Holoplankton and meroplankton communities in surf zone waters of a temperate SW Atlantic sandy beach: Seasonal patterns
Abstract
Sandy beaches and their associated surf zones are the most common type of open shoreline, with significant ecological and socio-economic importance. In this study, we explored the seasonal fluctuations of the surf zone holo- and meroplankton communities to understand the underlying environmental variables driving the temporal dynamics of each community. We also defined a surf-zooplankton community on a temperate sandy beach (Monte Hermoso, Argentina) and related the organisms to those reported in a nearby estuary for a better understanding of the connection between adjacent coastal ecosystems. Holoplankton was very abundant (13.52–11070.4 ind m−3) and showed a strong seasonality, mainly related to the specific pattern of the two most abundant species: Acartia tonsa (>autumn) and Paracalanus parvus (>winter). This community was affected by seasonal changes in water temperature and salinity and variations in the velocity of currents, which showed that hydrodynamic forces were more significant on a short-term time scale than seasonal ones. Meroplankton abundances (1.3–48.05 ind m−3) were significantly lower than those recorded for the holoplankton and were mainly dominated by Medusae and larvae of Bivalvia and Decapoda. This fraction also showed a clear seasonal pattern (>abundances in summer), primarily related to the seasonal changes in temperature. The abundances of the main surf-zooplanktonic taxa were high and constant, emphasizing the importance of these waters as habitats for well-established communities rather than being used as a transient area. Consequently, we proposed a “specific” surf-zooplankton community for a temperate sandy beach, considering resident and non-resident taxa. Finally, the comparison of the surf and estuarine communities indicated a faunal relationship between seascapes, highlighting the ecological role of coastal waters in planktonic life.
1 INTRODUCTION
The surf zone is the area from the outermost breakers to the limit of wave uprush on the beach face (swash). Sandy beaches and their associated surf zones represent approximately three-quarters of the world's open shorelines (McLachlan & Brown, 2006). Historically, they have been considered simple environments with low species diversity. However, in recent decades, several studies have shown that surf waters harbour a heterogeneous and diverse benthic and pelagic fauna (e.g., Marin Jarrin et al., 2015, 2016; Rodrigues et al., 2019; Stull et al., 2016). Moreover, they are critical areas in the life cycle of many coastal fish species, most of them of commercial importance (e.g., Strydom & d'Hotman, 2005). Even though surf zones are one of the most extended ecosystems worldwide, with significant ecological and socio-economic significance, they are under-represented in the scientific literature.
The surf zone biota is also understudied, mainly because of the turbulent nature of these waters. In general terms, many species represented by a low number of individuals and a few dominant ones characterize the surf zone faunistic assemblages, many of them shared with nearby coastal ecosystems like estuaries (Fiori et al., 2022). Concerning the general ecology of surf-zooplankton, there are a few studies concentrated in Brazil (Avila et al., 2009; Costa et al., 2011; Oliveira-Santos et al., 2016; Pinheiro et al., 2011, 2013; Rodrigues et al., 2019), Argentina (Baleani et al., 2020, 2021; Menéndez et al., 2019), USA (De Lancey, 1987; Morgan et al., 2017; Stull et al., 2016), Egypt (Aboul Ezz et al., 2014), and Portugal (Guerreiro et al., 2021). Holoplankton (organisms that spend their entire life cycle in the water column) generally dominate the surf zone waters; however, meroplankton (those with only part of their lives in the plankton) can also be relevant in terms of abundance and the number of species. Thus, surf-zooplankton is mainly represented by crustaceans, especially copepods, with larval stages of benthic adults (bivalves, cirripeds, polychaetes) and gelatinous forms as intermittent visitors and with sporadic dominance, among others (e.g., Costa et al., 2011; Guerreiro et al., 2021; Menéndez et al., 2019; Pinheiro et al., 2013). Mysidacea and benthic organisms, such as amphipods and isopods, are frequently observed (e.g., Marin Jarrin et al., 2015, 2016). On Brazilian beaches, common and occasionally dominant copepods include Paracalanidae, Ctenocalanus vanus, and the benthopelagic Euterpina acutifrons (Avila et al., 2009; Costa et al., 2011; Pinheiro et al., 2011, 2013), probably linked to high concentrations of suspended particulate matter and strong resuspension (Pinheiro et al., 2013). Surf-zooplankton may also be subjected to strong seasonality associated with the temperature cycle, as seen in the SWA (Argentina, Menéndez et al., 2019) and the Mediterranean Sea (Egypt, Aboul Ezz et al., 2014). In turn, on tropical and subtropical Brazilian beaches, the seasonal variation in precipitation and the associated changes in salinity seem to be the main drivers of zooplankton community structure (Avila et al., 2009; Costa et al., 2011; Pinheiro et al., 2011, 2013; Rodrigues et al., 2019). Until now, the surf-zooplankton and its links with the environment have been addressed at the community (zooplankton as a whole) or species level. Therefore, individual analysis of the holo- and meroplankton, considering their temporal dynamics and the role of the main environmental variables, will contribute to a better understanding of the ecology of these turbulent coastal waters.
Animals move in the seascapes to feed, reproduce and disperse, connecting populations, food webs, and ecosystems (Vargas-Fonseca et al., 2016). Well-known examples that illustrate the connectivity between coastal habitats include the movements of larval and adult of fish between surf waters, estuaries and nearby oceans (e.g., Olds et al., 2018; Strydom, 2003, 2007), regional dispersal of invertebrates along coastlines (e.g., Schoeman et al., 2015), and local movements between surf- and deeper waters behind the breakers (e.g., McLachlan & Defeo, 2018; Strydom, 2007). In particular, surf zones provide rich food resources (zooplankton) and protection for larvae, juvenile and adult fish, which several planktivorous fish evidence in these waters (e.g., Strydom, 2003). The relationship between surf-, an inner shelf- and estuarine waters depends on the state of the estuarine mouth, the tidal rhythms and the transport of sediments, nutrients, chemicals and even organisms by the estuarine plumes (Delgado et al., 2017). It has been suggested that neritic and estuarine zooplanktonic species coexist in the surf zone of sandy beaches, which is evidence of the connectivity between adjacent systems (Menéndez et al., 2019; Stull et al., 2016). Some authors compared surf planktonic communities with those in deeper coastal waters; however, the degree of similarity between the surf- and adjacent estuaries is a topic that remains unexplored.
This study aims to characterize holo- and meroplankton communities in the surf zone waters of a temperate sandy beach in the southwest Atlantic. We also attempt to answer the following questions: (1) which taxa in the holo- and meroplankton can be considered community descriptors?, (2) which environmental factors relate to temporal variations of each one? and (3) is it possible to define a specific surf-zooplankton community on this temperate SW Atlantic sandy beach? Finally, we compare the surf communities to those previously reported in the near estuarine area to better understand the relationship between adjacent coastal ecosystems.
2 METHODS
2.1 Study area
This study was conducted in Monte Hermoso (MH, 38°59′ S, 61°06′ W), a temperate sandy beach located in the southwestern Atlantic (Figure 1). MH has a mesotidal regime with semidiurnal tides (mean amplitude: 2.32 m; SHN, 2022) and is considered a tide-modified reflective to the intermediate beach under calm conditions, but wave-dominated intermediate to dissipative during storms periods (Fiori et al., 2022). The mean significant wave height ranges from 0.25 (winter) to 1.5 m (spring), associated with wave periods of 1–16 s (Delgado et al., 2012).
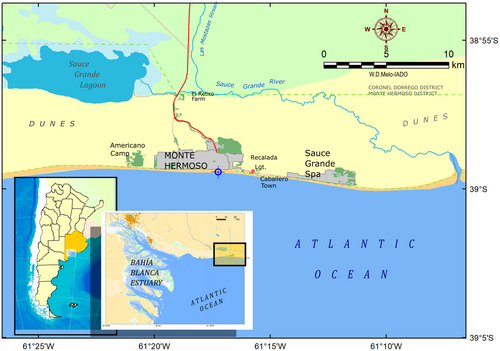
MH is a small coastal town with an economy based mainly on tourist activities and artisanal fisheries (Rojas et al., 2014). The area has a dry/sub-humid temperate climate, characterized by warm summers (21.4°C), cold winters (8.5°C), and moderate springs and autumns (14.2 and 16.7°C, respectively; e.g., Delgado et al., 2017). Therefore, coastal waters show a strong seasonality (6.1–22.9°C). Bahía Blanca Estuary frames the western limit of the study area (BBE, 38°45′–30°40′ S, 61°45′–62°30′ W), whose plume influences the coastal waters of MH, especially in terms of salinity (33.5–36) and turbidity (24–64 NTU; Delgado et al., 2017; Figure 1). The annual mean rainfall is ~650 mm, and the prevailing wind directions are from the N and NW, blowing with greater intensity in the spring and summer months.
2.2 Sampling and laboratory procedures
Zooplankton was sampled at one station (38°59′22.8″ S, 61°18′42.1″ W) twice per season (austral- spring, summer, autumn and winter) during two annual cycles: 2009–2010 (October and February 2009, December 2009 and February 2010, March and May 2010, June and August 2010) and 2015–2016 (October and February 2015, December 2015 and February 2016, April and May 2016, June and August 2016). Samples were collected by pulling a conical net (0.5 m in diameter, 300 μm mesh) by hand, parallel to the shoreline for 200 m during high tide (mean depths: 0.7–1.2 m). The net was equipped with a mechanical flowmeter to calculate the volume filtered. The net samples were immediately preserved in a 4% borax-buffered formaldehyde seawater solution for further analysis. Water temperature and salinity were measured in situ using a digital multisensor. Water samples were collected in the surf zone and transported to the laboratory in cool, dark conditions to determine chlorophyll-a and suspended particulate matter (SPM) concentrations. The velocity (VLC) and direction of the littoral current were measured in the surf waters using a floating buoy. Thirty continuous current readings were taken at a fixed static point 0.5 m above the sand bottom, following Schneider (1981). The wave height (WH) was estimated visually considering the distance between the top of the sea surface and the crest (McLachlan et al., 2018).
Planktonic organisms were identified to the lowest possible taxonomic level and classified according to their time of residency in the pelagic environment: (1) holoplankton, that are in the water column for their entire life cycle and (2) meroplankton, temporary residents spending only a part of their life cycle in the plankton community. According to Boltovskoy (1999), the final volumes were standardized for very high-abundant samples (more than 50 individuals per 5 mL) and then, they were subsampled (1/10). For those with fewer organisms, the entire sample was counted entirely. Holo- and meroplankton abundances were expressed as ind m−3. The mesh size used in the present study (300 μm) certainly underestimated tiny organisms of the mesozooplankton (e.g., early life stages of copepods, early meroplanktonic larvae). Thus, mainly adult copepods and more prominent copepodites and planktonic larvae are considered in this study, which should be discussed with caution. The SPM (mg L−1) was obtained gravimetrically, filtering 300 mL of water through Whatman GF/F filters previously dried and weighed, then dried (60°C, 24 h) and weighed. The chlorophyll-a (μm m−3) was measured by spectrophotometry following APHA-AWWA-WEF (1998). Water samples (300 mL) were filtered through Whatman GF/C filters, frozen and stored at −20°C. Pigment extraction was done in 90% acetone for about 20–24 h, placing the tubes in a fridge in complete darkness.
2.3 Statistical analysis
Non-metric Multidimensional Scaling analyses (nMDS) were performed to explore the structure of the holo- and meroplankton communities. These analyses were based on triangular matrices of the Bray-Curtis similarities on fourth-root-transformed abundance data to reduce the influence of very abundant organisms. An indicator species analysis (ISA, Dufrene & Legendre, 1997) was used to determine and describe the typical taxa in each community per season. The ISA combines the abundance of taxa in a particular group and the fidelity of their occurrence. The analysis provides an indicator species value (ISV) that expresses how consistently present the taxa are in their sample group, based on overall taxa abundance and frequency of occurrence. The index used in the analysis was the correlation index ‘r.g.’, which assesses the positive or negative preference of the taxa for the environmental conditions prevailing in the samples belonging to a particular group (De Cáceres et al., 2010). Also, the index will show the pattern that creates the highest inside/outside differences between groups. The ISV values of less than 0.25 were omitted as the association at this level was considered too weak (Dufrene & Legendre, 1997). Permutation tests (n = 999) were carried out to determine the significance of the taxa as indicators (α = .05). We applied Permutational Univariate Analysis of Variance (PERMANOVA) to test for significant differences in the holo- and meroplankton abundances between seasons (Anderson et al., 2008). The test was used to square-root transform data based on Bray–Curtis similarity between samples. All the factors were considered fixed and with the unrestricted permutation of raw data. When significant results were found, pairwise post hoc comparisons were conducted using permutational pseudo-t-tests (Anderson et al., 2008). Considering the similarity between the two sampled periods (2009–10, 2015–16) from an Analysis of Variance (ANOVA), all the samples were considered replicates in the PERMANOVA. The ANOVA was performed to compare holo- and meroplankton abundances (response variables) between seasons and years (predictor variables). Normal distribution was assessed using Q-Q plots, and square root transformation was applied to meet the normality. The interaction term between the factors was discarded from the models because non-significant effects (p > .25) were detected.
Finally, a distance-based linear model (DistLM) was performed to analyze the relationship between the holo- and meroplankton and the environmental variables (water temperature, salinity, SPM and chlorophyll-a concentrations, WH, VLC; Anderson et al., 2008; Clarke & Gorley, 2006). The DistLM was constructed using the step-wise selection procedure and the adjusted R2 based on 999 permutations as a selection criterion to enable the best explanatory environmental variables to be fitted into the model (Anderson et al., 2008). Results were visualized through a distance-based redundancy analysis (dbRDA). A redundancy analysis (RDA) was performed simultaneously with the analysis. Previously, zooplankton abundances were square-root-transformed and environmental variables were normalized (Legendre & Gallagher, 2001). Bray Curtis and Euclidean distance were used as resemblance measures in biological (abundances) and environmental procedures, respectively.
3 RESULTS
3.1 Environmental data in surf zone waters
Water temperature varied between 11.7 ± 0.07 and 23.8 ± 3.8°C during the period 2009–10 and between 11.6 ± 0.07 and 20.1 ± 1.7°C in 2015–16 (Figure 2a). As expected for the region, the highest values were recorded in summer and the lowest in winter. The mean salinity for the study period was 33.4 ± 0.45, which ranged between 33.1 ± 0.56 (spring) and 34.4 ± 0.63 (autumn) in 2009–10, and between 32.8 ± 0.21 (spring) and 33.7 ± 0.07 (summer) in 2015–16 (Figure 2b). SPM varied from 31.4 ± 1.9 to 70.9 ± 44.6 mg L−1 in 2009–10, and from 50.9 ± 7.5 to 94.2 ± 12.7 mg L−1 in 2015–16 (Figure 2c). The highest concentrations were recorded during autumn-10 (102.5 mg L−1) and winter-16 (103.2 mg L−1), and the lowest ones were during the summer in both periods (41.1 and 42.5 mg L−1, respectively). Chlorophyll-a ranged from 3.5 ± 0.57 to 7.6 ± 3.2 mg m−3 and was slightly higher during 2015–16 (Figure 2d). The highest concentrations were recorded during the winter in both periods.
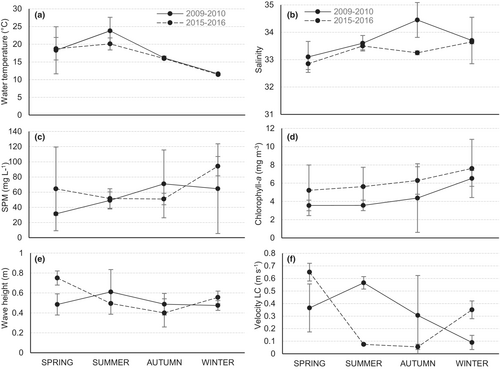
The lowest significant WH were recorded in winter-10 (0.41 m) and autumn-16 (0.4 m), whereas the highest was in summer 2009–10 (0.77 m) and spring-15 (0.8 m; Figure 2e). In 2009–10, the maximum VLC was detected in summer (0.77 m seg−1) and the minimum in winter (0.05 m seg−1). The highest velocities during 2015–16 were recorded in spring (0.8 m seg−1) and the lowest in the summer months (0.08 m seg−1; Figure 2f). Littoral currents flow mainly westwards (80% of the samplings), with only 20% of the measurements moving eastwards.
3.2 Holoplankton temporal dynamics
Of the 34 taxonomic groups or categories of zooplankton recorded, 14 belonged to holoplankton. Copepods (occurrence frequency, OF = 56.3%–100%) were the most commonly observed, followed by the chaetognath Parasagitta friderici (OF = 81.3%), Appendicularia (OF = 37.5%), and larvae of the shrimp Artemesia longinaris (OF = 37.5%). In the Copepoda, Acartia tonsa and Paracalanus parvus were the most consistently observed taxa (OF = 100%), and then Ctenocalanus vanus (OF = 62.5%), Labidocera fluviatilis (OF = 43.8%), and Calanoides carinatus (OF = 43.8%).
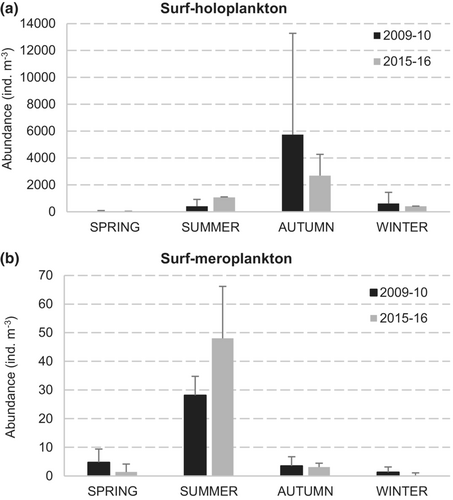
Holoplankton abundance displayed a consistent seasonal variation (pseudo-F = 5.28, p < .001; Figure 3a). According to the post hoc comparisons, abundances in spring were significantly different from winter and autumn (p < .05), whereas the winter abundances were substantially different from summer and autumn (p < .05). For both periods, abundances were the lowest during spring (<45 ind m−3), increasing in summer (up to 772.1 ind m−3), and they peaked in autumn (up to 11,070.4 ind m−3). A decline followed these peaks in the winter (<1205.5 ind m−3). ANOVA showed that holoplankton abundances were marginally significantly different between seasons (F = 3.4040, p = .057) but not significant between years (F = 0.1756, p = .6833).
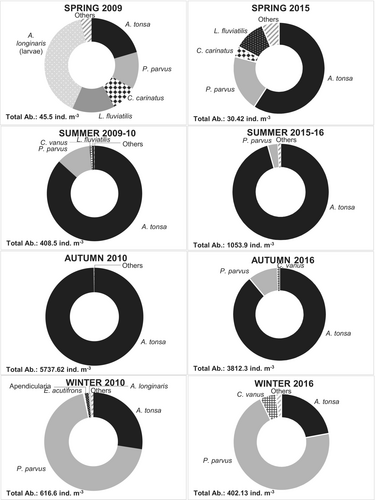
Acartia tonsa (>>) and P. parvus comprised most of the holoplankton abundance (up to 96.7%) and due to their numerosity, they strongly influenced the overall seasonal pattern of this community. A. tonsa was the dominant species, and it was recorded in all seasons with its highest abundance during autumn (2009–10: 5720.34 ± 7521.1 ind m−3, 2015–16: 3384.5 ± 1676.8 ind m−3; Figure 4). P. parvus was also recorded for most of the study period, being dominant in winter (2009–10: 283.8 ± 33.13 ind m−3, 2015–16: 341.9 ± 183.7 ind m−3). The abundance of the other taxa was much lower than these copepods (especially than A. tonsa) and varied from this overall seasonal pattern. For instance, C. vanus peaked in autumn-16 and winter-16, with abundance up to 41 and 32 ind m−3, respectively (Figure 4). Large calanoids, such as C. carinatus and L. fluviatilis, showed the highest abundance in spring in both periods (up to 13 ind m−3). The abundance of small copepods, such as Oithona nana and Euterpina acutifrons, was very low, probably due to the size of the net employed. Holoplankton, other than copepods, also showed a low abundance (0.03–35.9 ind m−3), A. longinaris (spring-09) and P. friderici being the most abundant (in autumn of both periods; Figure 4). Finally, Neomysis americana was the most frequent mysid recorded in these waters, with abundance up to 0.61 ind m−3.
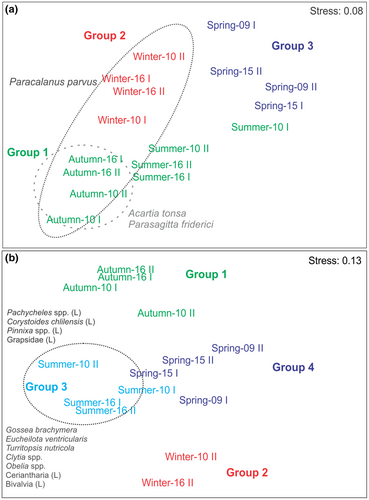
The nMDS corresponding to the holoplankton separated the samples into three main groups, corresponding to the seasons (Figure 5a). Group 1 represented the austral summer-autumn samples taken when the waters were warmer. Group 2 included winter samples, taken from cooler waters, and spring samples formed Group 3. The ISA identified three taxa with ISV > 0.25 as significant indicators (p < .05) only for two seasons: A. tonsa and P. friderici were the indicator taxa in autumn, and P. parvus in samples corresponding to winter and autumn (Figure 5a).
3.3 Meroplankton temporal dynamics
The number of meroplankton taxa was significant in warmer seasons (>>summer), which was composed of larvae of benthic adults of Bivalvia (OF = 56.3%), Spionidae (OF = 56.3%), Grapsidae (OF = 43.8%), and planktonic eggs of Teleostei (OF = 43.8%). The medusae Clytia spp. (OF = 37.5%) and Obelia spp. (OF = 37.5%), and larvae of Ceriantharia (OF = 37.5%) were also observed in the samples, especially in the summer.
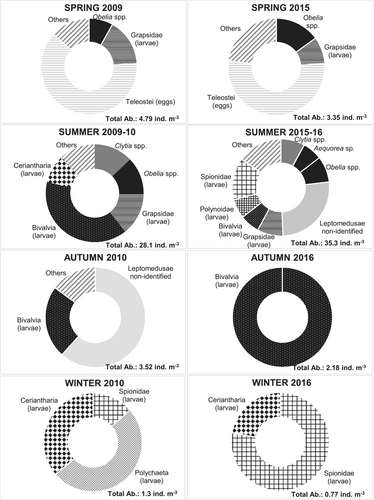
Abundances were low and ranged between 1.3 and 28.2 ind m−3 during 2009–10, and between 1.38 and 48.05 ind m−3 during 2015–16. Meroplanktonic forms exhibited seasonal differences (pseudo-F = 3.48, p < .01), being significantly higher in summer (Figure 3b). The post hoc comparisons showed that all the seasons were substantially different (p < .05). During the summer, the meroplankton showed a predominance of Medusae, with a total of 16.8 and 37.9 ind m−3 in 2009–10, and 2015–16, respectively. The species recorded were Obelia spp. (up to 7.1 ind m−3), Clytia spp. (7.1 ind m−3), Aequorea sp. (3.4 ind m−3), Gossea brachymera (0.65 ind m−3), Turritopsis nutricula (0.1 ind m−3), Eucheilota ventricularis (1.75 ind m−3) and non-identified species of Leptomedusae (18.5 ind m−3; Figure 6). Veligers of Bivalvia (>>2009–10), nectochaetae of Polychaeta (2015–16), zoeae of Decapoda (both periods) and cerinula larvae of Ceriantharia (both periods) were also abundant in the summer samples. In spring, the most abundant taxa were Teleostei eggs (2009–10, up to 3 ind m−3), Obelia spp. (2015–16, 0.7 ind m−3) and larvae of Grapsidae (both periods, up to 1.4 ind m−3), followed by larvae of Ceriantharia (2015–16, 0.25 ind m−3). During the autumn and winter of both periods, the total meroplankton abundances were also very low (1.3–3.5 ind m−3), with no organisms recorded during winter-16. Bivalvia larvae (2015–16, up to 3.21 ind m−3) and non-identified Leptomedusae (2009–10, up to 2.17 ind m−3) were the most abundant taxa in autumn, whereas larvae of Polychaeta (0.80 ind m−3) and Ceriantharia (up to 0.47 ind m−3) were recorded in winter 2009–10 (Figure 6). ANOVA also showed significant differences in meroplankton abundance between seasons (F = 5.6075, p < .05); however, differences between years were not evident (F = 0.1135, p = .7448). The nMDS separated the samples into four groups corresponding to each of the austral seasons (Figure 5b). The ISA identified 11 taxa with ISV > 0.25 as significant indicators (p < .05), all of them for the summer group: the medusae Obelia spp., Clytia spp., G. brachymera, E. ventricularis and T. nutricola, as well as the larvae of Pachycheles spp., Corystoides chlilensis, Pinnixa spp., Grapsidae, Ceriantharia and Bivalvia (Figure 5b).
3.4 Environmental regulation of the holo- and meroplankton communities
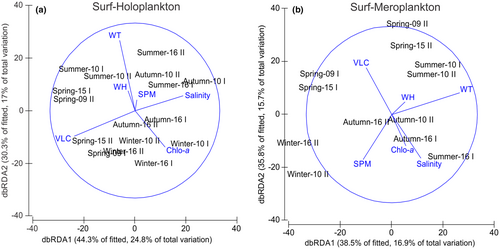
The distLM analysis found that the best model for the holoplankton abundances explained 44.3% of the total variability. This model included the water temperature (Pseudo-F = 2.45, p < .05), salinity (Pseudo-F = 2.34, p < .05), and littoral current velocities (Pseudo-F = 3.19, p < .01; Figure 7). Seasonal differences in the holoplankton community were mainly related to water temperature and salinity, whereas the VLC was linked to variations over a shorter period. The distLM analysis found that the best model of the meroplankton abundances included water temperature as an explanatory variable (Pseudo-F = 2.15, p < .05), which explained 39.5% of the total variation (Figure 7).
4 DISCUSSION
4.1 Holoplankton in surf zone waters
The Copepoda dominated the composition and abundance of the holoplankton in the waters of the surf zone. This community showed the highest densities during autumn (especially at the beginning), with A. tonsa being the dominant taxon. In the nearby estuary, this copepod has an annual mean abundance close to 700 ind m−3, with maximum values in warmer months (up to 6000 ind m−3 in late summer-autumn; see Table 1). The seasonal cycle and the highest abundance concentrated in late summer-autumn agree with our findings, suggesting that the estuary influences or regulates the dynamic of A. tonsa on the adjacent beaches. This species seems to be well adapted to instantaneous variations in salinity (not exceeding 10‰) and is commonly found in values ranging from 5‰ to 30‰, with the optimal ones for developmental stages < to 25‰ (Cervetto et al., 1999). It has been suggested that the BBE populations probably belong to a different lineage (Drillet et al., 2008), which may have gained tolerance to higher salinity levels over the generations (the salinity range of the estuary is 27–36; Berasategui et al., 2018). In the study area, the salinity values were consistently higher than 32.9, indicating the high salinity tolerance of A. tonsa and the presence of well-established populations with slow progressive transformations of their physiological tolerances (Cervetto et al., 1999). P. parvus dominated the surf during the winter months, a clear difference with the adjacent estuary, in which A. tonsa shares this temporal ecological niche with the exotic Eurytemora americana (Table 1). P. parvus was also recorded as dominant during winter on the west coast of the Iberian Peninsula (Portugal; Guerreiro et al., 2021). Species belonging to the genus Paracalanus have already been reported in similar abundances in other surf zones (Costa et al., 2011; Pinheiro et al., 2011, 2013) and associated with A. tonsa (Stull et al., 2016). Other copepod species, such as C. carinatus, L. fluviatilis, C. vanus and E. acutifrons, were also recorded in the surf, although in low numbers. Most of them are typical of neritic waters, and their occurrence seemed to be highly variable, which could be due to a fluctuating influx of offshore waters into the surf ecosystem, bringing plankton with them. The observed dominance of copepods in the surf waters is relevant considering that they are the main prey of larger predators, such as Sagitta spp (Giesecke & González, 2008) and larval fish (e.g., Sánchez-Velasco, 1998), common habitants of these turbulent waters. Overall, the overwhelming dominance of copepods in surf waters is a crucial factor for the annual productivity of sandy beaches, being an essential link in the carbon flow from phyto- and microzooplankton to larger zooplankton and nekton.
| Estuarine waters | Surf zone | |||
|---|---|---|---|---|
| 2009–2010 (Annual mean) | 2014–2018 (Abundance range) | 2009–2010 (Mean abundance) | 2014–2015 (Mean abundance) | |
| Total zooplankton | 557.84–11,704 | 13.52–11,070 | 24.71–4935.92 | |
| Total holoplankton | 45.5–5737.62 | 30.42–3812.3 | ||
| Acartia tonsa | 695.48 | >1000 | 9.31–5720 | 18–1009.32 |
| Eurytemora americana | 9.63 | 10–100 | Not registered | Not registered |
| Paracalanus parvus | 9.63 | 10–100 | 5.8–241.85 | 6–391 |
| Euterpina acutifrons | 3.62 | 100–1000 | 0.08–3.46 | 0.08–2.18 |
| Total meroplankton | 1.3–28.2 | 1.38–48.05 | ||
| Neohelice granulata | 81.97 | 100–1000 | Not registered | Not registered |
| Balanus glandula | 28.89 | 100–1000 | Not registered | Not registered |
| Balanus amphitrite | 13.35 | 100–1000 | Not registered | Not registered |
| Medusae | 10–100 | 1.5–15 | 0.77–30 | |
| Teleostei eggs | 10–100 | 0.24–6 | 1.77–3.54 | |
| Bivalvia larvae | <10 | 0.24–16.9 | 0.12–3.4 | |
| Spionidae spp. | 1.15 | 10–100 | <0.2 | <4.95 |
Despite the careful selection of sampling days with calm conditions, our results showed an evident influence of the local hydrodynamic conditions on the holoplankton community. Higher abundances coincided with sampling days with lower VLC, which is evidence that the physical dynamics of the surf zone may be relevant at shorter time scales than seasonal. Both the WH (Baleani et al., 2020; Guerreiro et al., 2021) and the VLC (Baleani et al., 2020) have been mentioned as driving forces of zooplankton on other beaches worldwide. Baleani et al. (2020) assumed the occurrence of some habitat selection, which allows organisms to avoid turbulence during rough conditions. Thus, holoplankton living in the surf zones are prone to shifts in community structure in more complex ways than just seasonally. Regular sampling in only one month to characterize a season, or in one week to characterize a month, will hardly represent the actual condition of the holoplankton community in qualitative/quantitative terms.
4.2 Meroplankton in surf zone waters
Our results suggest that the surf zone of the MH beach has a rich meroplankton community, which was especially abundant during the summer. Abundances were lower than those recorded for the holoplankton, but similar to those reported on other sandy beaches (Avila et al., 2009; Pinheiro et al., 2013). Only a few studies recorded higher meroplankton abundances: up to 1000 ind m−3 in North Carolina, USA (Stull et al., 2016) and the Rio Grande do Soul, Brazil (Rodrigues et al., 2019). In the nearby estuary, the meroplankton is generally represented by decapods, cirripeds, polychaetes, and mollusks, the Decapoda being the most abundant group during summer (Berasategui et al., 2018; Table 1). The highest abundances in this group are attributed to the zoeae of the crab Neohelice granulata, the dominant crab species in the saltmarshes, and also to the larvae of P. petrunkevitchi and A. longinaris. During winter, the larvae of the cirriped Balanus glandula dominate estuarine waters. Neither N. granulata nor B. glandula was recorded on the adjacent beach.
The meroplankton temporal pattern could be related to (1) the breeding of benthic organisms, (2) the supply of larvae from nearby environments and (3) the environmental variables affecting the surf waters. The groups having the highest contribution to the meroplankton were Decapoda, Medusae and Bivalvia. Not surprisingly, the local benthic communities in the surf zone of MH are composed of some Decapoda populations, which have life histories that include a planktonic larval phase (e.g., Pachycheles laevidactylus, Libinia spinosa, Austinixa patagoniensis; Carcedo et al., 2014). Larval stages seasonally make up a significant proportion of plankton communities in coastal systems worldwide, and they determine the distribution of their benthic adult populations (e.g., Ershova et al., 2019). The ecological significance of planktonic larvae is, therefore, very relevant considering that they are a dispersal stage for benthic organisms, that determine the potential of the species to colonize adjacent habitats (Shanks, 2009). Larvae and other zooplankters could enter the surf zones in near-surface onshore wind-driven currents, Stokes drift, and internal waves, whereas those near the bottom may be transported shorewards by benthic streaming (Morgan et al., 2017). The supply of larvae establishes the densities of communities. In contrast, the supply of zooplankton determines the food supply for the growth and reproduction of filter-feeding foundation species of the shore communities (Morgan et al., 2016). The sporadic appearance of planktonic organisms in the surf waters may be related not only to the marine influx but also to their feeding strategy. For instance, Obelia is a microphagous and filter-feeding medusa at the onset of its life; therefore, surf waters will be crucial for them because the microbial loop represents a biological force driving energy fluxes (Mann, 2000). In this study, the gelatinous species showed abundance peaks during the summer months, which could significantly impact the zooplankton community (Azeiteiro et al., 1999). When jellyfish occur in high numbers, their collective prey-consumption rate can be so high, directly or indirectly controlling the population size of other zooplankton organisms (Hansson et al., 2005). This may lead to a shift in the trophic structure of the pelagic community due to a trophic cascade effect. Also, they could be competing for resources with holoplankton species and serving as a food source for planktonic predators.
In this study, water temperature fluctuation was directly associated with the temporal variations of the surf meroplankton. The exclusive occurrence and high abundance of some taxa in the summer months show the crucial role of water temperature in this community and with the breeding pattern of adult populations. Other studies in surf waters concluded that the primary variable affecting meroplankton is the concentration of chlorophyll-a (Rodrigues et al., 2019; Stull et al., 2016), a clear association between the spawning of benthic invertebrates at times of high food availability for the larvae (Stull et al., 2016). For instance, Bivalvia larvae on the northern coast of Rio Grande do Sul (Brazil) peaked in association with chlorophyll concentrations, due to their role as phytoplankton grazers (Rodrigues et al., 2019). The contribution of meroplankton to the productivity of surf waters demonstrates the significant interaction between biotic/abiotic variables and between pelagic/benthic environments (pelagic-benthic coupling) in these coastal ecosystems.
4.3 Understanding the role of the surf waters of Monte Hermoso beach and the nearby estuary in planktonic life
Nowadays, knowledge about surf zones is related to their use, especially by fish larvae, as transitional or migratory pathways, as well as feeding or breeding habitats (Senta & Kinoshita, 1985; Strydom & d'Hotman, 2005; Watt-Pringle & Strydom, 2003). Moreover, in temperate and subtropical regions, riverine and estuarine plumes strongly influence the physical-biological characteristics of nearby beaches, sometimes evidencing a continuity of surf assemblages, especially for larval fish (Strydom & d'Hotman, 2005). However, the use that the smaller plankton made of these waters remains poorly understood. Some taxa recorded in the present study are also commonly reported in estuaries worldwide (e.g., David et al., 2005; Marques et al., 2009), showing a faunal connection between the surf- and estuarine waters. In addition, this relationship has also been suggested between surf- and adjacent neritic coastal waters (<50 m; Menéndez et al., 2019). In the study area, the abundances of common taxa were similar or slightly higher than those reported in the nearby estuary (Table 1; e.g., Berasategui et al., 2018; Menéndez et al., 2012), emphasizing the importance of surf waters as a habitat rather than being used as a transient area between the estuary and offshore environments. Surprisingly, the composition of zooplankton in the MH surf zone was similar to that in the BBE two decades ago (Hoffmeyer, 2004). In the 90s, several copepods dominated the estuarine system (A. tonsa, P. parvus, L. fluviatilis, C. carinatus, among others), but meroplankton were scarce. A recent study evidenced the restructuring of the community towards the dominance of typical estuarine species (A. tonsa, grapsid, and cirriped larvae, and the invader E. americana) and a decrease of those characteristics of the adjacent shelf area, such as L. fluviatilis and C. carinatus (Table 1; Berasategui et al., 2018). Nowadays, the species that dominate the estuary appear to be more adapted to coexisting in a polluted and eutrophic environment than the organisms that inhabit the surrounding shelf areas. Overall, this study showed evidence of a strong relationship between the two coastal systems and that surf waters are important habitats for many faunal groups—especially copepods—highlighting the ecological role of surf zones in planktonic life.
According to McLachlan and Defeo (2018), two categories may be recognized among the surf-zooplankton: resident species, which may be strictly planktonic or bentho-planktonic, and non-residents that may be holo- or meroplankton. These authors defined residence as a permanent occurrence of the organisms in the surf. Both main copepod species—A. tonsa, P. parvus—and the larval stages of local benthic populations were assumed to be resident species (Table 2). Mysids and small prawns were also considered truly planktonic resident species as they are frequently abundant but easily undersampled (McLachlan & Defeo, 2018). Bentho-planktonic included benthic organisms (isopods, amphipods) that only occurred circumstantially in the water column (see Table S1). Future studies with more detailed meroplanktonic taxonomic categories would be helpful to know if all recorded larvae belong to local or remote adult populations (e.g., larvae from nearby estuaries, rocky shores or offshore ecosystems). The non-resident holoplankton include the rest of the copepods, chaetognaths, and even medusae, which were highly variable in occurrence, and their sporadic appearance was associated with advection by wind-, wave-, and tide-induced currents. Finally, the meroplankton in the MH surf waters was mainly composed of medusae, eggs, and fish larvae.
| Resident taxa (26) | Non-resident taxa (19) | ||
|---|---|---|---|
| Planktonic (33.33%) | Bentho-planktonic (24.4%) | Holoplankton (20%) | Meroplankton (22.2%) |
| Acartia tonsa | Serolidae | Ctenocalanus vanus | Gossea brachymera |
| Paracalanus parvus | Chaetilidae | Calanoides carinatus | Turritopsis nutricula |
| Arthromysis magellanica? | Ancinidae | Labidocera fluviatilis | Eucheilota ventricularis |
| Neomysis americana? | Idoteidae | Euterpina acutifrons | Aequorea sp. |
| Peisos petrunkevitchi? | Oedicerotidae | Oithona nana | Clytia spp. |
| Pinnixa spp. (L) | Lilljeborgidae | Parasagitta friderici | Obelia spp. |
| Corystoides chilensis (L) | Phoxocephalopsidae | Liriope tetraphylla | Leptomedusae |
| Pachycheles spp. (L) | Monocorophium insidiosum | Apendicularia | Teleostei (E, L) |
| Grapsidae (L)? | Amphipoda | Artemesia longinaris (L) | Ophiuroidea (L) |
| Bivalvia (L)? | Cumacea | ||
| Gastropoda (L)? | Foraminifera | ||
| Polynoidae (L) | |||
| Spionidae (L) | |||
| Polychaeta (L)? | |||
| Ceriantharia (L)? | |||
Finally, it is worth noting that future studies in surf zones should consider smaller pore sizes than 300 μm, which will better represent smaller organisms and early planktonic life stages. It is imperative to design an appropriate and generalized methodology for these ecosystems that allow comparing similar studies on different beaches. Also, we emphasize the need to contemplate finer time scales than seasonal, different weather conditions, and physical data such as wave (height, period) and current velocity, to understand better the holo- and meroplankton communities' structure in such turbulent waters.
5 CONCLUSIONS
Studies on surf zone zooplankton are scarce, with substantial variability in sampling methods (e.g., net pore size, sampling frequency), taxonomic focus and beach types (Guerreiro et al., 2021). The interaction between these environments and biological communities, especially zooplankton, needs a better understanding. The present study shows that a diverse and abundant zooplanktonic community is well adapted to the surf waters of the MH sandy beach. Zooplankton composition also indicates a clear relationship between adjacent coastal ecosystems. The abundances of the main taxa were high and constant, emphasizing the importance of these environments as well-established habitats for the zooplankton. Changes in the zooplankton communities, mainly in terms of species composition and number, were highly influenced by the seasons. The main physical variables influencing the temporal distribution of the holoplankton are water temperature, salinity and hydrodynamic features. The correlation between VLC and abundance indicates that hydrodynamic forces are more significant in a shorter time scale than seasonal ones. More detailed studies that take into account the measurements of this physical variable would significantly complete the present evidence. Our results also suggest that when temperatures rise during the summer, meroplankton (especially medusas and Decapoda larvae) thrive on this temperate sandy beach. By emphasizing the importance of a separate analysis of the holo- and meroplankton communities and their environmental regulation, this study contributes to the knowledge of surf zone ecology. It will be helpful for the design of future research in these highly dynamic areas.
ACKNOWLEDGEMENTS
Partial support for this research was provided by grants from the Agencia Nacional de Promoción Científca y Tecnológica (ANPCYT, PICT2019-03349) and Consejo Nacional de Investigaciones Científcas y Técnicas (CONICET PIP11220200102444CO).
CONFLICT OF INTEREST STATEMENT
None.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




