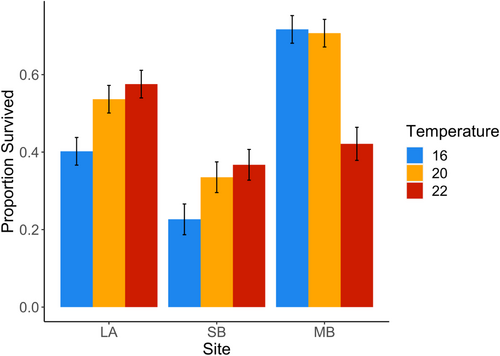
Individuals from different sites responded differently to temperature (Table 2). Despite having the highest settlement, spores from SB individuals tended to have the lowest survival rates (Figure 3). Spores from LA and SB showed increased survival with increasing temperature, but the survival of spores from MB showed a sharp decrease in survival at the highest temperature (22°C). Although there was significant variation in survival among individuals within a site, individuals within a site showed no significant difference in responses to temperature (Table 2 and Figure S2).
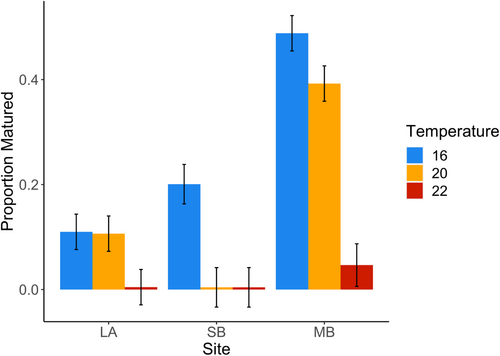
Surviving spores from all sites had lower rates of gametogenesis and maturation with increasing temperature, but the pattern differed among sites (Figure 4 and Table 2). Spores from LA and SB had overall lower rates of maturation relative to MB. Individuals from LA showed little response to temperatures between 16 and 20°C, but very few spores matured at 22°C (Figure 4). Spores from SB had intermediate maturation rates at 16°C, but very few spores matured at either 20 or 22°C. Spores from MB tended to mature more often than spores from other sites at each temperature, but also experienced declines in maturation with increasing temperature. We found no significant variation in maturation among individuals within a site and no significant difference in response to temperature among individuals within a site (Table 2 and Figure S3).
4 DISCUSSION
Giant kelp (Macrocystis pyrifera) is a critically important foundation species along the west coast of North America, as well as along many coastlines around the world. Rising ocean temperatures are expected to decrease kelp abundance at the local scale (Graham et al., 2007), which is also likely to decrease the diversity of species that rely on this habitat and the function of this ecosystem (Ellison et al., 2005; Thomson et al., 2015). Our results confirmed that settlement and maturation of early-life history stages of M. pyrifera tend to respond negatively to warming. Increased temperature tends to increase the survival of newly settled spores, which might allow gametophyte populations to persist as a “seed bank” for some period of time. However, we found variable responses to temperature among populations, which suggests that, at least at the regional scale, there is an opportunity for selection and potential for evolutionary rescue of M. pyrifera from warming if those populations that respond best to increased temperature increase the frequency of temperature-tolerant genes in the regional population.
As with all organisms, phenotypic variation in kelp is the result of a combination of genetic and environmental effects. By growing all of our individuals in the same environment, we minimized environmental effects as a source of trait variation, suggesting that our observed trait differences among populations and individuals are due to genetic differences. The fact that we observed large differences among populations and that these populations belong to different genetic demes supports that conclusion (Johansson et al., 2015). Nevertheless, we acknowledge that environmental effects can persist beyond one generation via maternal effects or epigenetics, in which the environment of the parent affects the traits of the offspring (Adrian-Kalchhauser et al., 2020; Mousseau, 1998). Although our common environments should minimize such effects, we cannot rule out that maternal provisioning of spores or DNA methylation that temporarily activates genes that regulate thermal tolerance may have played a role in the response of individuals to temperature. However, here we interpret site and individual-level trait differences as the result of genetic differences. Our results support the family-level variation in temperature responses observed in another kelp species, Ecklonia radiata (Mabin et al., 2019).
4.1 Spore settlement and maturation
On average, the increasing temperature decreased settlement and maturation rate, suggesting that the anticipated increases in ocean temperatures associated with climate change will result in fewer gametophytes in kelp populations. The effects of temperature on maturation rates were even more severe than those on settlement, with two sites experiencing almost no mature gametophytes at the highest temperature. This confirms the results of previous work that found decreased gametophyte success with increasing water temperature (Shukla & Edwards, 2017). That work examined increases in water temperature from 12 to 15°C and our results suggest that further increases in water temperature will continue to have adverse consequences for early life history stages of kelp. However, despite overall negative effects of increased temperature, the magnitude of the response to increased temperatures is largely dependent on the site of origin of kelp individuals.
Because Los Angeles experiences the warmest average temperatures of our three sites, we expected that individuals from this site might be the most adapted to higher temperatures and have the least response to increasing temperature. Surprisingly, individuals from Monterey had the highest maturation rates at every temperature, even though our lowest treatment temperature was beyond the highest average temperature experienced by individuals at this site. However, Santa Barbara had the highest settlement overall, showed little decrease in settlement between 16 and 20°C, and had the highest average settlement of the three sites at 22°C. Although the Los Angeles population experiences the highest average and maximum temperatures, the Santa Barbara population experiences the most variable temperatures (Table 1). Oceanographic patterns and seasonal upwelling lead to variable warm and cold currents in the Santa Barbara Channel and the largest temperature fluctuations among our three sites (Table 1, Harms & Winant, 1998). Such temperature fluctuation in SB may facilitate coexistence of individuals with an array of thermal tolerances during settlement, particularly if early life history stages can persist for extended periods of time. Just as a storage effect can maintain coexistence among species (Chesson, 2000), it may similarly facilitate coexistence among genotypes. For example, heat-tolerant genotypes may settle well in SB during warm conditions, whereas less heat-tolerant genotypes settle more often during cooler periods, resulting in a population of warm and cold-tolerant individuals. Such variation could also buffer the population against environmental change. Alternatively, we collected sporophylls during an abnormally warm year on the California coast and average temperature differences among sites may not reflect conditions in 2016, as such heat waves can result in habitat compression of upwelling and nutrient availability (Santora et al., 2020).
We also observed significant effects of individuals on settlement and survival. However, the interaction between individuals and temperature explained little variation in any of our fitness estimates. This suggests that there is little opportunity for selection of individuals in response to temperature within populations. However, the considerable variation among individuals that we observed may offer an opportunity for selection in response to other factors not considered in this study. It is possible that such genetic variation affects individual responses to other abiotic conditions, such as increasing CO2 concentrations, or biotic interactions, such as herbivory, but this requires further investigation.
We observed little variation in response to temperature among individuals within a site, suggesting a lack of opportunity for selection in response to temperature at the local scale. However, we observed significant variation among sites in survival and maturation, indicating that some populations are more adapted to changes in temperature than others. If there is little to no dispersal among sites, this suggests that with increasing ocean temperatures, kelp abundance will decline at some sites more than other sites. However, if there is sufficient dispersal to consider these three sites as one regional population upon which selection can act, then differences among our populations suggest that there may be sufficient variation to allow for natural selection in response to temperature. If temperature-tolerant individuals have relatively higher fitness at increased temperature and gene flow introduces those alleles to other sites, then there may be adaptation to temperature at the regional scale. Although the three sites in this study were identified as belonging to different genetic demes, there is also some admixture among these demes (Johansson et al., 2015). Long-distance dispersal of spores or gametes along water currents may be infrequency but can occur during marine heatwaves that greatly shift population ranges (Sanford et al., 2019). Rafting kelp or spores and gametophytes attached to other rafting objects may also regularly facilitate dispersal among sites (Haye et al., 2012; Hernández-Carmona et al., 2006).
4.2 Gametophyte survival
The survival of spores from different populations responded differently to increases in temperature. Survival of spores from Monterey was higher than other sites at 16 and 20°C but decreased considerably at 22°C. This is not surprising given that Monterey never experiences such high temperatures and individuals there have not had an opportunity to adapt or acclimate to high temperatures in the past. In contrast, spores from Los Angeles and Santa Barbara responded similarly to each other and showed increases in survival with increasing temperature. It is important to note though that our experiment manipulated temperature constantly and did not include the variation in temperatures that individuals experience in natural environments; decreases in temperature overnight may provide somewhat of a reprieve from stressful daytime temperatures, particularly in shallow water.
Spores that survived but did not mature may provide additional adaptive potential if those individuals delay development until temperatures return to a less stressful level. Dormancy, or delayed development, is a mechanism used by many organisms across a broad range of taxa (Caceres, 1997; Finch-Savage & Leubner-Metzger, 2006). M. pyrifera gametophytes are known to remain dormant or delay development for months until favorable conditions emerge, which has been suggested as an adaptive strategy (Carney & Edwards, 2010). Because storms and marine heatwaves can remove a huge number of sporophytes from kelp forests (Rogers-Bennett & Catton, 2019), underlying gametophyte banks can facilitate kelp forest recovery in subsequent seasons, so variation in survival may be critical for predicting long-term population dynamics of kelp (Dayton & Tegner, 1984; Ladah et al., 1999; Ladah & Zertuche-González, 2007). We did not test whether such spores would mature further if we had later lowered the treatment temperatures, although previous work suggests that even spores that survive temperature stress may not go on to successfully produce sporophytes (Ladah & Zertuche-González, 2007).
4.3 Potential for evolutionary rescue
Successful completion of the kelp life cycle requires settlement, survival, maturation, and many more stages and components of fitness not examined in this study. The variable responses of each life stage must be considered together when attempting to predict individual fitness. For example, settled spores from Monterey Bay are likely to experience a decrease in spore survival at 22°C, but those surviving spores are more likely to mature at that temperature than spores from Los Angeles or Santa Barbara. Although we expect that the relative differences between populations and individuals we observed in the laboratory are applicable to natural environments, the exact estimates for settlement, survival, and maturation are certain to differ in the field. Temperature variability is likely to be at least as important as mean temperature differences and in California, seasonal temperature differences are tightly tied to upwelling, which also drives nutrient availability. Quantifying fitness under different temperature conditions in the field and considering the collective effects of these different fitness components will be required to ultimately predict evolutionary trajectories in nature. However, the differences in response to temperature observed among sites suggest that there is potential for evolutionary rescue at the regional scale. However, the scope for evolutionary rescue within populations appears to be minimal, at least at these early life history stages. This does not preclude potential differences in individual traits at later life history stages though.
Given its importance as a foundation species, there are considerable efforts at kelp restoration underway (Layton et al., 2020). Recent calls for assisted evolution may be especially important for marine foundation species, such as kelps or corals (Baums, 2008; van Oppen et al., 2015). Given the limited opportunity for selection within kelp populations, genetic rescue via transplantation may be an important conservation effort in the face of declining kelp populations in response to increasing ocean temperature or other threats. If conservationists can identify, breed, and outplant resistant individuals, this may increase success of the outplanted individuals and introduce heat-tolerant alleles to populations that would be subject to natural selection, thus increasing the speed of evolutionary rescue (Mussmann et al., 2017; Tallmon et al., 2004).
5 CONCLUSIONS
This study highlights the value of examining individual variation that is hidden in average trait estimates of species. The variation in traits may be as important as the average in traits, as it provides potential for evolutionary rescue from environmental threats. Our results find little potential for evolutionary rescue from increased temperature within populations in California, although there is some variation among populations that may allow for genetic rescue through natural or human-assisted efforts. Although this research focused on California Macrocystis pyrifera populations, it also highlights the potential for standing allelic variation to facilitate evolutionary rescue for the survival of other populations, species, and ecosystems.
ACKNOWLEDGMENTS
This project would not have been possible without collection assistance from Jamie Canepa, Erin Jaco, Hannah Nelson, Leah Reidenbach, and Sigfrido Zimmerman. Invaluable guidance was provided by Drs. Steve Dudgeon, Jacqueline Padilla-Gamiño, Jeanne Robertson, and two anonymous reviewers. This work was supported with funding from the National Science Foundation (OCE-1559105, DEB-1754449), the California State University, Northridge (CSUN) College of Science and Mathematics, the CSUN Office of Graduate Studies, and the CSU Council on Ocean Affairs, Science, and Technology.
CONFLICT OF INTEREST STATEMENT
None.
Open Research
DATA AVAILABILITY STATEMENT
Data from this experiment have been archived at BCO-DMO through the National Science Foundation and are available at https://www.bco-dmo.org/dataset/878555/data.




