Bottom-up and top-down control of seagrass overgrazing by the sea urchin Tripneustes gratilla
Abstract
The lack of top-down control on Tripneustes gratilla, a sea urchin commonly known to graze on seagrass, and the bottom-up control of its feeding preference, led to the overgrazing of seagrass meadows of the species Thalassodendron ciliatum in Changuu Island (Zanzibar Archipelago). The impact of overgrazing on seagrasses was assessed by mapping the presence of grazed versus non-grazed seagrass patches in the study site, while the top-down control on T. gratilla was assessed by measuring the abundance of its fish predators. The feeding preference and distribution of T. gratilla were characterized by calculating the electivity indexes for each seagrass species and measuring sea urchin density, respectively. Approximately half of the patches of T. ciliatum were overgrazed, while predatory fishes of T. gratilla were absent from the site. The Vanderploeg and Scavia's Relativized Electivity Index indicated that T. gratilla had a feeding preference for T. ciliatum, which was also supported by higher urchin densities within T. ciliatum dominated patches. Bottom-up control of grazing activity was observed by quantifying and analyzing morphological, nutritional, and the chemical defense traits of the seagrass in relation to feeding preference and urchin density. Feeding was positively correlated to the seagrass tissue C:P ratio (ρ = 0.9), whereas urchin density showed no correlations. The bottom-up control of the feeding preference and agglomeration of T. gratilla in T. ciliatum meadows, together with the lack of evidence of substantial top-down control and the long recovery time of T. ciliatum led to the overgrazing of this species at this site. Overgrazing, therefore, was shown to be the result of multiple factors ranging from the traits of the seagrass and feeding preference of T. gratilla, to the abundance of predators in this area.
1 INTRODUCTION
Sea urchins are one of the main invertebrate grazers in tropical seagrass meadows (Lawrence, 1975), which play a fundamental role in shaping the structure and abundance of macroalgae and seagrasses (Alcoverro & Mariani, 2002). In tropical seagrass ecosystems, 24% of seagrass production is consumed by sea urchin grazing (Klumpp et al., 1992), which shows that the accumulation of plant biomass in these ecosystems is heavily controlled by consumers (Heck & Valentine, 2007). Grazing pressure can be compensated initially by the stimulation of seagrass growth, promoted by the translocation of carbohydrates; however, after exhaustion of carbohydrate reserves, loss of seagrass tissue can occur (Cebrián et al., 1998). An increase in abundance of sea urchin populations, generally due to the absence of top-down control, can lead to increased grazing pressure and, consequently, overgrazing of seagrass meadows (Eklöf et al., 2008). This has been reported in several cases in East Africa where populations of Tripneustes gratilla have been observed to be 127 times greater in areas that are devoid of top predators (generally due to fishing activities) than in marine protected areas (Eklöf et al., 2009).
Tripneustes gratilla is a subtropical sea urchin species and a well-known seagrass grazer (Klumpp et al., 1993, Vaïtilingon et al., 2003). Its distribution includes East Africa, the South Sea Islands, Australia, and southern Japan (Mortensen, 1943). Population outbreaks of T. gratilla can lead to serious consequences in the ecosystem structure and functioning, like a change in the dominant primary producers from foliose algae to crustose coralline algae (Valentine & Edgar, 2010) or a severe reduction in seagrass densities (Alcoverro & Mariani, 2002). A common behavior in population outbreaks of T. gratilla is the appearance of urchin grazing fronts or aggregations of high urchin density (Alcoverro & Mariani, 2002; Eklöf et al., 2008; Valentine & Edgar, 2010), which advance together grazing the primary producers along the front path. These fronts can gather an average of 10 urchins/m2 (Mombasa Bay, Alcoverro & Mariani, 2002). In the last 40 years, several cases of overgrazing by sea urchins have been reported. A review by Eklöf et al. (2008) reports 16 known overgrazing events, three of them caused by the species Tripneustes gratilla on two different seagrass species: Thalassodendron ciliatum (Kenya, Alcoverro & Mariani, 2002; Crona, 2006) and Halophila stipulacea (Red Sea, Jafari & Mahasneh, 1987).
Feeding preference of the grazer may play a role in the extent of over-grazing events as it determines the target of the grazers when several feeding options are available. In the case of T. gratilla, its feeding preference varies depending on the region (Lawrence & Agatsuma, 2013). Food availability (Stimson et al., 2007) and habitat preference (Herring, 1972) are the main factors reported to control the diet of T. gratilla, which characterize this species as a generalist feeder. Other studies have also discussed seagrass traits as being responsible for the bottom-up control of feeding preference, including lipid content, morphology, and texture (Floreto et al., 1996; Stimson et al., 2007; Vaïtilingon et al., 2003), nutritional content (Seymour et al., 2013) or epiphytation (Lyimo et al., 2011). Nevertheless, due to interactive effects of different characteristics of the food and the variety of preferences and available feeding sources, the cause for the election of a specific feeding source by T. gratilla remains unclear.
Local population dynamics of sea urchins may play a role in the occurrence of overgrazing in seagrass meadows. First, the success in the recruitment of urchins and their post-settlement survival can be controlled by predation and structural complexity (Bonaviri et al., 2012; Hereu et al., 2005), as well as hydrodynamics (Nishizaki & Ackerman, 2007), among other factors (Balch & Scheibling, 2001; Hunt & Scheibling, 1997). In the case of adult sea urchins, factors like wave exposure (Clemente & Hernández, 2008), structural complexity, and predation pressure also shape their abundance (Farina et al., 2014). Local differences in these factors can lead to higher densities of urchins in certain patches, thereby increasing overgrazing risk.
In November of 2015, we observed large patches of overgrazed T. ciliatum together with aggregations of the sea urchin T. gratilla (Figure 1) in Changuu Island, located off the west coast of Unguja Island, Zanzibar Archipelago, Tanzania. While the internal patches of T. ciliatum were largely affected, other seagrass species present in the area seemed unaffected. Similarly, outer isolated patches of T. ciliatum seemed also unaffected. These observations contrasted with previous observations made in the same site in previous years of study (Dieuwke Hoeijmakers, personal observation) in which no signs of overgrazing on T. ciliatum nor aggregations of T. gratilla were apparent. We, therefore, assessed the grazing activity of T. gratilla on the seagrass meadows of Changuu Island during the overgrazing event.

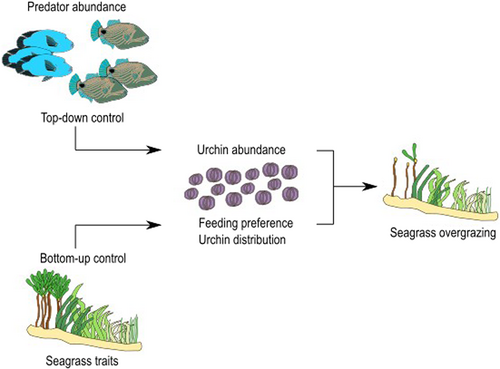
First, we quantified the magnitude of the overgrazing of seagrass by T. gratilla by mapping the seagrass meadows (overgrazed and non-overgrazed) in the study site, the east coast of Changuu Island. Secondly, prior studies have shown that indicators of biomass and diversity of fishes in Changuu Island were below critical thresholds (Rehren et al., 2020). Therefore, we hypothesized that there was a lack of top-down control on the sea urchin population which allowed for the aggregation of sea urchins in the study site (Figure 2). To test this hypothesis, we assessed the abundance of predatory fishes by deploying underwater cameras to measure fish abundance (Conn, 2011). Thirdly, due to the exclusive overgrazing of T. ciliatum, we hypothesized that seagrass traits and cover may exert a bottom-up control on the feeding preference of T. gratilla and its distribution (Figure 2). To test this hypothesis, we measured the distribution of T. gratilla through the measurement of its density in seagrass patches dominated by different seagrass species. Feeding preference was assessed by the calculation of the electivity index of the different seagrass feeding options (Vanderploeg & Scavia, 1979). The traits considered to exert bottom-up control on feeding preference and urchin distribution were seagrass morphology (canopy height and leaf area), tissue nutrient content (carbon, nitrogen, and phosphorus ratios), and chemical defenses (free phenolics content).
2 MATERIALS AND METHODS
2.1 Study area
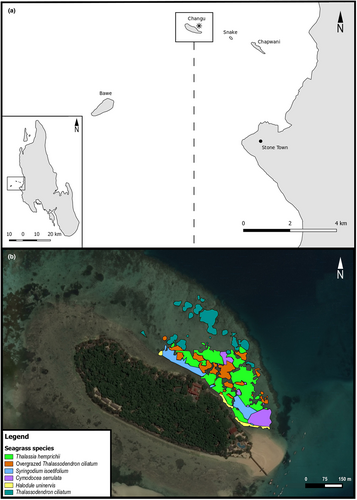
Changuu Island (Figure 3a) is one of several islands on the coast of Stone Town, the biggest city in Unguja Island (Zanzibar Archipelago, Tanzania). It is approximately 4.5 km off the coast. The sediment is carbonate and forms a shallow platform surrounded by a fringing reef. This shallow platform has a depth of <0.5 m in low tide and around 2 m in high tide, increasing to >1 m in low tide and 4 to 5 m in high tide toward the fringing reef. Seagrass meadows cover the carbonate platform from the low intertidal area out to a patchy reef area before the fringing reef. The seagrass meadows are mainly composed of five species: Thalassia hemprichii, Thalassodendron ciliatum, Syringodium isoetifolium, Halodule uninervis, and Cymodocea serrulata. The island is also a touristic spot, with boats arriving every day for the transport of tourists to a small resort and a tortoise's zoo. It is also a popular area for snorkel excursions and is used as a fishing ground both inside and outside the fringing reef.
The sampling for this study was done after an initial visual observation of the meadow on Autumn 2015 confirmed that large areas of T. ciliatum meadows were disturbed, possibly due to heavy grazing pressure. This visual observation contrasted from previous observations we had made in the area in 2013, in which seagrass meadows were generally intact and urchin abundances were negligible. The difference in observations over a short time period alerted us to a potentially new disturbance in the area.
2.2 Study area characterization
2.2.1 Seascape configuration and magnitude of overgrazing
We mapped the seagrass meadows in the study area to calculate the total area covered by each seagrass species and the extent of the overgrazing of T. gratilla. The mapping was performed in low tide with a Garmin GPSmap 78 s and a transect tape, making a total of six transects parallel to the coast and 20 perpendiculars to the coast to form a grid. We recorded patches of seagrass species directly in the GPS by marking the change in the benthic communities along each transect. The patches were categorized by species and further sub-categorized as overgrazed or not overgrazed. A patch was defined as overgrazed when the shoots in the patch were lacking leaves and meristems except for sparsely distributed ungrazed individual shoots (as shown in Figure 1a).
All data points were transferred to the software Quantum GIS (Version 2.10.1 Pisa). We constructed polygons over the seagrass meadows with the data and the plug-in Open Layers and calculated the total cover area per seagrass species.
2.2.2 Predatory fish community
To determine whether there was a lack of top-down control on sea urchin densities in this site, we deployed underwater cameras (GoPro Hero3) to measure the abundance of predators of T. gratilla. According to previous literature, the main fish predators of T. gratilla in this region were Balistapus undulatus (Triggerfish, family Balistidae) and Cheilinus undulatus (Wrasse, family Labridae) (Eklöf et al., 2009; McClanahan & Muthiga, 1989; McClanahan & Shafir, 1990). Both B. undulatus and C. undulatus are diurnal species, feeding during the day in seagrass beds (Chateau & Wantiez, 2007; Young & Bellwood, 2012). C. undulatus tends to show site fidelity and has a home range of approximately 0.5 km2, which only leaves to reproduce (Chateau & Wantiez, 2007). Both of the species are commercially fished (Rehren, 2017). Abundances of these predators in our study site were the main focus of these surveys. We deployed the cameras in four sampling days in the dry season of 2017 in the internal seagrass patches where overgrazing of T. ciliatum occurred. As these surveys were performed approximately a year and a half after our initial seagrass mapping, we are making the assumption that there were no significant changes in the driving factors affecting fish abundances in the region over this time period. The change in the number of cameras deployed per day was subjected to the absence of technical problems (cameras running out of battery and cameras not recording the intended amount of time). We deployed three cameras simultaneously, attached to a 50 cm tall wooden pole. Each camera recorded a mean (± standard error) of 53 ± 2 min and an area of 10 m2, resulting in a total of ~20 h of video and a total surveyed area of ~220 m2. We used the maximum count method for counting fishes in the video surveys (Conn, 2011). We counted the fishes as the number of times they appear in the recorded area (i.e., if one fish left the area recorded and appeared again, it was counted as a new fish, as defined by Conn, 2011). This method was used to ensure that any predatory fish of T. gratilla would be detected despite the likely over-estimation of fish counts of abundant fish families. The fishes that appeared in the videos were screenshotted and identified using the fish identification guide developed by Allen et al. (2003). We created one category per fish family identified, two individual categories for the fish species that are predators of T. gratilla (B. undulatus and C. undulatus) and one category for unidentifiable fishes (generally due to suboptimal visibility).
We additionally assessed if the fish families were overfished in Changuu Island by collecting data of overfishing indicators from Rehren (2017). Overfishing was defined as the level of fishing effort that reduces stock levels below safe biological or sustainable limits. The indicator we selected from Rehren (2017) was the difference between the stock size B (biomass) and B50, which is the size of the stock reduced to half of the unexploited stock size (Gulland, 1973). The fish families were considered overfished when the stock size was significantly lower than B50.
2.3 Feeding preference and density of T. gratilla
2.3.1 Density of T. gratilla in different seagrass patches
To assess the sea urchin distribution across the seagrass species, we measured the sea urchin density in three monospecific patches (C. serrulata H. uninervis and overgrazed T. ciliatum) and two mixed patches (dominated by T. hemprichii and by S. isoetifolium) in the study site. We selected three random patches per patch type. With a transect tape, we delimited square seagrass patches with a length of 5 m per side and an area of 25 square meters per patch. We counted the number of sea urchins at high tide and divided this number by the surface of the patch to calculate sea urchin density per square meter.
2.3.2 Vanderploeg and Scavia's relativized electivity index (E*) of T. gratilla
To determine the gut content of T. gratilla, we sampled 10 sea urchins along a transect parallel to the coastline and transported them to the Institute of Marine Sciences (Stone Town, Zanzibar). The urchins were collected upon encounter along the transect in the internal seagrass patches (were the overgrazing on T. ciliatum occurred) with the rule of not collecting urchins from the same seagrass patch. This rule was established to ensure independent replicates (i.e., urchins sharing the same patch would not be independent replicates). Before collection of the sea urchin, a square quadrat with a side length of 0.25 m was deployed on top of the sea urchin, the number of shoots per seagrass species counted and the aboveground biomass collected (see Supplementary Material 1). We then weighed the sea urchins (with their spines), measured their diameter to the nearest millimeter with a ruler, and stored them in a 90% ethanol solution. For the gut content analysis, the sea urchins were opened and the content from the whole digestive tract was extracted (Vaïtilingon et al., 2003). The gut content was placed in a Petri dish and then transferred to a 250 μm mesh to remove the sediment. After the sediment removal, we weighed the gut content fixed sample weight (FSW). Approximately 1 g FSW was taken as a sub-sample from the gut content and placed in a new Petri dish to observe under the magnifying glass. Each sample was compared to an example of seagrass material that was prepared specifically for identification after the grazing and digestion process of each species. We imitated this effect by grinding the seagrass species to a similar size and storing them in 90% ethanol solution. Using this method, we could separate the gut contents into the different seagrass species. Contents were then dried in the oven at 60 degrees for 24 h to obtain their dry weight, and percentage of each seagrass species in the gut was calculated.
This electivity index is a measure of the feeder's perception of a food's value as a function of both its abundance and the abundance of other food types present (Lechowicz, 1982; Vanderploeg & Scavia, 1979) and it ranges from +1, which indicates that is preferentially consumed, to −1, which indicates that the food source is avoided. Values close to zero indicate random feeding, with the food source being consumed upon availability.
2.4 Seagrass traits
2.4.1 Carbon, nitrogen, and phosphorus content (%) and nutrient ratios of seagrass tissues
To estimate whether nutrient content of seagrass influenced the feeding preference of T. gratilla, we measured the carbon (C), nitrogen (N), and phosphorus (P) content of seagrass leaves. We took three seagrass leaf samples in five meadows of each seagrass species (T. ciliatum, T. hemprichii, H. uninervis, S. isoetifolium, and C. serrulata) and transported them in a cooled container to the Institute of Marine Sciences (Stone Town, Zanzibar). The leaves were cleaned of epiphytes and rinsed with distilled water. We separated the second leaf of each shoot for the measurement, dried them in the oven at 60 degrees for 48 h, and ground them to a fine powder with mortar and pestle. The samples were then transported to the Leibniz Centre of Tropical Marine Research in Bremen (Germany).
C and N content was measured in a Euro EA 3000 (EuroVector) analyser. For the determination of the % P content of the leaves, we used an alkaline persulphate oxidation method (Koroleff, 1983). We transferred 1 mg of ground sample into a vial and added 4.5 ml of distilled water and 0.5 ml of Oxisolv reagent (Merck). The vials were closed and placed in the oven for 1 h at 120°C. After letting the samples cool down at room temperature, they were centrifuged at 4700 rpm for 10 min. Three ml of supernatant were placed into another vial and 0.0626 ml of ascorbic acid reagent were added. After mixing, 0.0626 ml of molybdate mix-reagent was added. After a reaction time of 10 min, the samples were transferred to a 1 cm macrocuvette. We then measured absorbance at 880 nm in a Shimadzu UV-1700 UV–VIS dual-beam photometer for the % P determination. Finally, we calculated the ratios between C, N, and P.
2.4.2 Free phenolics content
To assess whether chemical defenses of seagrasses have an effect on the feeding behavior of T. gratilla, we measured the free phenolics content of the seagrass leaves of the different species. Seagrass samples for this analysis were taken in a nearby meadow in Snake Island, an island approximately 1 km away from our study site (Figure 3a, see Supplementary Material 2 for a comparison of the environmental conditions in Changuu and Snake Island). We took three seagrass leaf samples in three meadows of each seagrass species (T. ciliatum, T. hemprichii, H. uninervis, S. isoetifolium, and C. serrulata) and transported them in a cold container to the laboratory in the Institute of Marine Sciences (Stone Town, Zanzibar).
We cleaned the leaves from epiphytes and rinsed them with water. The cleaned leaves were dried in an oven at 38°C for approximately 3 days and transported to the Leibniz Centre for Tropical Marine Research in Bremen (Germany). The leaves were crushed to a fine powder using a mortar and pestle with liquid nitrogen, and the content of free phenolic compounds was measured using photometric determination of the phenolic compounds as tannic acid equivalents, following a modified protocol by Ainsworth and Gillespie (2007). To create the calibration curve, we prepared a standard tannic acid solution and transferred it to Eppendorf tubes in increasing volumes (from 0 ml to 0.450 ml in 0.050 ml intervals) filling up to a volume of 0.5 ml with 70% ethanol. We extracted 10 mg of dried sample with 2 ml of 70% ethanol for 24 h on a shaker at 225 rpm and centrifuged them from 10 to 20 min between 10,000 g and 20,000 g until a clear supernatant was obtained. An aliquot of the supernatant (between 0.02 and 0.1 ml) was transferred to 2 ml Eppendorf tubes, filling up to a volume of 0.5 ml with distilled water. We added 1250 μl of Na2CO3-NaOH-solution (0.7 N Na2CO3 and 0.1 N NaOH) to standards and samples. We added 250 μl 1 N Folin–Ciocalteu reagent and placed 340 μl of the standards and samples into a 96-well plate in the dark and measured after 2 h with TECAN Infinite M200 Pro plate reader at 760 nm. The concentration of tannic acid equivalents in the samples was calculated following the calibration curve by multiplying the concentration of tannic acid equivalent to the factor of volume extract containing 1 g of leaf powder and the dilution factor of extract used. Final results are shown in mg of free phenolics (as tannic acid equivalents) per g of seagrass dry weight (mg/g DW).
2.4.3 Seagrass morphology
To determine whether differences in seagrass morphological characteristics could be important drivers of the feeding preference and density of T. gratilla, we measured seagrass leaf area and canopy height. We took six biomass cores (20 cm diameter) at random points in the study area. We then measured the canopy height (cm) of each seagrass species in each core. For T. ciliatum, we measured the length of the stem separately and coupled it to the corresponding shoot. Stem and leaf lengths were then summed to obtain canopy height. The length and width of each leaf were measured and then multiplied to calculate the leaf area per species (cm2).
2.5 Statistical analysis
The statistical analyses were performed using R statistical software (Version 3.5.3, R Core Team, 2021).
To test the feeding preference of T. gratilla and its differences in distribution among seagrass species, the electivity index and the density of T. gratilla were analysed using a linear model in which seagrass species was used as the categorical explanatory variable. The significance of the categorical variables was obtained with analysis of variance (Type II sum of squares) using the R package “car” (Fox & Weisberg, 2011). Post hoc pairwise comparisons were performed by using complete permutations of the raw data in the data matrix. The assumptions of homoscedasticity, normality, and independence of the residuals were validated for the model, and no influential observations were found. In the case of the density of T. gratilla, we detected a problem with heteroscedasticity in the residuals that was solved adding a variance structure to the model. For this reason, the model was changed from a linear to a generalized least squares model using the package “nlme” (Pinheiro et al., 2018).
To test the bottom-up control exerted by the traits and % cover of seagrass on the gut content and density of T. gratilla we built a correlation matrix. For this correlation matrix we used the percentage gut content of seagrass in T. gratilla instead of the electivity index to avoid the confounding effect of the standardization of this index with seagrass abundance in the study area. We calculated the average trait value per seagrass species for all seagrass traits (canopy height, leaf area, leaf nitrogen, leaf phosphorus, leaf carbon, and leaf free phenolics content), seagrass % cover and for the gut content and density of T. gratilla and used these as input values for the correlation matrix. We used a Spearman rank-based test (see Table 1 for the input values). Spearman's correlation coefficient (ρ) measures the strength and direction of the monotonic association between the two ranked variables. The R packages “Hmisc” (Harrell Jr, 2020) and “PerformanceAnalytics” (Peterson et al., 2018) were used for these analyses.
| Variables | Seagrass species | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cymodocea serrulata | Halodule uninervies | Syringodium isoetifolium | Thalassodendron ciliatum | Thalassia hemprichii | |||||||
| n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | ||
| Morphological | Canopy height (cm) | 8 | 6.21 ± 1.16 | 2 | 2.10 ± 1.00 | 14 | 5.62 ± 0.81 | 8 | 7.87 ± 2.64 | 15 | 6.09 ± 0.48 |
| Leaf area (cm2) | 5.99 ± 1.34 | 0.44 ± 0.33 | 0.63 ± 0.11 | 5.18 ± 1.19 | 3.45 ± 0.64 | ||||||
| Nutritional content | %C | 9 | 32.74 ± 0.21 | 9 | 31.77 ± 0.45 | 9 | 19.49 ± 0.59 | 9 | 35.55 ± 0.78 | 9 | 31.81 ± 0.46 |
| %N | 1.35 ± 0.05 | 1.43 ± 0.06 | 0.56 ± 0.03 | 1.47 ± 0.04 | 2.24 ± 0.05 | ||||||
| %P | 0.22 ± 0.01 | 0.26 ± 0.02 | 0.09 ± 0.01 | 0.13 ± 0.02 | 0.21 ± 0.01 | ||||||
| C:N ratio | 28.32 ± 0.54 | 26.06 ± 0.45 | 40.66 ± 0.99 | 28.47 ± 1.03 | 16.59 ± 0.27 | ||||||
| C:P ratio | 380.34 ± 10.80 | 328.85 ± 20.59 | 568.61 ± 53.31 | 750.96 ± 37.24 | 387.93 ± 11.05 | ||||||
| N:P ratio | 13.45 ± 0.42 | 12.63 ± 0.76 | 14.16 ± 1.48 | 26.60 ± 1.60 | 23.39 ± 0.63 | ||||||
| Chemical defense | Free phenolics (mg/g DW) | 12 | 4.98 ± 0.30 | 7 | 5.24 ± 0.83 | 7 | 0.50 ± 0.16 | 7 | 6.22 ± 0.23 | 14 | 2.91 ± 0.99 |
Seagrass abundance and overgrazing data and fish abundance data were not statistically analysed, as they were quantifications of the effect of overgrazing and the presence or absence of top-down control. The nominal Type I error rate (alpha) was set at 0.05. Figure 4 was plotted using the R package “ggplot2” (Wickham, 2016). The figures were aesthetically modified using InkScape (version 0.92).
3 RESULTS
3.1 Seascape configuration and magnitude of overgrazing
The total area of seagrass meadows monitored around Changuu Island (Figure 3) was 59,662 m2 (Table 2). The seagrass community was mainly dominated by T. hemprichii (40.5%) and T. ciliatum (33.9%). Nevertheless, 43% of T. ciliatum patches were overgrazed, accounting for 14.8% of the total seagrass cover and a grazed surface of 8847 m2. The grazed T. ciliatum area was located mainly in the patches surrounded by other seagrass species, while the patches that were isolated or on the edges of the area were less affected (Figure 3b). We did not find overgrazed patches of any other of the seagrass species present in the study area.
| Seagrass species | Area (m2) | Percentage cover (%) |
|---|---|---|
| Thalassia hemprichii | 24,144 | 40.5 |
| Thalassodendron ciliatum (Total) | 20,258 | 33.9 |
| Thalassodendron ciliatum (Not grazed) | 11,411 | 19.1 |
| Thalassodendron ciliatum (Overgrazed) | 8847 | 14.8 |
| Syringodium isoetifolium | 7635 | 12.8 |
| Cymodocea serrulata | 6175 | 10.4 |
| Halodule uninervis | 1448 | 2.4 |
| Total | 59,662 | - |
3.2 Predatory fish community
The predators of T. gratilla, B. undulatus and C. undulatus, were absent in the fish survey (Table 3). We found a total of 13 fish families represented in the fish survey. The most abundant fish family (count mean ± SE) was Labridae (21.86 ± 6.43) followed by Pomacentridae (13.73 ± 8.67) and Apogonidae (8.55 ± 1.93). The rest of the families were irregularly present and generally in low numbers. The fishery status obtained from Rehren (2017) showed that the families Balistidae, Pomacentridae, Chaetodontidae, and Mullidae were overfished in Changuu Island.
| Fish families | Fish count (mean ± SE) | Fishery state | |
|---|---|---|---|
| Balistidae | 0.00 ± 0.00 | ||
| Labridae | 21.86 ± 6.43 | ||
| Pomacentridae | 13.73 ± 8.67 | ||
| Apogonidae | 8.55 ± 1.93 | NA | |
| Lethrinidae | 3.86 ± 1.00 | NA | |
| Lutjanidae | 2.86 ± 0.58 | ||
| Siganidae | 3.36 ± 2.47 | ||
| Carangidae | 0.14 ± 0.07 | NA | |
| Sphyraenidae | 2.73 ± 2.73 | NA | |
| Chaetodontidae | 0.09 ± 0.09 | ||
| Mullidae | 0.18 ± 0.13 | ||
| Tetraodontidae | 0.18 ± 0.11 | NA | |
| Monacanthidae | 0.41 ± 0.21 | NA | |
| Gobiidae | 1.00 ± 0.86 | NA | |
| Small fish school | 3.91 ± 0.93 | NA | |
| Unidentified | 12.41 ± 4.84 | NA | |
| Total | Total fish | 75.27 ± 14.17 | NA |
3.3 Feeding preference and density of T. gratilla
3.3.1 Density of T. gratilla in different seagrass patches
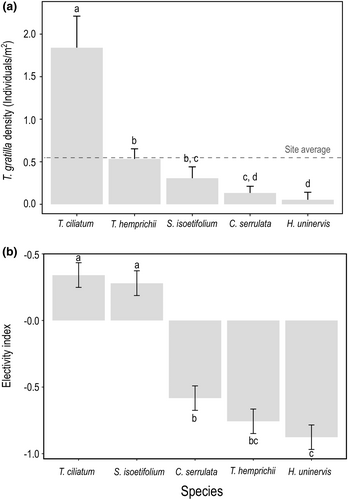
The density of T. gratilla (ind m−2; Figure 4a) was significantly different among patches dominated by different seagrass species (F4, 14 = 12.045, p-value = .001). The density (average ± SE) was highest for patches dominated by T. ciliatum (1.84 ± 0.36 ind m−2) and gradually decreased for T. hemprichii (0.53 ± 0.09 ind m−2), S. isoetifolium (0.31 ± 0.10 ind m−2), C. serrulata (0.13 ± 0.07 ind m−2) and H. uninervis (0.05 ± 0.03 ind m−2). When compared to the grand mean of T. gratilla density for the study site (0.57 ± 0.18 ind m−2), only T. ciliatum was above this mean, whereas the rest were similar or below it. For pairwise comparisons in the density of T. gratilla between species, please see Figure 4a.
3.3.2 Vanderploeg and Scavia's relativized electivity index (E*) of T. gratilla
The electivity index of T. gratilla (, Figure 4b) was significantly different among seagrass species (F4, 45 = 40.358, p-value = 2.422∙10−14). The electivity index was positive for T. ciliatum and S. isoetifolium, indicating a feeding preference for these two species, whereas C. serrulata, T. hemprichii, and H. uninervis showed negative electivity indices, indicating that these species were avoided by T. gratilla. For pairwise comparisons in preference between species, please see Figure 4b.
3.4 Seagrass traits
Seagrass morphology differed among species (Table 1). T. ciliatum had the highest canopy height (7.87 ± 2.64 cm), closely followed by C. serrulata, T. hemprichii, and S. isoetifolium. H. uninervis showed the lowest height (2.10 ± 1.00 cm). Similarly, H. uninervis had the smallest leaf area (0.44 ± 0.33 cm2), while C. serrulata (5.99 ± 1.34 cm2), and T. ciliatum (5.18 ± 1.19 cm2) had the largest leaf area. Concerning the seagrass nutritional content, the two bigger seagrass species (T. ciliatum and C. serrulata) had the highest %C content (35.55 ± 0.78 and 32.74 ± 0.21 respectively), while S. isoetifolium had the lowest (19.49 ± 0.59). T. hemprichii had the highest %N content (2.24 ± 0.05) and S. isoetifolium the lowest (0.56 ± 0.03), with the other species showing similar intermediate values (Table 1). S. isoetifolium (0.09 ± 0.01) and T. ciliatum (0.13 ± 0.02) had the lowest %P content, while the rest of the species had higher and similar %P values (Table 1). Seagrass free phenolics content was the highest for T. ciliatum (6.22 ± 0.23 mg gDW−1) and the lowest for S. isoetifolium (0.50 ± 0.16 mg gDW−1).
3.5 Correlation of seagrass traits with the gut content and density of T. gratilla
The gut content of T. gratilla was significantly and positively correlated with the C:P ratio of seagrass leaves (r = 0.9, p = 0.01), indicating a preference for P-depleted tissues (Table 4). Gut content did not show significant correlations with any other seagrass trait. In the case of the density of T. gratilla, there was no significant correlation with seagrass traits. However, there were strong (albeit non-significant) correlations with C:P ratio (r = 0.9, p = 0.08) and seagrass abundance (r = 0.9, p = 0.08).
| T. gratilla variables | Seagrass variables | |||||
|---|---|---|---|---|---|---|
| Biochemical traits | Seagrass morphology | Seagrass cover (%) | ||||
| C:N | C:P | Free phenolics (mg/g DW) | Canopy height (cm) | Leaf area (cm2) | ||
| Gut content % | 0.6 | 0.9* | 0.0 | 0.6 | 0.3 | 0.7 |
| Sea urchin density (individuals m−2) | 0.2 | 0.9 | 0.1 | 0.7 | 0.4 | 0.9 |
4 DISCUSSION
4.1 Overgrazing, urchin distribution, and top-down control of T. gratilla in Changuu Island
Overgrazing of seagrass by sea urchins in Changuu Island was only evident for the seagrass species T. ciliatum (Figures 1a and 3b). Although it is a reported common behavior for T. gratilla to overgraze seagrass meadows (Eklöf et al., 2008), the behavioral mechanisms behind it have remained partially unclear. Our spatial analysis indicated that the area of meadow closer to the coast was the most affected and also that isolated patches were generally not grazed (Figure 3b). This could be attributed to specific local population dynamics in the study site. First, recruitment success and survival post-settlement may be higher in the internal patches due to higher structural complexity (Bonaviri et al., 2012; Hereu et al., 2005) with the presence of mixed seagrass patches and the rocky shore. In addition, it has been reported that recruitment of urchins tends to happen in shallow areas (Ouréns et al., 2013), possibly causing that the internal patches right next to the coastline (with a depth of <0.5 m at low tide and around 2 m at high tide) have a higher recruitment success than the outer, deeper patches (with a depth of >1 m at low tide and 3 to 5 m at high tide). Secondly, the external T. ciliatum patches are isolated from the internal patches by a bare sandy bottom. Sand and corals can act as barriers to sea urchins (Alcoverro & Mariani, 2002; Laur et al., 1986; Stimson et al., 2007), and they could impede urchin migration from the internal, highly populated patches to the external ones.
The urchin density measured in this study site (Changuu Island: 0.57 ± 0.18 ind m−2) falls in a similar range as previous studies on T. gratilla (ind m−2): 0.1–0.33 (Mukai & Nojima, 1985), 0–2 (Juinio-Meñez et al., 1998), 1.5 (Uy et al., 2000), 0.1–4.7 (Beldia et al., 2003), 1.55 ± 0.07 (Vonk et al., 2008) and 0.06–0.58 (Regalado et al., 2010). However, this was highly dependent on which seagrass species dominated the meadow. The urchin density was more than three times higher in meadows dominated by T. ciliatum. Additionally, sea urchins distributed in the study area in two different ways: as individuals and as aggregations (also called urchin fronts by Alcoverro & Mariani, 2002). Unfortunately, urchin fronts were observed in the overgrazed internal seagrass patches but not systematically measured and, therefore, we cannot directly establish causality between the overgrazing of the T. ciliatum patches and the presence of sea urchin aggregations. However, it is shown in two reports of T. gratilla where severe grazing of seagrass and macroalgae occurred that, despite the presence of urchin fronts, the overall density of sea urchins was similar to densities of T. gratilla in absence of these fronts. Alcoverro and Mariani (2002) reports an overall abundance of 1.6 ± 0.99 urchins m−2, with presence of fronts with a density of 10.3 ± 2.2 urchins m−2. Similarly, Valentine and Edgar (2010) report the presence of urchin fronts (no direct density measured), with an overall density of 0.2–0.7 urchins m−2. It is therefore important to differentiate between these two forms of distribution. Presence of urchins does not mean risk of severe grazing on meadows, but the presence of urchin aggregations or fronts is an indication that, potentially, meadows are under a higher grazing pressure.
The lack of observations of B. Undulatus and C. undulatus, two of the main predators of T. gratilla in the region, and the overfishing status of several fish families (Balitidae, Pomacentridae, Chaetodontidae, and Mullidae) suggests a limited of top-down control on the population of T. gratilla at the study site. However, individuals of the Labridae family were observed, which have been reported to feed on juvenile sea urchins (Lawrence & Agatsuma, 2013). This indicates that there could still be some top-down control on the juvenile urchins, but not in the adult population. We also observed the presence of two other predators, the sea stars Protoreaster linki and Pentaceraster mammilatus (Eklöf et al., 2009), which were not quantified. In view of the intense overgrazing observed in the internal T. ciliatum patches, they did not exert enough predation pressure for successful top-down control of the urchin population. This seems to indicate that, even when benthic predators are present, fish predators of adult sea urchin individuals would be necessary to control the population and grazing of sea urchins. Our hypothesis that Changuu Island is under a regime in which the grazing of T. ciliatum by T. gratilla is not penalized by higher predation is partially confirmed by the absence of B. undulatus and C. undulatus. Therefore, successful recruitment coupled with low top-down control of T. gratilla may explain the large urchin aggregations in the study site, as they were less penalized by predation while eating their own shelter (Eklöf et al., 2009), which likely resulted in the extensive overgrazing of T. ciliatum.
Tripneustes gratilla formed urchin fronts despite an overall density within previous observations in which urchin agglomerations were not reported. Local differences in the study site (depth, structural complexity and sand barriers) may have impeded the propagation of urchin fronts to the external seagrass patches, causing the overgrazing exclusively in the internal patches. The study of local differences, the behavioral mechanism of urchin aggregation in fronts and its control deserves further study in the future, as it will help to understand how and when overgrazing on seagrass meadows or macroalgae could potentially occur, damaging ecosystem functioning or changing the dominant primary producer species in the area (Valentine & Edgar, 2010).
4.2 Bottom-up control of the behavior of T. gratilla by seagrass traits
Due to its wide range distribution (Lawrence & Agatsuma, 2013), there is a big variety of feeding sources reported for T. gratilla. In the case of this study, we showed that T. gratilla had a feeding preference for T. ciliatum and S. isoetifolium. This preference has been previously reported for T. ciliatum in Zanzibar (Herring, 1972) and for S isoetifolium (Vaïtilingon et al., 2003) and both species (Maharavo et al., 1994) in Madagascar. H. uninervis has been reported as the preferred feeding source for T. gratilla in South Sulawesi (Vonk et al., 2008), while in another study T. hemprichii was the preferred source (Kasim & Mukai, 2009). These reports indicate that feeding preference of T. gratilla is highly contextual and contingent to the feeding options present in the study area and, therefore, the extrapolation of the results reported in this and previous studies is difficult.
A review of the scientific literature on the causes of feeding stimulation or deterrence by sea urchins indicated that, for the species T. gratilla and other sea urchins as well, there is no consensus of the drivers behind feeding preference. There are reported deterrents like terpene geranylacetone (Steinberg & van Altena, 1992) and acrylic acid (Van Alstyne, Wolfe, et al., 2001), and reported stimulants or attractants like dimethyl sulfoniopropionate (DMSP) (Van Alstyne, Wolfe, et al., 2001), aldehydes (Scholtz, 2008) or even the epiphytation of the leaves (Lyimo et al., 2011). Phenolic content, despite deterring the herbivory from other primary consumers, does not seem to deter T. gratilla (Steinberg & van Altena, 1992), a result that is also supported by this study, as we did not find any significant correlation between feeding preference and free phenolics content. Additionally, while it has been discussed that palatability, in terms of morphology, texture (Floreto et al., 1996), and fragility (Vaïtilingon et al., 2003) of the tissues may be behind attraction or deterrence, this study found no evidence that palatability of seagrass affected feeding preference.
The nutritional content of primary producers is a tool generally used to assess feeding preference of grazers. In the case of sea urchins, there are contradictory reports suggesting that N may or may not play a role in grazing behavior. Some reports suggest that N has no effects (Van Alstyne, Whitman, et al., 2001), whereas others showed that nutrient enrichment can increase grazing (de Loma et al., 2002). In this study, the C:N ratio of seagrass leaves had no effect on feeding preference of T. gratilla. However, our study adds C:P ratio as a new variable to the list of possible explanations for the electivity of certain food sources by T. gratilla, partially confirming our hypothesis of a bottom-up control of feeding by seagrass traits. Both T. ciliatum and S. isoetifolium had the highest C:P ratio and they were the most preferred food sources, while the C:N ratio was approximately1.5 higher for S. isoetifolium than T. ciliatum.
To our knowledge, there are no previous reports indicating a relationship between feeding preference of T. gratilla and C:P ratio in seagrass, and there is generally a lack of studies relating P to grazing by other marine herbivores. Burkholder et al. (2012) reported that species with a lower C:P ratio were preferred feeding sources by green turtles, dugongs, and fishes with fundamental effects on the seagrass abundances in Shark Bay (Australia). More studies were found studying the relation of P content and grazing in terrestrial ecology, generally indicating a preference for P-enriched plants by animals like sheep and termites (Botch et al., 2010; Ozanne & Howes, 1971) and by P-rich grasshoppers (Ibanez et al., 2017). This result sets T. gratilla apart from other grazers (both marine and terrestrial) and poses the question of why T. gratilla has an eventual preference for P-depleted (or higher C:P ratio) seagrass species like T. ciliatum. There is no direct explanation in the literature to answer this question. However, calcifying organisms like coccolithophorids, tridacnid clams, and corals can give us some indications of the effects of P on calcification and its potential effects on T. gratilla. Coccolithophorids have shown a significant increase in calcification under P-limitation, simultaneously decreasing their population growth rate (Langer et al., 2012; Paasche, 1998; Paasche & Brubak, 1994). In the case of triacnid clams (Belda, 1994) and corals (Dunn et al., 2012), increases in P have shown increases in growth, but also the formation of more fragile and porous shells and skeletons, respectively. If P-limitation translates into higher calcification and a more robust and dense test in T. gratilla, then a diet involving P-depleted plants would be evolutionarily selected, as it would increase the survival of T. gratilla due to better protection against predation and physical disturbances. This result indicates the need of further research in the effects of P consumption in sea urchin calcification and its potential effects on its survival and the evolutionary selection of its diet.
We found that the density of T. gratilla varied across patches dominated by different seagrass species, with the highest density in patches dominated by T. ciliatum. However, there were no significant correlations between density and any seagrass trait. The fact that both feeding preference and urchin density are the highest for T. ciliatum suggests that urchin distribution is driven by feeding preference. Although this behavior has not been explicitly suggested for T. gratilla, it has been described for another sea urchin species (Strongylocentrotus droebachiensis, Vadas et al., 1986).
Our hypothesis suggesting that seagrass traits exert a bottom-up control on the behavior of T. gratilla is, therefore, partially confirmed by the influence of C:P ratio on its feeding preference. However, no connections between traits and urchin distribution have been found. Further research should address the effects of the C:P ratio of the feeding sources of T. gratilla on its calcification and general fitness in order to unravel the mechanism behind this trait and its relationship to feeding preference.
4.3 Overgrazing of T. ciliatum: A consequence of multiple interactive factors at different levels
The original motivation for this study was to determine the driving factors leading to overgrazing on the seagrass species T. ciliatum. We discovered that, despite the presence of fishes of the order Labridae and predatory sea stars, there was limited top-down control due to the lack of big predatory fishes. It was also found that the C:P ratio of the seagrass tissues exerted bottom-up control on the feeding preference of T. gratilla. This brings the question of why the only seagrass overgrazed was T. ciliatum, while S. isoetifolium was not overgrazed despite also being one of the preferred feeding sources. This can be explained by two different factors. The density of T. gratilla was significantly higher in meadows dominated by T. ciliatum and therefore more grazing could have occurred in them. It is a reported behavior that sea urchins tend to choose habitats with a higher complexity (e.g., Pinna et al., 2012) for shelter and for food availability, which calls for further research on the reasons behind the distribution of this sea urchin species. We cannot, however, rule out that grazing pressure was the same for both species, but that at the time of our surveys, T. ciliatum had not yet recovered from overgrazing while S. isoetifolium recovered more quickly. The morphology of T. ciliatum's leaves expose the meristem of the plant to grazing (schematic representation of this morphology in Alcoverro & Mariani, 2002), which obliges the plant to produce new shoots to increase its photosynthetic capacity. This is not the case for the rest of the seagrass species in the area, for which the meristem is generally belowground. The shoot plastochrone interval (defined as the average number of days between the formation of two successive shoots) for T. ciliatum is as long as 174 days (calculated from the average of the shoot recruitment data from Kamermans et al., 2001), explaining the lack of shoots with leaves in the overgrazed patches. However, further research is needed to disentangle both the effects of the sea urchin distribution and recovery time of seagrass.
5 CONCLUSIONS
This study shows that overgrazing of the seagrass Thalassodendron ciliatum by the sea urchin Tripneustes gratilla is a consequence of several interacting factors. The limited top-down control likely allowed for the formation of urchin fronts and a higher density of T. gratilla in meadows dominated by T. ciliatum. T. gratilla showed a feeding preference for both T. ciliatum and S. isoetifolium possibly due to the high C:P ratio in their tissues. The observed overgrazing of T. ciliatum within the meadow at the time of our study may be due to the time needed to recover from grazing by this seagrass species. Future research on urchin agglomerations and fronts, and the factors that lead to their appearance can shed light on the mismatch between low general urchin density, lack of strong evidence of top-down control, and overgrazing. Additionally, the possible influence of the C:P ratio on the feeding preference of T. gratilla should be further investigated to understand the mechanism behind it. Finally, the study of the time scales at which grazing and seagrass recovery happen will shed more light on the reasons behind overgrazing only on one specific seagrass species.
ACKNOWLEDGMENTS
The authors want to thank the staff of the Institute of Marine Sciences (IMS) in Stone Town (Zanzibar) for their support both administratively and scientifically, specifically Mtumwa Mwadini for his advice and help. We would like to thank Aoife O'Sullivan for her help in the samplings. We would like to thank Elizabeth Fay Belshe and Inés G. Viana for their feedback on the manuscript. Leibniz Centre for Tropical Marine Research Master Thesis grant and the PROMOS scholarship (University of Bremen) awarded to Agustín Moreira-Saporiti partially funded the field work. This project was developed under the SUTAS program (Sustainable Use of Tropical Aquatic Systems-funding to Dieuwke Hoeijmakers). The Seagrass and Macroalgal Community Dynamics and Performance under Environmental Change (SEAMAC) project (DFG, TE 1046/3-1) awarded to Mirta Teichberg partially funded this project. Open Access funding enabled and organized by Projekt DEAL.
Open Research
DATA AVAILABILITY STATEMENT
All the data used in this article is accessible in the PANGAEA database. The applicable links are listed below:
Moreira-Saporiti, Agustín; Teichberg, Mirta (2022): Seagrass canopy height and leaf area in Changuu Island (Unguja Island, Zanzibar Archipelago, Tanzania). PANGAEA, https://doi.pangaea.de/10.1594/PANGAEA.942660
Moreira-Saporiti, Agustín; Teichberg, Mirta (2022): Density of Tripneustes gratilla in different seagrass meadows in Changuu Island (Unguja Island, Zanzibar Archipelago, Tanzania). PANGAEA, https://doi.pangaea.de/10.1594/PANGAEA.942661
Moreira-Saporiti, Agustín; Teichberg, Mirta (2022): Seagrass leaf carbon, nitrogen and phosphorus content and their ratios in Changuu Island (Unguja Island, Zanzibar Archipelago, Tanzania). PANGAEA, https://doi.pangaea.de/10.1594/PANGAEA.942662
Moreira-Saporiti, Agustín; Teichberg, Mirta (2022): Gut content of Tripneustes gratilla in Changuu Island (Unguja Island, Zanzibar Archipelago, Tanzania). PANGAEA, https://doi.pangaea.de/10.1594/PANGAEA.942663
Moreira-Saporiti, Agustín; Teichberg, Mirta (2022): Seagrass abundance in Changuu Island (Unguja Island, Zanzibar Archipelago, Tanzania). PANGAEA, https://doi.pangaea.de/10.1594/PANGAEA.942664
Moreira-Saporiti, Agustín; Teichberg, Mirta (2022): Fish abundance in Changuu Island (Unguja Island, Zanzibar Archipelago, Tanzania). PANGAEA, https://doi.pangaea.de/10.1594/PANGAEA.942665
Moreira-Saporiti, Agustín; Teichberg, Mirta (2022): Free phenolics content of seagrass leaves in Snake Island (Unguja Island, Zanzibar Archipelago, Tanzania). PANGAEA, https://doi.pangaea.de/10.1594/PANGAEA.942666




