Octospy: What Octopus insularis do in their dens
Abstract
Octopus insularis is a benthic octopod from the tropical and subtropical Western Atlantic Ocean that inhabits semi-permanent “dens” (small crevices) in hard substrate. We used visual surveys to assess den occupancy and remote cameras at den entrances to assess activity patterns of O. insularis of South Caicos (21.5112° N, 71.5190° W) in the Turks and Caicos Islands (May–August, 2020 and July–December, 2021). Dens were occupied for a median of 4 days, but occupancy ranged widely (1 day–2 months), indicating migration between dens at erratic intervals. The two most prevalent behaviors were Away from the Den, time presumably spent mostly foraging, and Quiescence, when the octopus was sleeping or otherwise unreactive. Our data indicate that Octopus insularis is diurnal, with time Away from the Den peaking between mid-morning (0700–1000) and late afternoon (1600–2000), and with Quiescence peaking at night (2000–0600) and also in the middle of the day (1100–1600), although adherence to this pattern was not strict. High interindividual variation and high within-individual stability in the proportions of time Away from the Den and Quiescent suggest that individuals of this species vary greatly from each other in their hunting and resting patterns, while also showing high levels of internal consistency for at least a week. These observations will guide future research with this commercially important species, further differentiates O. insularis from congeners, and demonstrate the efficacy of minimally disruptive field techniques in studying behavior.
1 INTRODUCTION
Interest in octopus behavior by both professional animal behaviorists and by the non-scientific public has grown tremendously as their impressive flexibility and capacity for adaptation become increasingly well known (for review, see Godfrey-Smith, 2016; Hanlon & Messenger, 2018; O'Brien et al., 2018). In the past dozen years, laboratory studies have been conducted on a broad range of octopus behaviors, including sociality (Edsinger & Dölen, 2018), sleep (Medeiros et al., 2020), motor control (Gutnick et al., 2020), problem-solving (Richter et al., 2016), personality (Pronk et al., 2010), and the ability to recognize individual conspecifics (Tricarico et al., 2011) as well as individual humans (Anderson et al., 2010), among others. Octopus have also been studied in their natural habitats, including determination of prey choice and its correlates with individual personality (Scheel, Leite, et al., 2017), responses to novel objects as well as simulated prey items (realistic rubber crab) and conspecifics (mirror; O'Brien, Di Miccoli, & Fiorito, 2021), interaction and communication with conspecifics (Godfrey-Smith et al., 2022; Humbert et al., 2022; O'Brien, Di Miccoli, & Fiorito, 2021; O'Brien, Taylor, et al., 2021; Scheel et al., 2016; Scheel, Chancellor, et al., 2017), interactions and niche partitioning with sympatric octopus species (Bennice et al., 2019; Huffard & Bartick, 2015), interactions with fish (Humbert et al., 2022; Sampaio et al., 2020), ecosystem engineering (Scheel et al., 2014, 2018), and habitat use (Scheel & Bisson, 2012).
Despite this growing body of field literature, there are many difficulties associated with accessing subjects in the ocean and collecting data in the field (e.g., the need for specialized marine equipment and transportation, dependency on weather, currents and tides, etc.), leaving much to be learned about octopus behavior and activity in the wild. Such information is crucial to designing good experiments (e.g., to determine what point in a species' natural activity cycle is the ideal time to conduct certain types of tests, or what food types are “preferred” items that can be used as experimental rewards), to explore topics that cannot be simulated or addressed in captivity (e.g., inter-specific interactions, niche partitioning, long distance navigation), and to validate the findings from laboratory studies. Moreover, octopus behavior observed in artificial settings is likely to differ widely from behavior in the natural environment, given the confined space and lack of natural light and tidal flux, the presence of unnatural sensory cues (such as glass, plastic, and metal) and the absence of other organisms, particularly of predators, which can influence octopus behavior (e.g., Meisel et al., 2013).
With this in mind, we conducted extensive field observations of Octopus insularis (Leite et al., 2008), a mid-sized and short-lived (<1 year) species of benthic octopod occurring along the shallow tropical and subtropical coasts of the northwestern and southwestern Atlantic Ocean (Avendaño, Roura, et al., 2020; Batista et al., 2021; Leite, Haimovici, Mather, & Oliveira, 2009; Lima et al., 2014; O'Brien, Bennice, & Leite, 2021), including the coastal waters of Brazil, Gulf of Mexico, and West Indies. It can be found on hard substrates, particularly reefs, rubble, and bedrock, where it inhabits natural holes and crevices (dens) and is a predator of benthic invertebrates (Leite, et al., 2009, b). O. insularis has long been a significant component of Brazilian and Mexican fisheries (Flores-Valle et al., 2018; Gonzalez-Gomez et al., 2020; González-Gómez et al., 2018; Leite et al., 2008; Lima et al., 2017; Lopes et al., 2021) and it co-occurs with at least seven other species of octopus throughout its geographic range (Avendaño, Roura, et al., 2020; Hanlon, 1988; Jereb et al., 2014; Jesus et al., 2021; Leite & Haimovici, 2006). Due to a proclivity for warm (23°C and 30°C) shallow (0.5–40 m) water (Leite et al., 2008; Leite, Haimovici, Mather, & Oliveira, 2009; Rosas-Luis et al., 2020), its high abundance (Batista & Leite, 2016; Bouth et al., 2011), and its tendency to scatter highly visible prey debris in the vicinity of its den (Leite et al., 2016; Mather., 1991), O. insularis are relatively easy to locate and observe, making it an ideal species in which to study behavior in the wild.
In the past, O. insularis (along with a number of other species across the globe, see Avendaño, Roura, et al., 2020) was mistaken for O. vulgaris (O'Brien, Bennice, & Leite, 2021). The historical conflation of these species contributed to the misconception that O. vulgaris is globally distributed and adopts different lifestyles in different regions, including diurnal behavior in the western Atlantic (Mather, 1988), and nocturnal behavior in the eastern Atlantic and Mediterranean (Altman, 1966; Kayes, 1973; Lane, 1960; Woods, 1965). The establishment of O. insularis as a species distinct from O. vulgaris by Leite et al. (2008) clarified the reasons for these regional differences in the Atlantic, and other work has established that “O. vulgaris” is actually a species complex composed of at least six species (see Avendaño, Roura, et al., 2020 and references therein). While similar looking to O. vulgaris, O. insularis is not part of the O. vulgaris species complex. It does, however, overlap in geographic range with a similar-looking member of the O. vulgaris species complex, O. americanus, another species that was historically conflated with O. vulgaris and a further source of confusion (Avendaño, Roura, et al., 2020; O'Brien, Bennice, & Leite, 2021).
The behavior and activity of O. insularis have been documented in the field in both Brazil (Leite, Haimovici, & Mather, 2009) and Bermuda, under the name O. vulgaris (Mather, 1988, 1991, 1994; Mather & O'Dor, 1991). Long-term monitoring showed that individual octopuses will inhabit a single den for a median of 6 days (reported as a mean of 10) before moving to a new one (Mather, 1994). In Brazil, O. insularis activity has only been formally documented during diurnal excursions from dens. During these observations, octopuses spent 80% of time searching for food (Leite, Haimovici, & Mather, 2009). In Bermuda, where octopuses were observed both in and Away from the Den by snorkelers, octopuses spent half of the daytime sleeping and a sixth of the time resting but awake, while the remaining time was spent foraging (11%), consuming prey (13%), and performing “home maintenance” and other miscellaneous activities (5%) (Mather, 1988). A more recent study of O. insularis in captivity, using specimens collected from Brazil, found that during 12 h of artificial light, 55.73% of the time was spent quiescent, 37.85% alert, and 6.42% of the time active (Medeiros et al., 2020). During quiescence, octopuses spent most of the time in “Quiet sleep,” with little movement and dulled reactions to external stimuli. However, these periods of Quiet sleep were punctuated regularly by short bursts of “Active sleep,” a state analogous to REM sleep in vertebrates in which the octopus twitches and shows changes in body patterning, with the time between these bursts usually lasting around half an hour (Medeiros et al., 2020).
In order to determine the den occupancy habits and behavioral patterns of O. insularis around South Caicos (Turks and Caicos Islands), we assessed O. insularis behavior using minimally disruptive techniques (visual surveys and remote photography and videography). From our data, we calculated den occupancy time, activity budgets, whether octopuses rest and sleep or leave the den at particular times of day, if individuals differ in the amount of time they devote to certain behaviors and if they are stable in this over time. While O. insularis has been studied in the wild before, our study differs from these previous observations in its location (the West Indies) and in its use of primarily remote recording, rather than focal follows (Leite, Haimovici, & Mather, 2009; Mather, 1988), to assess behavior. This is also the first study to observe the nocturnal activities of O. insularis, since previous observations were restricted to daylight hours.
2 MATERIALS AND METHODS
We observed O. insularis behavior in situ off the island of South Caicos in the Turks and Caicos Islands (West Indies). Den occupancy patterns were tracked via daily snorkel surveys from April to August, 2020. In order to document activity patterns, six individuals were recorded with time lapse photography on multiple separate days between May and August, 2020 during daylight, and 10 individual octopus were recorded using a specially made device, the Octopus Monitoring Gadget (OMG; see Bennice et al., 2019) between July and December, 2021 during both daylight and darkness.
2.1 Field site and study subjects
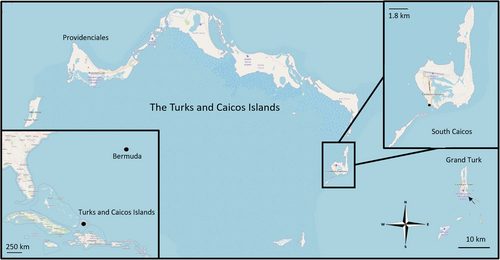
Field observations were conducted in a roughly 5600 m2 area consisting of two small coves in shallow (<2 m) subtidal waters adjacent to the Center for Marine Resource Studies (CMRS, operated by The School for Field Studies) on the southwestern coast of South Caicos in the Turks and Caicos Islands, West Indies (Figure 1; 21.7° N 71.8° W). The Turks and Caicos Islands are located on three shallow (< 20 m) carbonate plateaus (Caicos, Turks Island, and Mouchoir Banks) in the southeastern portion of the Lucayan archipelago (Logan & Sealey, 2013). The study site at the southwestern end of South Caicos lies at the margin of a deep (up 2200 m) channel (Turks Island Passage) between the Caicos and Turks Island banks. Surface seawater temperatures range from 22°C to 28°C according to season (Logan & Sealey, 2013) and salinity ranges from 36.4 to 36.5 ppt (Jury, 2013). The seafloor in the study area consists largely of limestone and dead coral heads, interspersed with patches of sand and seagrass (C.E. O'Brien, pers. obs.). Using characteristic body patterns (Leite et al., 2008; O'Brien, Bennice, & Leite, 2021) and estimated body size (Leite, Haimovici, & Mather, 2009) as species identifiers, we located O. insularis inhabiting natural holes and crevices in the hard substrate at depths ranging from half a meter to 3 m (Batista & Leite, 2016; Leite et al., 2008; Leite, Haimovici, Mather, & Oliveira, 2009). Based on comparison with objects of known size in the environment (e.g., bivalve shells, green algae, octocorals), octopus mantle lengths were estimated visually to range between 10 and 80 mm, largely corresponding with juvenile and sub-adult size dimensions (Lima et al., 2014). No attempt was made to measure or mark individuals as it has been found that doing so causes octopuses to flee from an area (Mather, 1994).
2.2 2020 Field data collection
2.2.1 Den occupancy
The presence of O. insularis in dens within this area was monitored daily from March 21 to August 27, 2020, with the exception of July 30–August 1 (Hurricane Isaias) and August 23 (Tropical Storm Laura). Visual surveys were conducted by snorkeling to all known den locations and recording the presence or absence of an octopus, along with the time and the presence or absence of fresh prey remains in front of the den (prey midden). In addition to these daily circuits, the study area was searched several times a week for new dens, which were easily detected by the presence of prey remains around the den, or simply by the presence of an octopus at the den entrance. When a newly occupied den was located, its location was marked with nearby natural materials (usually a rock) to assist in future relocation, and it was added to the regular daily survey route. Surveys and searches typically occurred in the late afternoon, but occasionally in the late morning or early afternoon, and were planned in accordance with weather conditions and local COVID-19 restrictions.
Den occupancy was calculated as the number of days between when an octopus was first found in a den until the last day it was seen in that den. Surveys located 34 distinct dens within the study area over the 5 months of observation. However, two of the dens were occupied on the first day of observation and five of these dens (including two of the individuals monitored with time lapse photography) were still occupied at the end of the study. Since the beginning or end date of occupancy of these dens were unknown, they were excluded from the calculations, for a final sample of 27.
Based on the number of individuals seen simultaneously in different dens on a single day, a minimum of six distinct individuals were observed over the course of the den occupancy surveys in 2020. The actual number of individuals observed is likely higher than that, theoretically up to the total number of occupied dens observed. However, given that some octopuses, upon vacating one den, sometimes appeared (based on size and proximity) to move to another den in the study area, it is likely less than that.
2.2.2 Field recording
Six individual octopus from this population were filmed from dawn to dusk (approximately 0600 to 1930 h) on multiple days between late May and late August (Table A1). Five individual octopuses were filmed on two separate days, while a sixth individual was filmed over 3 days. Eight other filming attempts which failed or were incomplete due to technical issues with the camera or because the octopus physically displaced or destroyed the camera were excluded from analysis. Octopuses were recorded using the time lapse photography function on five Lightdow® HD 1080P action cameras sealed in underwater housings. The cameras were mounted onto a three- or four-pound dive weight and placed approximately 30–50 cm away from the entrance to an octopus den. Battery life of the cameras varied from 2.5 to 4 h, necessitating that the camera be changed three to four times over the course of the day (see Table A1). The cameras were set to take a single 5 Mp photographer every 20 s (although aberrations in the timing device of the cameras resulted in actual intervals of 19–21 s), yielding about 2500 photographs for each daytime sequence.
2.3 2021 Field data collection
2.3.1 Field recording
Nine individual octopus were filmed for a 24-h period, and one for 22.67 h, from late July to early December, 2021 (Table A2). Two other filming attempts failed and were excluded from analysis because either the octopus was occluded by den angle and macro algae for the majority of the time or left the den soon after filming commenced and did not return. Filming dates were selected opportunistically based on having moderate to low wave activity, in order to minimize the likelihood of camera displacement or obscured visibility. Individuals were selected for filming based on their availability during favorable filming conditions. Octopuses were recorded on video with GoPro 3+ cameras connected to an external power bank (Cygnett 20,000 mAh 5 V Portable Power Bank) along with a red (640 nm) light inside a sealed PVC tube. This “Octopus Monitoring Gadget,” or “OMG” (see Bennice et al., 2019), was weighted with two rubber-coated 3 lb dive weights and placed approximately 30–50 cm from the entrance to an octopus den. Automatic shutdown of the GoPros after 21.3 h of continuous filming necessitated that the camera be changed once to record a 24-h period. These changes occurred after approximately 8 or 16 h after initial placement of the OMG (see Table A2). The red light enabled octopus behavior to be observed at night. This light was composed of five 5 mm red LED bulbs and was powered by USB connection to the power bank. Red light (620–750 nm) is considered minimally intrusive for observation of marine organisms in general since they are typically insensitive to wavelengths over 600 nm (Weiss et al., 2006), and for octopus in particular, as the opsin in the related O. vulgaris has a maximum absorption of 475 nm (Hanke & Kelber, 2020).
2.4 Behavioral analysis of Timelapse photographs and videos
The behavior of the octopus in each time lapse sequence and video was categorized according to Table 1, noting the start and stop time of each behavior to the second. Analysis was conducted by the first and second authors separately, and upon comparison of these, any discrepancies between behavioral categorizations of the two analyzers were re-examined and rectified through consensus or designated as unknown. Note that for both experimental and methodological reasons, the behavioral categories defined in Table 1 differ in some cases from the categories and definitions used in other studies of O. insularis (e.g., Mather, 1988; Medeiros et al., 2020). Time spent Away from the Den and Quiescent are of particular interest to researchers of octopus behavior (e.g., Medeiros et al., 2020), and thus were isolated for further analysis.
| Behavior | Definition |
|---|---|
| Away from the den | The body of the octopus (with the occasional exception of one or two arm tips) is at least one mantle length from the den or not visible in photograph/video, and subsequently re-enters the den |
| Active | Octopus changes its body's position, usually by crawling, or moves its arms or siphon without changing positions, including to manipulate objects, defecate, or groom itself |
| Alert | Octopus is stationary and unmoving, or moves very slightly without changing positions, with eyes open and reactive to stimuli |
| Quiescence | Octopus is mostly stationary, making only brief movements, with eyes narrowed and minimally reactive to external stimuli or is fully withdrawn into its den. Note that this term largely consists of behaviors that other authors have termed “asleep” (Mather, 1988) and “eyes closed” (Brown et al., 2006), as well as “quiet sleep” and “active sleep” (Medeiros et al., 2020). However, because sleep could not always be visibly differentiated from restful wakefulness, we opted to use the more conservative term “Quiescence” to denote a general lack of engagement with its environment |
| Prey handling | Octopus feeding on or attempting to subdue prey |
| Unknown | Behavior unknown because octopus was entirely withdrawn into the den, obscured by poor water quality/an intervening object or footage is missing |
2.5 Statistical analyses
All statistical analyses were conducted in RStudio version 3.6.2 (RStudio Team, 2019) with an alpha of 0.05. In most cases, the data are not normally distributed. Thus, median and interquartile range (IQR) were used as summary statistics, and nonparametric statistical methods applied.
2.5.1 Activity budgets
From the time lapse photography sequences taken in 2020, the proportion of time engaged in each activity was calculated as the total number of seconds that elapsed between the first photograph of an octopus engaged in that activity and the last, summed across all occurrences of that activity in a single day, and divided by the total number of seconds the octopus was observed for that day. From the OMG sequences recorded in 2021, the proportion of time engaged in each activity was calculated as the total time that each behavior could be seen in videos. The proportions of time devoted to each activity during 2020 and 2021 was then summed across all individuals and days and divided by the total amount of observation time to yield a summary activity budget for each year.
2.5.2 Away from the Den and Quiescence
Away from the Den and Quiescence were summarized by the proportion of individuals engaged in each behavior during each hour over all of the observation periods (2020 and 2021, correcting for daylight savings), by the minimum, maximum, and median (IQR) number of occurrences per observation period, and by the median lengths of instances of Away from the Den and Quiescence across all individuals, days, and years. Since Quiescence was often punctuated by short periods in which the octopus would briefly shift positions or be Alert, the total span of time which Quiescence was not interrupted by another activity for more than 10 min was used for these calculations.
The degree of difference between individuals in time spent Away from the Den and Quiescent in 2020 was quantified using the intraclass correlation coefficient (ICC). The ICC (also known as “Repeatability”) is a standardized metric that expresses the variation between individuals as a proportion of total variation (interindividual plus intraindividual variation; Lessells & Boag, 1987). In this case, a high ICC for an activity would indicate that there are strong interindividual differences in the proportion of time each individual devotes to it (Wolak et al., 2012). Data were first checked for normality and equality of variance using Shapiro–Wilk tests and Levene's tests, respectively. The data for Quiescence were normally distributed and had equal variance (Shapiro–Wilk and Levene's tests, p > .05), but data for Away from the Den needed to be log-transformed to achieve a normal distribution. The rptR package (Stoffel et al., 2017) was then used to fit mixed effect models to the activity budget data in order to estimate the ICC of each activity assuming a Gaussian distribution. Confidence intervals were estimated with parametric bootstrapping (N = 1000), and p-values via likelihood ratio tests (LRT; Stoffel et al., 2017). In this analysis, individual identity was included as a random factor and the Nest function in the package ICC (Wolak et al., 2012) was used to calculate the minimum necessary sample size given the value of the ICC, the number of repeated measures, and the width of the confidence interval (Wolak et al., 2012).
Individual stability in time spent Away from the Den and Quiescent from the first day of observation to the second in 2020 was assessed with the individual stability statistic (ISS; Asendorpf, 1990). A Z-transformation ((x − group mean)/group standard deviation) was first performed on the proportion data, and the ISS was then calculated according to the equation: ISS = 1 − (Zt1 − Zt2)2/2, with “Zt1” being the Z-transformed value for the first day of observation. The resulting metric ranges between 0 and 1 and quantifies the consistency of an individual in a particular behavior, with high values indicating high stability (Asendorpf, 1990; Dingemanse et al., 2010). ISS values cannot be compared across different studies (Cleasby et al., 2015), but give a sense of the relative stability of behaviors and individuals within a single study.
2.6 Ethical Statement
Permission to conduct these observations was granted by the Turks and Caicos Department of Environment and Coastal Resources (DECR, permit number D2-001). No special permission is required to record octopus in the wild, although every effort was made to limit the impact of the study on the animals involved, in particular by minimizing the time the experimenter spent in proximity to the dens and by not handling the animals.
3 RESULTS
3.1 Den occupancy
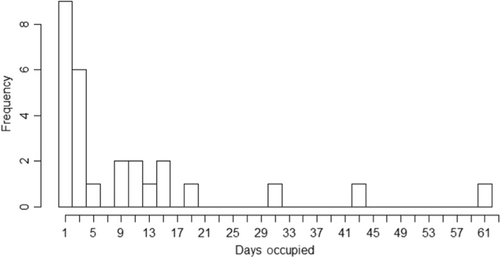
During each day of observation in 2020, 0–6 individual octopuses inhabited the 5600 m2 study area, with a median (IQR) of 2 (2) per day. Thus, the population density of the study area ranged from 0 to 0.0004 individuals per m2 between April and August, 2020. In total, 34 distinct O. insularis den were observed in the study area over the 160 days of monitoring. The dens included in the calculations for den occupancy in 2020 (N = 27) were inhabited for a median (IQR) of 4 (10) days in a row, with a minimum of 1 and a maximum of 62 days (Figure 2).
3.2 Activity budgets and patterns
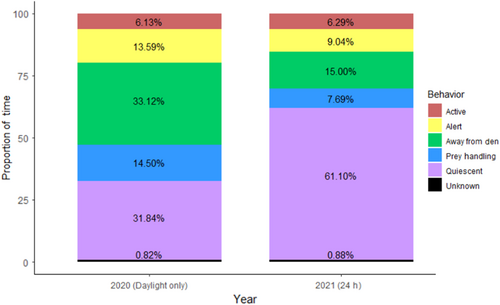
Pooled across all individuals and observation days, the six O. insularis photographed in daylight over 13 observation periods in 2020 spent 6.01% of the observation time Active, 13.59% Alert, 33.12% Away from the Den, 14.5% Prey Handling, 31.84% Quiescent, and 0.82% in unknown activities (Figure 3). Individual octopuses' percentages of time spent engaged in each activity across its 2–3 days of observation are presented in Table 2. Pooled across all individuals (N = 10), the O. insularis video recorded during 10 24-h periods in 2021 spent 6.29% of the observation time Active, 9.04% Alert, 15.00% Away from the Den, 7.69% Prey Handling, 61.10% Quiescent, and 0.88% in unknown activities (Figure 3). Individual octopuses' percentages of time spent engaged in each activity are presented in Table 3.
| Individual | Date | Active | Alert | Away from the den | Quiescent | Prey handling | Unknown |
|---|---|---|---|---|---|---|---|
| 1 | May 21 | 5.25% | 27.40% | 38.10% | 23.88% | 5.17% | 0.20% |
| 1 | May 25 | 6.19% | 24.13% | 44.28% | 23.58% | 1.79% | 0.04% |
| 2 | June 2 | 9.29% | 12.43% | 10.06% | 26.72% | 41.40% | 0.11% |
| 2 | June 9 | 9.36% | 11.67% | 16.03% | 46.39% | 14.89% | 1.65% |
| 2 | June 13 | 16.23% | 11.08% | 26.07% | 38.21% | 8.26% | 0.15% |
| 3 | July 15 | 3.56% | 7.66% | 24.15% | 57.24% | 5.29% | 2.09% |
| 3 | July 17 | 4.32% | 11.74% | 28.58% | 38.68% | 15.93% | 0.75% |
| 4 | August 9 | 1.39% | 2.12% | 94.15% | 0.00% | 0.00% | 2.35% |
| 4 | August 14 | 2.21% | 2.02% | 95.78% | 0.00% | 0.00% | 0.00% |
| 5 | August 9 | 5.30% | 16.01% | 6.34% | 31.20% | 40.76% | 0.40% |
| 5 | August 12 | 3.43% | 14.12% | 9.66% | 45.82% | 26.82% | 0.15% |
| 6 | August 25 | 4.40% | 10.95% | 24.19% | 48.72% | 8.92% | 2.82% |
| 6 | August 26 | 8.39% | 25.94% | 14.72% | 32.67% | 18.29% | 0.00% |
- Note: See Table 1 for definitions of each behavior.
| Individual | Dates | Active | Alert | Away from the den | Quiescent | Prey handling | Unknown |
|---|---|---|---|---|---|---|---|
| 1 | July 24–25 | 4.33% | 13.31% | 5.71% | 67.53% | 9.13% | 0.00% |
| 2 | August 29–30 | 8.90% | 9.41% | 3.08% | 71.21% | 7.41% | 0.00% |
| 3 | August 30–31 | 6.19% | 8.76% | 40.22% | 42.31% | 2.53% | 0.00% |
| 4 | August 31–September 1 | 6.29% | 8.87% | 21.77% | 61.63% | 1.45% | 0.00% |
| 5 | September 9–10 | 8.41% | 22.94% | 3.84% | 48.59% | 11.62% | 4.61% |
| 6 | September 25–26a | 3.63% | 6.03% | 17.27% | 61.96% | 10.76% | 0.36% |
| 7 | October 13–14 | 6.16% | 5.20% | 18.07% | 59.14% | 11.42% | 0.00% |
| 8 | October 14–15 | 4.83% | 7.25% | 1.86% | 68.10% | 13.83% | 4.14% |
| 9 | November 2–3 | 9.46% | 6.53% | 7.35% | 67.98% | 8.66% | 0.02% |
| 10 | December 2–3 | 4.81% | 3.14% | 30.33% | 61.25% | 0.46% | 0.00% |
- Note: See Table 1 for definitions of each behavior.
- a 22.67 h monitoring period.
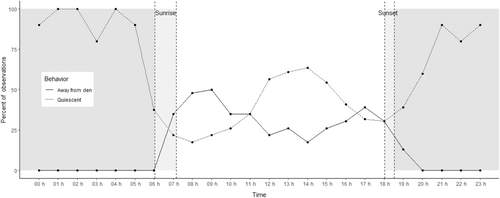
Across all individuals, observation periods and years, the proportion of individuals that were away from the Den or Quiescent during each hour of observation is plotted in Figure 4. Between 2000 and 0500 h (night time), no octopus left the den, while all individuals (n = 10) engaged in at least one Quiescent episode during each of these hours. During daylight (approximately 0600 to 1830 h), the number away from the Den peaked in the hour preceding, during and in the 3 h following the range of sunrise times (0500–1000 h) and the 2 h preceding, during and in the roughly hour and a half following the range of sunset times (1600–1900 h). Quiescence peaked from 2000 and 0500 h and from 1000 to 1700 h. Spearman correlation showed that over the 23 observation periods, the proportions of time engaged in these two behaviors were strongly negatively correlated with each other (R = −.61, S = 1,015,966, N = 23, p < .01).
With six behaviors, there were 30 transition pairs possible, and Figure 5 depicts the frequency of transitions between each of the behaviors, with percentages indicating the proportion of instances in which each behavior was preceded by (inputs) or followed by (outputs) each of the five other behaviors. Since individuals in 2020 were not filmed continuously, but rather photographed every ca. 20 s, meaning that a certain percentage of behavioral transitions were missed, only 2021 data were used for this analysis. A total of 1386 behavioral transitions were recorded across the 10 individuals and days of 2021 video observation. Eight behavioral transition pairs never occurred (Quiescent to Away from Den, Prey Handling to Away from Den, Prey Handling to Unknown, Unknown to Prey Handling, Away from Den to Alert, Away from Den to Unknown, Away from Den to Prey Handling and Away from Den to Quiescent), while Away from Den was followed by Active 100% of the time.
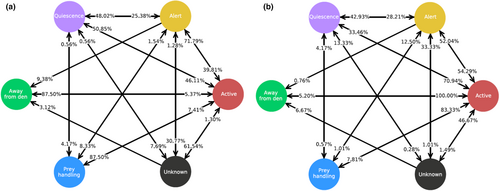
3.3 Away from the Den and quiescence
Pooling data across all individuals, days, and years, the 16 octopuses observed were Away from the Den a total of 40 times across the 23 days of observation, corresponding to 22.68% of the total observation time. Between two and seven separate excursions Away from the Den were made during each 14-h observation period in 2020, with a median (IQR) of 2.00 (1.00) per observation sequence. Between one and five separate excursions Away from the Den were made during each 24-h observation period in 2021, with a median (IQR) of 3.00 (2.00) per observation sequence. Instances of Away from the Den ranged between 4.00 s and 12.93 h in length, with a median (IQR) duration of 23.72 (71.77) min.
There were 81 episodes of Quiescence across all 16 individuals, 23 observation periods, and 2 years, corresponding to 51.7% of the total observation time. Individuals had zero to five episodes of Quiescence per 14-h observation period in 2020, with a median (IQR) of 2.00 (3.00) per observation period. Individuals had two to nine episodes of Quiescence per 24-h observation period in 2021, with a median (IQR) of 5.00 (3.25) per observation period. All but one individual (individual 4 in 2020) was observed engaging in Quiescence at least once per day. Individual 4 spent almost the entire observation time Away from the Den on the 2 days it was observed. It is likely that Individual 4 did engage in Quiescence on these days, but that it did so in another location out of view of the camera, perhaps utilizing multiple dens as Enteroctopus dofleini does (Mather et al., 1985). Time spent Quiescent ranged between 34.00 s and 14.28 h in length, with a median (IQR) duration across all episodes of 54.65 min (235.85).
Interindividual variation in the proportion of time spent Away from the Den and Quiescent in the six individuals tested at least twice in 2020 was assessed by calculating the Intraclass Correlation Coefficient (ICC). The ICC for the log-transformed Away from the Den data was 0.87 (CI = 0.40–0.98, N = 6, p = .002) and the ICC for the untransformed Quiescence data was 0.75 (CI = 0.02–0.95, N = 6, p = .02), showing strong variance among individuals in the amount of time devoted to these behaviors. While estimates based on transformed values can diverge from those based on the original data (see Nakagawa & Schielzeth, 2010 and references therein), the “true” ICC in this case would still be very high given the high ISS (see next paragraph) and wide variance between individuals in the time spent Away from the Den (see Table 2).
Intraindividual variation in the proportion of time spent Away from the Den and Quiescent in the six individuals tested two or more times in 2020 was assessed by calculating the Individual Stability Statistic (ISS). The individual stability statistics (ISS) for the time spent Away from the Den and Quiescent by the six individuals across their first 2 days of observation are listed in Table 4. The median (IQR) values were high for both: 0.99 (0.01) for Away from the Den and 0.64 (0.42) for Quiescence.
| Individual | Away from the Den | Quiescence | Days between observations |
|---|---|---|---|
| 1 | 0.98 | 1.00 | 4 |
| 2a | 0.98 | 0.40 | 7 |
| 3 | 0.99 | 0.47 | 3 |
| 4 | 1.00 | 1.00 | 5 |
| 5 | 0.99 | 0.67 | 2 |
| 6 | 0.95 | 0.60 | 1 |
| Median (IQR) | 0.99 (0.01) | 0.64 (0.42) |
- Note: Summarized by group median (IQR). The time between the first and second observation period is included for reference.
- a The third day of observations for this individual was excluded from the ISS calculation for consistency.
4 DISCUSSION
One of the most dramatic changes in the evolutionary history of the octopods and other coleoid cephalopods was the transition from a hard-shelled ancestor to a mostly soft body. This left coleoids vulnerable to predation by fishes, a selective pressure which likely drove the development of alternative ways to evade these predators, including crypsis, advanced sensory abilities, and cognitive complexity (Amodio et al., 2019a, 2019b; Grasso & Basil, 2009; Jaitly et al., 2022). Among those octopods that have adopted a benthic lifestyle, many (if not most) utilize a crevice or hard object as a protected refuge (Hanlon & Messenger, 2018), commonly referred to as a “den.” But what do octopuses do in their dens? How much of their time do they spend there? How long do they stay at any particular den? These have been difficult questions to answer. In this study, we utilized minimally disruptive methods (visual surveys and remote photography and videography) to “spy” on O. insularis in dens in its natural habitat, documenting for the first time the 24-h activity cycle of this species.
4.1 Den occupancy
Our results suggest that O. insularis have a nomadic lifestyle, only transiently inhabiting any particular crevice or hole, with a length of stay that varies widely (1 day to a few months), but typically lasting less than a week. This movement between dens at erratic intervals is in accordance with several other species observed in the wild that span a range of body sizes and geographic regions. These include E. dofleini (Hartwick et al., 1984; Mather et al., 1985), O. bimaculatus (Ambrose, 1982), O. cyanea (Forsythe & Hanlon, 1997), O. joubini (Mather, 1982), and O. vulgaris (Altman, 1966; Kayes, 1973). Thus far, it appears that temporary site fidelity of widely varying lengths within species is a widespread trait in the order Octopoda.
What prompts O. insularis to leave a den and seek a new one is not entirely clear, but den size and prey availability in the near vicinity are likely a factors, as Mather (1994) found that O. insularis in Bermuda stayed longer in dens that were larger and had more preferred prey species around (as indicated by shell middens). Mather and O'Dor (1991) posited that O. insularis employ a “win-switch” strategy (Stephens & Krebs, 2019), in which they remain in a den until they can no longer locate enough prey in the surrounding area and move on to a new one. The data in the present study do not address this hypothesis directly, but a win-switch strategy could explain the variation observed in den occupancy times. However, the fact that individuals sometimes appeared to move to a nearby den, rather than away from the study area entirely, suggesting that factors besides the prey availability in an area, such as the presence of predators or researchers, influenced den occupancy. Note that while we strove to be as unintrusive as possible, it is also possible that the periodic surveys by the snorkeling observer affected den occupancy patterns to some degree, potentially increasing the rate of octopus relocation.
Another possibility suggested by Mather and O'Dor (1991) is that O. insularis moves dens frequently to prevent predators, such as large fishes, sharks, rays, or other cannibalistic octopuses (Dantas et al., 2022), from learning their location. While the population density of potential predators was not quantified in the study, nurse sharks (Ginglymostoma cirratum), barracuda (Sphyraena barracuda), Southern sting rays (Hypanus americanus), and eagle rays (Aetobatus narinari) were occasionally observed in the study area during the periods of observation. Spotted morays (Gymnothorax moringa) were particularly abundant in the area, and night time footage from 2021 recorded two separate (unsuccessful) attacks by this species on O. insularis. In addition, visits by researchers may also have been interpreted as a threat, although the continued presence of individuals in the same den despite repeated visits in most cases (two-thirds of dens were occupied for 3 or more days) suggests that this did not cause most octopuses to change dens.
It was surmised that octopus found in the same den on different days were the same individual. This conclusion was based on the fact that during the period of observation, there was no evidence that octopuses evicted one another from dens: no altercations were witnessed or recorded and in no instance did octopus inhabiting a den change noticeably in appearance (e.g., relative size, wounds). Moreover, since numerous natural crevices in the substrate and empty conch shells (Aliger gigas) went unused over the course of the study, dens did not appear to be a limiting factor to O. insularis in this area, as it can be to other species in some similar habitats (e.g., O. briareus in Sweetings Pond; Aronson, 1986). This is further evidenced by the fact that once a den had been vacated, it was not re-inhabited by any other octopus, at least during the period of observation, a finding which is consistent with those of Mather (1994). Moreover, many crevices which appeared to be suitable for octopus habitation went unused during the course of this study. It is likely that in areas with fewer suitable crevices, where octopuses may compete physically to forcibly evict each other from prime dens, the den occupancy pattern observed here may not hold. Indeed, in Noronha, Brazil, fishermen will revisit the same dens and find them reoccupied by new octopus within a week (T. Leite, pers. obs.).
4.2 Activity budget and patterns
The activity budget of O. insularis is dominated by Away from the Den and Quiescence, with about a third or less of observation periods occupied by Active, Alert, and Prey Handling. The low proportion of time spent outside the den (33% during daylight, 15% over a 24-h period) by most octopuses in this study supports the characterization of O. insularis as a “lazy” (Mather, 1988) “time-minimizing forager” (Leite, Haimovici, & Mather, 2009; Mather & O'Dor, 1991) that trades faster growth and potential mating opportunities for a reduction in predation risk by spending as little time out of the den (and thus exposed to predators) as possible. This proportion of time Away from the Day is very similar to that observed in O. cyanea (24% and 35% of daylight in two individuals; Forsythe & Hanlon, 1997) and perhaps E. dofleini. (Data for E. dofleini are conflicting: Mather et al. (1985) report 45% Away from the Den over 24-h monitoring periods while Scheel and Bisson (2012) report they were away only 14% of 24-h periods.)
One factor we could not account for in our assessment of activity budgets was the possibility that some individuals inhabited and utilized multiple dens during an observation period. This might be the case for individual #4 in 2020 and #3 in 2021, both of which spent more than twice as much time away from the observed den than the other octopuses in their cohort. If a portion of this time was spent at another protected crevice, then the activity budgets reported here slightly inflate time spent Away from the Den and deflating time spent in all other activities.
Based on the proportion of individuals Away from the Den and Quiescent during each hour of observation across the 2 years (Figure 4), O. insularis can be classified as a diurnal species, with excursions Away from the Den peaking in the morning and evening and Quiescence peaking in the middle of the day and throughout the night. Adherence to this diurnal pattern is not strict however, as excursions Away from the Den occurred all throughout the day, and Quiescence occurred all throughout the day and night. Although the peaks in excursions during the early morning and evening might suggest that O. insularis is crepuscular, these peaks were not restricted to the brief low light periods around dawn and dusk, which is characteristic of crepuscular species by definition (Hammerschlag et al., 2017). Moreover, excursions occurred at a some level at every hour of the day, as is the case with O. insularis in Brazil (Leite, Haimovici, & Mather, 2009). This diurnal activity pattern has important implications for future work, as researchers can use this information to aid in species identification, for planning fieldwork to maximize their chances of observing a particular behavior of interest (e.g. foraging or sleeping), or to optimize the feeding schedule of captive O. insularis.
This is the first study demonstrating that O. insularis is diurnal in its natural environment. This trait distinguishes O. insularis from O. vulgaris sensu stricto and other members of the O. vulgaris complex, such as the geographically co-occurring O. americanus, both of which are nocturnal in the field (Altman, 1966; Bennice et al., 2021; Kayes, 1973; Lane, 1960; Woods, 1965). Indeed, despite overlapping geographic ranges (Avendaño, Hernández-Flores, et al., 2020; Avendaño, Roura, et al., 2020; O'Brien, Bennice, & Leite, 2021) and their similar size and appearance, the diurnal behavior observed in this study further reinforces the idea that O. insularis and O. americanus occupy distinct ecological niches: O. insularis is adapted to warmer (23°C–29°C), generally shallower (0.5–40 m) water, and is active mostly during the day (Leite, Haimovici, & Mather, 2009; Leite et al., 2008; Rosas-Luis et al., 2019; present study), while O. americanus prefers colder (18°C–25°C), usually deeper (up to 200 m) water and is active at night (Amado et al., 2015; Bastos, 2018; Lima et al., 2017). As with Macrotritopus cf. defilippi and O. americanus (referred to as O. vulgaris) at Blue Heron Bridge in Florida (Bennice et al., 2019, 2021), these differences likely facilitate resource partitioning between O. insularis and O. americanus in areas of geographic overlap, reducing competition over prey and habitat and direct confrontations between the two species.
Because it was long-confounded with the O. vulgaris complex (O'Brien, Bennice, & Leite, 2021), the diurnal habits established here for O. insularis help explain the historical misconception that O. vulgaris sensu stricto has different activity patterns in tropical and temperate locations (e.g., Mather, 1988). Additionally, along with the data presented in O'Brien, Bennice, and Leite (2021), our observations support the fact that the geographic range of O. insularis extends to Bermuda, and that the species observed by Mather (1991); Mather (1988); Mather (1992); Mather (1994), Mather and Mather (1995), Mather and O'Dor (1991), and Mather et al. (2012) in Bermuda, which exhibited similar diurnal behavior and the body patterning differences discussed in O'Brien, Bennice, and Leite (2021), was in fact O. insularis. This demonstrates the potential utility of activity patterns, in addition to morphology and body patterning, in distinguishing species of octopus from each other.
There were three potential sources of bias inherent to our observation methods. The first was the fact that the camera changes during time-lapse and video surveillance likely disrupted natural activity cycles to some degree. However, of 48 camera changes in 2020, only eight (17%) were followed by a change in activity 5 min after the camera was replaced. In 38 of the camera changes, the octopuses resumed whatever activity it had been engaged in prior to the camera change after a brief period (<5 min) or with no interruption at all (in the remaining two instances, behavior immediately before or after the change was unknown). Out of 10 camera changes in 2021, only two (20%) were followed by a change in activity 5 min after the camera was replaced. The presence of the camera alone also affected behavior, as shown by the fact that most of the octopuses reached out and “investigated” the camera at some point during the observation sequences, perhaps seeing it as a potential food item or possible threat. In 2020, this occurred in four of the six octopuses on one to three separate occasions (total n = 9), and in 2021, all 10 of the octopuses touched the camera one to four times over the 24 h of observation (total n = 18). These investigations, however, were typically brief (less than 6 min per instance) and rare (one to four instances per observation period), representing only a small fraction of the observation time.
Another potential source of bias in the data was the fact that octopuses were only recorded on days with low to moderate wave activity, given the propensity for wave action in the shallow study area to displace the camera or obscure the frame with suspended particulates. It is possible that octopuses display entirely different activity patterns depending on wave activity. For instance, when visibility is low due to sediment-clouded water, they may spend more time Away from the Den to hunt, since these conditions might help obscure them from potential predators and prey. Unfortunately, this limitation was unavoidable given the shallowness of the study area and its susceptibility to wave action.
Finally, our study involved mostly younger O. insularis inhabiting shallow water. Larger, more mature individuals tend to inhabit deeper water farther from shore (Batista et al., 2021; Leite, Haimovici, Mather, & Oliveira, 2009), and therefore, our results are not necessarily representative of older octopuses' behavior. Older octopus would be expected to spend more time Away from the Den than juveniles, both because they may be more protected from potential predators by their larger size, and in order to look for mating opportunities. As younger individuals presumably still in a “learning phase” of their life cycle, the octopuses in our study thus might also need to spend a greater proportion of the time than adults sleeping in order process experiences and consolidate memories after spending time Away from the Den, as is the case in most mammals, although not cetaceans (Keene & Duboue, 2018).
4.3 Away from the Den and Quiescence
While the octopuses in the present study were not recorded when they were Away from the Den, studies in Bermuda found that O. insularis leave the den mainly to forage, spending roughly a third of the time actively hunting, as well as moving across the seafloor and resting (Mather, 1988), and also eating up to 30% of the prey they capture (Mather., 1991). Likewise, a study from Brazil showed that while away from the den, O. insularis engaged in food-searching behavior 80% of the time (Leite, Haimovici, & Mather, 2009). In the present study, at least 53.13% of the excursions made by the octopuses were successful foraging trips, as they were followed within 10 min by prey handling activity (data not shown). The other 46.87% may also have been successful, but the prey was consumed before the octopus returned to its den, as in Bermuda (Mather, 1991). Thus, we can surmise that while the octopus were Away from the Den they were searching for prey and perhaps mating opportunities.
The individuals monitored in this study left the den roughly three times per observation period on forays which ranged widely in length (minutes to hours). Multiple excursions lasting widely varying amounts of time is also seen in E. dofleini (mean of six 1–1.5 h trips per day; Mather et al., 1985), O. cyanea (Forsythe & Hanlon, 1997; multiple trips per day of 0–6 h; Yarnall, 1969), and O. vulgaris (multiple trips per day of 0–11 h; Altman, 1966; Kayes, 1973). The wide variation in the length of hunting trips may reflect differences in hunting success or predator encounter rates between excursions (Mather & O'Dor, 1991; Hofmeister & Voss, 2017). Octopuses may need to make multiple trips per day to obtain a sufficient amount of food to sustain themselves for a 24-h period, and the quantity of prey gathered on any particular trip is probably limited by the holding capacity of the arm web and the speed with which a prey item can be consumed.
Quiescence was the dominant behavior engaged in when the octopus was inside its den. Within this category, we observed all of the stages of sleep identified in laboratory observations of O. insularis, including quiet sleep and active sleep (Medeiros et al., 2020), although we did not differentiate between them in our analysis. Octopuses in this study observed over 24-h periods (2021) spent almost twice (61%) as much time Quiescent than those observed during daylight only (2020), reflecting greater Quiescence during the night time, and further reinforcing the diurnality of this species.
4.4 Between- and Within-Individual variation in Away from the Den and Quiescence
The intraclass correlation coefficient (ICC) values for time spent Away from the Den indicated exceptionally high variation between individuals in the time spent outside of their den. A meta-analysis of studies involving a wide range of vertebrate and invertebrate species found that interindividual differences account for about a third of the behavioral variability in a range of traits: across 114 studies of 98 species, the average ICC was 0.37 (Bell et al., 2009). Compared to that, the ICC of time allocated to Away from the Den in O. insularis is more than double this (0.87), suggesting that variability between individuals for this trait is much greater than is typical for animal behavior in general. Note also that since octopuses were not marked and moved between dens, it is possible that one or more individuals was recorded multiple times but counted as multiple individuals. This would result in the underestimation of interindividual variation, meaning that ICC could be even greater.
In contrast to the strong variation between individuals, the median (IQR) individual stability statistics (ISSs) for the proportion of time devoted to this behavior was extremely high (0.99 (0.01)), indicating that the six individuals spent nearly the same amount of time Away from the Den on both observation days. Indeed, the proportion of time allocated to this activity never differed by more than 10% from the first observation day to the second day 1–7 days later. Such interindividual differences in the proportion of time devoted to Away from the Den, paired with this strong within-individual consistency, suggests highly individualized foraging strategies (e.g., sit and wait vs. active search, Leite, Haimovici, & Mather, 2009) or differences in individuals' preferred prey and the time needed to transport and consume it (e.g., a large heavy clam that must be drilled or pulled open vs. a small crab that can be rapidly paralyzed and quickly consumed, Leite, Haimovici, & Mather, 2009; Mather et al., 2012). Differences in prey choice have been hypothesized as an expression of individual personality in three other octopus species, O. vulgaris, E. dofleini, and O. cyanea (Mather et al., 2012).
The ICC value for the time spent Quiescent (0.75 (CI = 0.041, 0.95)) indicated high variability between individuals (individual mean proportions for this behavior ranged from 24% to 48% of the daytime). Thus, like time spent Away from the Den, variability between individuals in daytime Quiescence is much greater than is typical for animal (mainly arthropods, fish, amphibians, birds, and mammals) behavior in general (Bell et al., 2009). At the same time, ISS values show that the amount of time allocated to Quiescence within a single individual was fairly stable from the first day of observation to the second (median (IQR) ISS = 0.64 (0.42)). As with Away from the Den, the combination of high ICC and ISSs for Quiescence suggests highly individualized sleeping patterns in O. insularis. Such individualized sleep patterns have been observed in a diversity of other taxa (e.g., rodents, birds, and humans; reviewed in Randler, 2014) and thus may be an evolutionarily conserved characteristic of sleep.
4.5 Comparison with other Octopus insularis studies and methods
Despite broad differences in the methodology (a snorkeling observer following octopus vs. one snorkel survey a day and remote cameras), years of study (1980s vs. 2020s), and distance between study sites (ca. 1300 km), the data collected in this study largely coincided with that collected from O. insularis (referred to as O. vulgaris) in previous studies (Mather, 1988, 1994) conducted in Bermuda (Table 5). The similarities between observations in Bermuda and South Caicos suggest that the two populations have similar ecological habits and temporal organization to their behavior which have not changed over the roughly three decades between observations. Thus, such habits may represent species-level behavioral strategies, and may have utility in differentiating co-occurring species, such as O. insularis and O. americanus.
| South Caicos, Turks, and Caicos | Bermuda | |
|---|---|---|
| Daytime field observation | Daytime field observation | |
| May–August, 2020 | July–August, 1985 | |
| Present study | Mather, 1988, 1994 | |
| Den occupancy | 1–62 days, median = 4 (N = 27) |
1–32 days, median = 6 (Mather, 1994; N = 133) |
| Daytime activity budget | 6% Active 14% Alert 33% Away from the Den 15% Prey Handling 32% Quiescent (N = 13 over 1–3 days) |
5% Active (“home maintenance” and “other”) 17% Alert (“rest”) 30% Away from the Den (includes some “active,” “hunt,” “alert” and “prey handling”a) 13% Prey Handling (“feed”) 53% Quiescent (“sleep“) (Mather, 1988; N = 4 over 5–32 days) |
| Activity pattern | Diurnal, with peaks in the morning and afternoon (N = 13 over 1–3 days) |
Diurnal, with peaks in the morning and afternoon (Mather, 1988; N = 4 over 5–32 days) |
| Quiescence | Multiple episodes per day (N = 13 over 1–3 days) |
Multiple episodes per day (Mather, 1988; N = 4 over 5–32 days) |
- Note: Differences from the present study are italicized. Note that methodology and behavior definitions vary between studies.
- a Percentages in this cell exceed 100% due to the combination of categories.
O. insularis in Bermuda spent more of the daytime Quiescent than O. insularis in South Caicos during daytime observation. This may reflect differences in the methodology and the ability of observers in each study to assess behavior: snorkelers observing octopuses from a distance as in Mather (1988) only noted behavior every 10 min and would likely have had great difficulty in discerning the subtle difference in iris width that differentiates Alertness from Quiescence, causing them to erroneously assume that octopuses were asleep much of the time that they were actually Alert. By contrast, the use of cameras placed close to the den in the present study allowed behavior to be monitored either every 20 s or continuously, and enabled more accurate assessments of behavior.
The use of remote photography and videography rather than sole reliance on direct observations by snorkel or SCUBA, or on acoustic tracking, contrasts with most other in situ studies that have been conducted with octopus. Remote videography has only been used in a few recent studies of octopus behavior (e.g., Bennice et al., 2021; Godfrey-Smith et al., 2022; Scheel et al., 2014), which is surprising given its use since the 1990s in monitoring wildlife for scientific research (Kays & Slauson, 2008), its efficacy as a less intrusive and less labor-intensive data collection method and its obvious potential to increase knowledge about the natural habits of octopods that reside in stationary dens. We urge greater use of this technique to study the behavior of benthic octopods in their natural habitats.
5 CONCLUSIONS
This study provides the first information about the 24-h activity cycle of O. insularis in its natural habitat and yields broader insights into the niche use, behavioral evolution, and time allocation of a molluscan group that has “lost” its hard protective shell, but also evolved a complex nervous system capable of learning and adaptation to change. The information gleaned here about the life habits of O. insularis will guide future research into the ecology and behavior of this species as well as other species of octopus, particularly studies of foraging and sleep. Our study also demonstrates the utility of minimally disruptive in situ remote time-lapse photography and videography in the study of behavior in animals that inhabit a den or other stationary shelter. We encourage other researchers to apply these techniques more broadly in the study of animal behavior, particularly that of octopus and other marine animals.
ACKNOWLEDGMENTS
We are deeply indebted to Heidi Hertler, Clarence Stringer, Ewa Krzyszczyk, Neil Oculi, Adelina Mkami, Willy Bolivard, Katrina Orthmann, John DeBuysser, Sydney O'Brien, Jooje Locke, Skylar Wuelfing, Tanner Reugg, Micah Strike, Theodore Bufferd, Andres Paredes-Vincent, Morgan Cleary, Katie Zagata, Izzy Kocher, and Michael O'Brien for logistical and technical support, as well as Drs. Jennifer Mather and Chelsea Bennice for their expertise and editorial assistance. This work was made possible by The School for Field Studies and the Turks and Caicos Department of Environment and Coastal Resources (DECR).
FUNDING INFORMATION
This work was supported by the School for Field Studies Center for Marine Resource Studies in the Turks and Caicos Islands.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
APPENDIX 1
| Individual | Dates filmed (2020) | Days den occupied | Times filmed | Camera changes | Individual size |
|---|---|---|---|---|---|
| 1 | May 21, 25 |
16 | 0559–1946; 0608–1945 | 0851, 1204, 1459, 1745 0854, 1155, 1451, 1801 |
Small |
| 2 | June 2, 9, 13 |
19 | 0546–1952; 0549–1955; 0548–1952 |
0854, 1150, 1455, 1752 0849, 1157, 1455, 1755 0852, 1158, 1444, 1732 |
Large |
| 3 | July 15, 17 |
9 | 0605–1956; 0559–2000 |
0921, 1212, 1348, 1649 0900, 1156, 1343, 1643 |
Small |
| 4 | August 9, 14 |
13 | 0604–1945; 0611–1948 | 0848, 1201, 1411, 1702 1001, 1302, 1604 |
Very small |
| 5 | August 9, 12 |
>22 | 0601–1951; 0608–1948 | 0902, 1159, 1414, 1704 1005, 1303, 1602 |
Very small |
| 6 | August 25, 26 |
>12 | 0606–1934; 0611–1936 | 0959, 1252,1556 0954, 1250, 1605 |
Medium |
- Note: Each den is assumed to have housed only a single individual during the course of the study. Individual size is based on visual estimations of relative differences between octopuses inhabiting the study area over the entire course of the study (very small: <50 mm mantle length (ML), small = 50–80 mm ML, medium = 80–100 mm ML, and large: >100 mm ML).
| Individual | Dates filmed (2021) | Times filmed | Camera changes | Individual size |
|---|---|---|---|---|
| 1 | July 24–25 | 1701–1720 | 0916 | Medium |
| 2 | August 29–30 | 1717–1741 | 0938 | Large |
| 3 | August 30–31 | 1747–1740 | 0906 | Medium |
| 4 | August 31–September 1 | 1748–1800 | 0954 | Medium |
| 5 | September 9–10 | 1540–1400 | 0911 | Very small |
| 6 | September 25–26 | 1752–1757 | 1211 | Medium |
| 7 | October 13–14 | 1733–1734 | 1052 | Medium |
| 8 | October 14–15 | 1745–1753 | 0909 | Medium |
| 9 | November 2–3 | 1112–1115 | 1740 | Medium |
| 10 | December 2–3 | 1621–1640 | 1042 | Small |
- Note: Individual size is based on visual estimations of relative differences between octopuses inhabiting the study area over the entire course of the study (very small: <50 mm ML, small = 50–80 mm ML, medium = 80–100 mm ML and large: >100 mm ML).
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.




