The paradox of the Hauraki Gulf snapper population: Testing the nursery habitat concept
Abstract
Juvenile nursery habitats are a critical requirement for the maintenance of some fish populations. Snapper in northern New Zealand is hypothesised to fit this nursery habitat model; however, nursery habitat association and dependency are poorly understood for New Zealand's largest snapper population in the Hauraki Gulf. To better understand habitat usage by post-settlement snapper and identify potential nursery habitats, we surveyed fish communities across a range of structured benthic habitats using fixed-position seabed cameras in Kawau Bay, in the northwestern Hauraki Gulf. Results indicated a low overall abundance of post-settlement snapper, relative to previous surveys at a nearby harbour outside of the Hauraki Gulf. There were also amongst-habitat differences in Kawau Bay, with post-settlement snapper and fish diversity higher in association with horse mussels relative to bare sediment. We anticipated high abundance of post-settlement snapper at restored green-lipped mussel bed sites, but abundance was again low relative to previous surveys outside of the Gulf. While Hauraki Gulf locations other than Kawau Bay may serve as nursery habitats with abundant populations of post-settlement snapper, another possibility is that post-settlement snapper habitat may not be as limiting within the expansive semi-sheltered Hauraki Gulf (potentially following an Effective Juvenile Habitat model). This hypothesis requires further investigation, as the answer could alter the emphasis of habitat conservation and restoration efforts in the area.
1 INTRODUCTION
Marine habitats that support high abundances of juvenile fish represent a critical life-history requirement for the maintenance of some fish populations (Beck et al., 2001; Heck, Hays, & Orth, 2003; Rosenberg, Bigford, Leathery, Hill, & Bickers, 2000), where life cycle ontogeny necessitates different, and potentially specialised, habitat requirements for juvenile compared to adult life stages (Dahlgren & Eggleston, 2000; Werner & Gilliam, 1984). Habitats that promote greater rates of juvenile growth and survival, and consequently provide a disproportionately greater contribution of individuals to adult populations on a per-unit-area basis have been defined as marine nurseries (Beck et al., 2001). This definition, however, excludes habitats that have a low per-unit-area contribution, but a high overall contribution by virtue of their large overall area, which has subsequently been termed as Effective Juvenile Habitats (Dahlgren et al., 2006). As such, both concepts provide a useful framework for assessing the importance of juvenile habitats to marine fish populations, which has subsequently been assessed multiple times since (Taylor, Fry, Becker, & Moltschaniwskyj, 2017; Vasconcelos, Reis-Santos, Costa, & Cabral, 2011).
In northern New Zealand, snapper (Chrysophrys auratus) is a highly valued fish species that is highly associated with structured habitats during early life stages (Parsons et al., 2014). Snapper larvae settle out of the water column and adopt a near-benthic life mode, a post-settlement stage that they occupy for a few months during summer/autumn at the start of their first (or 0+) year. This post-settlement stage is distinct from older juvenile stages in that (a) it feeds predominantly on pelagic copepods (Parsons et al., 2015), (b) its distribution is largely inshore/estuarine relative to older life stages (Parsons et al., 2014), and (c) it tends to have greater abundance in structured habitat types, although not in rocky reef habitat, where predation pressure (including from adult snapper) is thought to be highest (Parsons, Buckthought, Middleton, & MacKay, 2016; Parsons et al., 2013). Whereas some of the highest densities of post-settlement snapper have been observed amongst sub-tidal seagrass meadows (Morrison et al., 2014), structure type does not seem to be important (Parsons et al., 2016), whereas the density of that structure is (Parsons et al., 2013). The reason that post-settlement snapper are associated with non-rocky reef structured habitats is unclear, but it could be for energetic reasons whereby they expend less energy when sheltering from swift currents in the lee of structures, while simultaneously having higher contact rates with pelagic zooplanktonic prey items (Parsons et al., 2015). At the end of this post-settlement phase, around autumn of their first year or when they reach c. 60 mm Fork Length (FL), these post-settlement snapper disperse to either channels within estuaries or coastal environments for the remainder of their 0+ year and beyond (Parsons et al., 2014).
In the present study, we investigated post-settlement snapper habitat association within the Hauraki Gulf. The Hauraki Gulf is home to New Zealand's largest snapper population, with an estimated unfished biomass of 220,000 t, some 2.5 times greater than the next biggest snapper population in the Bay of Plenty (Francis & McKenzie, 2015a, 2015b). Despite the overall importance and abundance of snapper in the Hauraki Gulf, locations with high densities of post-settlement snapper have not been documented within the Gulf itself. Alternatively, high densities of post-settlement snapper have been recorded associated with structured habitats outside of the Hauraki Gulf, for example, in association with sub-tidal seagrass beds in the Parengarenga, Rangaunu and Kaipara harbours, and to some extent Whangarei Harbour (Evans, 2014; Lowe, Morrison, & Taylor, 2015; Morrison et al., 2014; Parsons et al., 2015). All these locations are discrete estuaries, with abrupt transitions at the estuary mouth from shallow and sheltered to a deeper and more exposed coastal environment.
Alternatively, the Hauraki Gulf is a large semi-sheltered and shallow embayment (the area inside the 30 m depth contour encompasses some c. 2,745 km2), with a more gradual transition to an open coastal environment. As a result, the contrast between post-settlement and adult habitats is less clear. Furthermore, many of the estuaries and harbours within the Hauraki Gulf are heavily impacted by land-based effects such as excess sediment and nutrient loading. These detrimental effects can manifest themselves indirectly by reducing the abundance and quality of important habitats (such as the disappearance of sub-tidal seagrass from the Hauraki Gulf during the early to mid-21st century, Inglis, 2003) or through direct effects on post-settlement snapper health and feeding success (Lowe et al., 2015; Morrison, Lowe, Parsons, Usmar, & McLeod, 2009). Surveys of post-settlement snapper that have been conducted within Hauraki Gulf estuaries have documented low post-settlement snapper density relative to other northern New Zealand estuaries (Lohrer, McCartain, Buckthought, MacDonald, & Parsons, 2018; Lowe et al., 2015; Morrison & Carbines, 2006). Alternatively, the surveys conducted within the coastal parts of the Hauraki Gulf have encountered high densities of larger and older (1+) juvenile snapper (Francis, 1995; Thrush, Schultz, Hewitt, & Talley, 2002). As a result, the source of post-settlement snapper that fuels New Zealand's largest snapper fishery is presently unknown. Two alternative explanations exist. There may be undocumented nursery habitats that make a disproportionately high per-unit-area contribution to the adult snapper population as per the nursery concept (Beck et al., 2001), or alternatively habitats with low per-unit-area densities may contribute the majority of snapper to the adult population through a large overall area as per the Effective Juvenile Habitat concept (Dahlgren et al., 2006).
We used the Hauraki Gulf post-settlement snapper population to test which of these two alternative concepts explaining juvenile habitat association was most likely. Specifically, we sampled post-settlement snapper abundance in a range of benthic habitat types in a sheltered coastal section of the northwestern Hauraki Gulf, an area that we judged as likely to contain important juvenile snapper habitat. Our objective was to discover whether a specific structured habitat type was supporting high densities of juvenile snapper, or whether low juvenile densities were more evenly spread amongst habitats within this area. We used an established video sampling method (Parsons et al., 2016; Parsons, MacDonald, Buckthought, & Middleton, 2018) that has good selectivity for small, cryptic post-settlement snapper. Because the method had been employed previously (Parsons et al., 2016), this also allowed us to compare the overall abundance and patterns of habitat use inside and outside of the Hauraki Gulf.
2 METHODS AND MATERIALS
Sampling was conducted in Kawau Bay, within the Hauraki Gulf, northeastern New Zealand (Figure 1). We chose Kawau Bay as our sampling location because it is a shallow area where a number of islands provide shelter from most wind and swell directions, has abundant populations of larger juvenile (i.e. older than the post-settlement stage) snapper (Francis, 1995) and is therefore a likely candidate as a coastal nursery location for post-settlement snapper within the Hauraki Gulf. The survey methods and analysis described below are similar to a previous study (Parsons et al., 2016) conducted in nearby Whangarei Harbour.
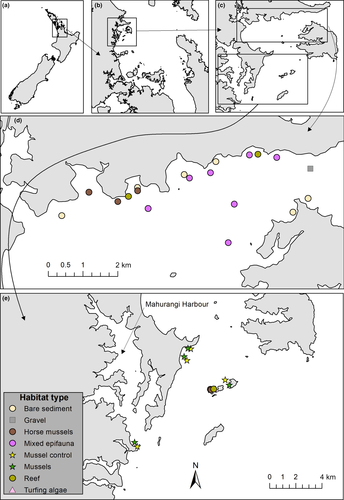
A survey of sub-tidal habitats was conducted within Kawau Bay in 2008 (Chiaroni, Hewitt, & Hancock, 2008), using towed camera sleds, drop cameras, diver observations and sediment cores to describe habitat types. We used the map produced by Chiaroni et al. (2008) to identify locations within Kawau Bay with structured habitats for our fish survey. The habitat types identified were bare sediments (mud, sand and compacted shells); turfing algae (expanses of sediment and compacted shells with a layer of turfing red and brown algae of <5 cm height); gravel (small rocks, cobbles and gravel with some coarse sediment and bivalve shells, may include some sparsely distributed individual brown algae, e.g. Ecklonia); reef (structurally complex rocky reefs dominated by large, potentially well over 1 m in height, brown algae, e.g. Ecklonia and Carpophyllum sp.); horse mussels (sediment containing patches of horse mussels [Atrina zealandica], up to 10 per m2 and of c. 5–15 cm in height); and mixed epifauna (sediment interspersed with sponges [e.g. Dactylia varia, and Chondropsis kirkii], ascidians, horse mussels, tubeworms [e.g. Chaetopterus sp., and Sabella spallanzanii] and rhodolith algae of c. 20 cm in height) (see Supporting Information for images of each habitat type). In addition, there are also a number of locations within Kawau Bay and Mahurangi Harbour where plots of adult green-lipped mussels (Perna canaliculus) were deployed in late 2016 as part of an experiment to assess the potential for restoring green-lipped mussel habitat within the Hauraki Gulf (Wilcox & Jeffs, 2017). We incorporated these mussel plots (patches of mussels at densities up to 100 per m2 and of c. 5–15 cm in height) and adjacent bare sediment (as controls) into our fish survey. Whereas sub-tidal seagrass is an important juvenile snapper habitat in other locations, it is not present within the survey area.
In March 2017, the time of year when post-settlement snapper abundance is at its peak (Parsons et al., 2014), a fish survey was conducted across the locations assessed above. Because our aim was to assess the nursery potential of sheltered coastal habitats (as opposed to estuarine habitats) within the Hauraki Gulf, we targeted sites known to have biogenic habitat structure present. At a potential site, we would often confirm the presence of this structure using a surface-deployed drop camera. Then at each site, three GoPro Hero 3 or Hero 4 cameras on 15 cm high steel stands would be deployed. Divers would search until habitat structure was found and then position a camera next to an individual structural element. Care was taken to ensure the structural element and seabed were in the field of view and the camera was placed 1 m away from the structure. This process was repeated for the other two cameras, ensuring that each camera was separated by at least 20 m. At reef sites, where habitat elements are more continuous, divers would position cameras 1 m from the edge of the reef. At bare sediment sites, divers confirmed the lack of structure before cameras were placed facing a patch of bare sediment. At green-lipped mussel sites, the extent of mussels was only large enough for one camera to provide an independent observation of the fish community at each mussel plot. We therefore deployed one camera observing the mussel habitat, and another camera positioned 20 m away and observing bare sediment (referred to as a “mussel control” replicate). At all sites, recording cameras were left undisturbed for an hour before being retrieved via a float and thin surface line attached to the metal stand.
During the fish survey itself, 79 camera replicates were deployed across 28 sites within Kawau Bay. Eight of these replicates (including both replicates at one of the green-lipped mussel sites) were not usable either because the camera fell over, became obscured or malfunctioned, or because water visibility at some sites was not sufficient to observe fish. This left 27 usable sites (displayed in Figure 1); however, at two sites, the habitat observed (gravel and turfing algae, see definition below) did not occur at any other site. These sites thus lacked replication and were therefore not included in statistical analyses. Overall statistical analyses were performed using 58 camera replicates across 21 natural habitat sites and eight camera replicates from four green-lipped mussel sites (and their bare sediment controls).
The first aspect of video analysis was to determine whether water visibility was adequate to enumerate fish abundance. If visibility was not ≥1 m (enabling a clear view of fish over the habitat element of interest), the video analysis from that replicate was not included in any subsequent analysis. Where water visibility was adequate, the habitat type was then categorised for each camera deployment. Next, fish abundance was analysed using a subsampling process similar to the mean count procedure described by Schobernd, Bacheler, and Conn (2014). Here, 10 random times were selected within the hour of video footage obtained from each camera deployment (excluding the first 10 min to remove the potential of diver disturbance). At each of these times, 2 min of video footage was watched (ensuring that the random times selected were ≥2 min apart), counting the maximum number of fish observed at one time (within a number of species and age classes described below), with the mean of these counts then used to represent fish abundance for that camera replicate. Only fish that were around the habitat element or habitat edge, or closer to the camera were counted. For bare sediment sites, a similar depth of field was estimated from footage taken while the diver set the camera in place. It is possible, however, that for these bare sites, fish were counted from a larger volume, which would make fish abundance estimates from sites with habitat structure conservative. Small benthic species (triplefins Forsterygion sp. and Grahamina sp. and goby Favonigobius exquisitus) and non-site attached pelagic fish (jack mackerel Trachurus novazelandiae) were not counted as they would not provide insight about the relative importance of juvenile fish habitat.
Whereas the primary focus of this survey was post-settlement snapper, the response of other fish species to habitat structure, and how that might differ with age was also of interest. Therefore, in addition to post-settlement snapper, we also counted other fish species observed. In terms of age class categorisation, this survey was conducted during March. For snapper at this time of year, the terms 0+ and post-settlement are synonymous because snapper spawned that year will not have yet transitioned out of their post-settlement stage. There should also be a c. 6 cm or c. 100% difference in the length of post-settlement and the next oldest age class (1+ snapper) (Francis, 1994), providing adequate resolution to categorise snapper into post-settlement and ≥1+ age classes. A similar methodology was followed for other common coastal fish species, although the terminology used to define age classes for these species was 0+ and ≥1+. The term post-settlement was reserved for only snapper as we have a more detailed understanding of the specific life-history requirements of snapper during this stage that may not be applicable to 0+ individuals of other fish species.
2.1 Statistical analysis
The three response variables of interest for analysis were the abundance of post-settlement snapper, fish diversity (the number of different fish species and age classes observed), and the 0+ proportion (the proportion of all fish observed that were in the 0+ year, which for snapper includes the post-settlement stage). For each video camera deployment, 10 × 2 min segments of video were viewed. The observations were averaged, resulting in one observation for each response variable for each camera deployment (eliminating temporal autocorrelation). Water visibility also precluded video analysis at one of the five restored green-lipped mussel habitat sites (and its bare sediment control).
After initial attempts to analyse these data with parametric statistical techniques (either log transforming data or directly fitting to a Poisson distribution), residuals from these models contained questionable distributions. As a result, non-parametric ANCOVA (within the PERMANOVA + routine, Primer v6) was used to analyse a resemblance matrix of Euclidian distances calculated from each response variable. Separate analyses were conducted for the restored green-lipped mussel sites (and their bare sediment controls) and the naturally occurring habitat sites. For the natural habitat analysis (where within site replication existed), we accounted for the likelihood that replicates within a site would be more similar than between sites, by treating site as a random variable, whereas habitat type was treated as a fixed variable. For the green-lipped mussel site analysis, habitat (mussel vs. bare sediment) was treated as a fixed variable, with site serving as the replicate (there was no replication within a site). For both analyses, water depth (m below chart datum at each site as indicated on the local hydrographic chart, 3–12 m range) was offered to these models as a continuous covariate. Non-significant terms were iteratively pooled, and each model was run with 9,999 permutations.
3 RESULTS
Fish communities were successfully surveyed at 27 sites within Kawau Bay across a range of habitats including bare sediment (n = 6), turfing algae (n = 1), gravel (n = 1), reef (n = 3), horse mussels (n = 5), mixed epifauna (n = 7), and green-lipped mussels and their bare sediment controls (n = 4). The fish communities observed at these sites were dominated by juveniles, sub-adults and adults from four species including: snapper, spotty (Notolabrus celidotus), goatfish (Upeneichthys lineatus) and leatherjacket (Parika scaber).
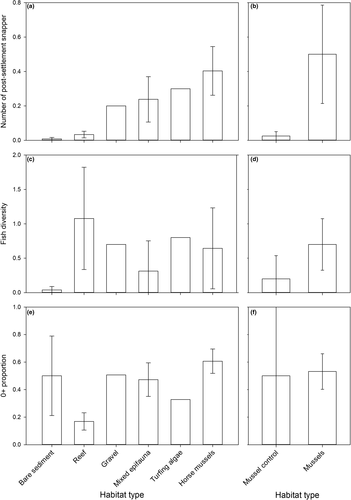
Overall, fish abundance was low. For example, the sum of the mean count response variable across the 71 useable camera replicates for all species and age classes was only 46 (mean count responses for replicates were usually <1). Post-settlement snapper were amongst the most commonly occurring fish observed in the video records, ranking 2nd (present in 33 of 71 useable replicates), just behind ≥1+ snapper (in 35 of 71 replicates). Post-settlement snapper also had the highest overall abundance (an average of 0.21 post-settlement snapper per 2 min of observation across all sites). Other fish species occurred much less frequently (≥1+ goatfish, ≥1+ spotty, 0+ spotty and ≥1+ leatherjacket, respectively, occurred at 16, 12, 11 and nine out of 71 replicates, respectively). In addition to overall abundance, we examined patterns of abundance across habitat types. Post-settlement snapper were rare in bare sediment (one of 15 replicates) and reef habitats (three of nine replicates) (Figure 2a), and more common in gravel, mixed epifauna, turfing algae and horse mussel habitats (occurring in 25 of 39 camera replicates deployed across these habitat types; Figure 2a). The final permutation-based ANCOVA model of post-settlement snapper abundance at the natural habitat sites retained a pooled variable containing the random factor site (df = 17, pseudo-F = 2.45, p = .024) and a separate variable for the fixed factor habitat (df = 3, pseudo-F = 3.39, p = .033). Pairwise comparisons of the different levels of the habitat variable suggested that the strongest differences were driven by higher abundance of post-settlement snapper at horse mussel compared with bare sediment sites (Table 1, Figure 2a). There was also some indication that horse mussel sites had more post-settlement snapper than rocky reef sites. While post-settlement snapper appeared to be more abundant at mixed epifauna sites compared to bare sediment or reef sites, these comparisons were not significantly different (Table 1). For the green-lipped mussel sites, post-settlement snapper were rare at the bare sediment control sites (one of four replicates) and more common within green-lipped mussel habitat (three of four replicates) (Figure 2b). This habitat difference was not significant, however, when tested by the permutation-based ANCOVA model of post-settlement snapper abundance within green-lipped mussel habitat (df = 1, pseudo-F = 3.35, p = .141).
| Habitat type comparison | t | p value | Significance |
|---|---|---|---|
| Bare sediment versus horse mussel | 3.3629 | .0037 | *** |
| Bare sediment versus mixed epifauna | 1.7764 | .1029 | n.s. |
| Bare sediment versus reef | 1.5518 | .1571 | n.s. |
| Horse mussel versus mixed epifauna | 1.1253 | .2856 | n.s. |
| Horse mussel versus reef | 2.2051 | .0675 | * |
| Mixed epifauna versus reef | 1.0899 | .3147 | n.s. |
Note
- p values are permutation based and have not been adjusted for multiple comparisons. The significance column represents our interpretation of these p values: *** strong evidence of a difference; * some evidence of a difference; n.s. = no evidence of a difference.
Fish diversity observed across the natural habitat sites was low at bare sediment sites, and higher at all other habitats, including reef, which had the highest overall average (1.1 fish species age classes per replicate) (Figure 2c). The final permutation-based ANCOVA model of diversity at the natural habitat sites retained a pooled variable containing the random factor site (df = 17, pseudo-F = 12.969, p = .0001) and a separate variable for the fixed factor habitat (df = 3, pseudo-F = 4.008, p = .024). Pairwise comparisons of the different levels of the habitat variable suggested that the strongest differences were driven by higher fish diversity at horse mussel and reef compared with bare sediment sites (Table 2). There was also some evidence that reef sites may have had higher fish diversity than mixed epifauna sites. For the green-lipped mussel sites, fish diversity was low at the bare sediment control sites relative to the green-lipped mussel habitat (Figure 2d). This habitat difference was not significant, however, when tested by the permutation-based ANCOVA model of fish diversity within green-lipped mussel habitat (df = 1, pseudo-F = 4.5692, p = .1109).
| Habitat type comparison | t | p value | Significance |
|---|---|---|---|
| Bare sediment versus horse mussel | 2.8220 | .0139 | *** |
| Bare sediment versus mixed epifauna | 1.6645 | .1184 | n.s. |
| Bare sediment versus reef | 3.8153 | .0079 | *** |
| Horse mussel versus mixed epifauna | 1.2169 | .2557 | n.s. |
| Horse mussel versus reef | 0.7809 | .4753 | n.s. |
| Mixed epifauna versus reef | 1.9885 | .0812 | * |
Note
- p values are permutation based and have not been adjusted for multiple comparisons. The significance column represents our interpretation of these p values: *** strong evidence of a difference; * some evidence of a difference; n.s. = no evidence of a difference.
The proportion of all fish in the 0+ year class observed across the natural habitat sites was generally lower at reef sites compared to all other habitats (Figure 2e). The final permutation-based ANCOVA model of the 0+ proportion at the natural habitat sites, however, did not reflect this. This model retained a pooled variable containing the random factor site (df = 14, pseudo-F = 2.5604, p = .0231), but the fixed factor habitat was not significant (df = 3, pseudo-F = 1.6432, p = .2203). Pairwise comparisons of the different levels of the habitat variable, however, did provide some evidence that the 0+ proportion was lower at reef compared to horse mussel sites (Table 3). For the green-lipped mussel sites, the 0+ proportion was similar amongst both bare sediment control and green-lipped mussel habitat (Figure 2f). The effect of habitat on the 0+ proportion at green-lipped mussel sites was not significant when tested by the permutation-based ANCOVA model (df = 1, pseudo-F = 0.079984, p = .8691).
| Habitat type comparison | t | p value | Significance |
|---|---|---|---|
| Bare sediment versus horse mussel | 0.53642 | .5989 | n.s. |
| Bare sediment versus mixed epifauna | 0.12373 | .8448 | n.s. |
| Bare sediment versus reef | 1.2230 | .2443 | n.s. |
| Horse mussel versus mixed epifauna | 0.9056 | .4229 | n.s. |
| Horse mussel versus reef | 2.4434 | .0470 | * |
| Mixed epifauna versus reef | 1.3059 | .2398 | n.s. |
Note
- p values are permutation based and have not been adjusted for multiple comparisons. The significance column represents our interpretation of these p values: *** strong evidence of a difference; * some evidence of a difference; n.s. = no evidence of a difference.
4 DISCUSSION
This study investigated fish communities associated with biogenic and other habitat structure in Kawau Bay, a sheltered coastal location that has the potential to have nursery habitat value for New Zealand's largest snapper population in the Hauraki Gulf (Parsons et al., 2014). The main observation from this study was the generally low overall abundance of post-settlement snapper relative to previous surveys conducted in Whangarei Harbour. For example, the highest average video sample count in Kawau Bay was <0.5 post-settlement snapper per 2 min, which occurred in a restored green-lipped mussel habitat. Alternatively, using identical methods in previous studies, all of the natural structured habitat types surveyed in Whangarei Harbour had average counts of >1 post-settlement snapper (Parsons et al., 2016), and for artificial seagrass units, also deployed in Whangarei Harbour, average counts were >6 post-settlement snapper (Parsons et al., 2018). This raises the possibility that post-settlement snapper conform with the nursery habitat concept (Beck et al., 2001) in some areas, and the Effective Juvenile Habitat concept in others (Dahlgren et al., 2006). A secondary objective of the present study was to describe patterns of habitat use displayed by post-settlement snapper within Kawau Bay. Compared to a previous investigation conducted in Whangarei Harbour (Parsons et al., 2016), post-settlement snapper habitat use was somewhat similar, where comparable habitats were present. Specifically, in Kawau Bay, post-settlement snapper abundance and fish diversity both responded to horse mussel habitat relative to bare sediment, with reef habitats also having high fish diversity relative to bare sediment. For the 0+ proportion of the fish species observed in Kawau Bay, there was some indication that it was lower at reef compared to horse mussel sites. Alternatively, in Whangarei Harbour all structured habitats had higher post-settlement snapper, fish diversity and 0+ proportion responses relative to bare sediment (with reef habitat also having high fish diversity but a low proportion of 0+ fish) (Parsons et al., 2016). Rather than reflecting an exclusive preference for horse mussel habitat in Kawau Bay, we suggest that the same mechanisms put forward by Parsons et al. (2016) apply here, but were partially obscured by low overall abundance (i.e. low effect size) and replication. Specifically, (a) that in the off reef habitats where we conducted our observations, many juvenile fish species responded to structure in general rather than to the type of structure (Heck et al., 2003) and (b) that the juvenile fish species we observed predominantly occupied non-reef habitats, whereas the reef habitats we observed were predominantly occupied by adult fish (Grol, Nagelkerken, Rypel, & Layman, 2011; Kimirei et al., 2015; Nagelkerken, Dorenbosch, Verberk, Cocheret de la Morinière, & Velde, 2000). It is important to note, however, that habitat occupancy and ontogeny patterns are often species specific and in other systems many species of juvenile fish do predominantly occupy rocky reef habitats (Félix-Hackradt, Hackradt, Treviño-Otón, Pérez-Ruzafaa, & García-Charton, 2014).
The low overall abundance of post-settlement snapper in Kawau Bay was potentially best illustrated by the minimal response to restored green-lipped mussel habitat. The introduction of structured habitat generally results in large increases in abundance via attraction of fish from surrounding areas, or through an increase in production. This has been documented for post-settlement snapper outside of the Hauraki Gulf (Parsons et al., 2018, 2013), and for fish communities more generally (Bohnsack, Harper, McClellan, & Hulsbeck, 1994; Powers, Grabowski, Peterson, & Lindberg, 2003). In Kawau Bay, however, while post-settlement snapper abundance was higher at green-lipped mussel sites relative to nearby bare sediment, the relatively small magnitude of increase and low number of restored mussel patches available to be sampled (n = 4) impeded detection of a statistically significant difference. Statistics aside, the low magnitude of the response of post-settlement snapper to added structure in Kawau Bay, relative to responses to artificial seagrass in Whangarei Harbour, stands out (Parsons et al., 2018). As the ecological function of green-lipped mussel compared to artificial seagrass habitat is unknown, we do not think that the detrital and invertebrate communities associated with the young green-lipped mussel beds were underdeveloped. Our work with artificial seagrass patches in other locations demonstrates the speed at which large numbers of post-settlement snapper can respond to small patches (c. 3.25 m2) of newly introduced structure, and also illustrates that their diet is dominated by pelagic invertebrates not associated with the structure itself (Parsons et al., 2015, 2013).
In addition to responses to habitat type, it is important to consider the potential influence of environmental variables on fish communities (Ley, McIvor, & Montague, 1999; Martinho et al., 2007; Parsons et al., 2015, 2013; Stoner, Manderson, & Pessutti, 2001). We had hoped to assess the response of fish to structured habitat across a gradient from sheltered to exposed sites within Kawau Bay. The limited interspersion of habitat types, the non-linearity of this gradient and the low abundance of fish communities in general, however, precluded us from assessing this gradient. Other potentially important environmental variables that we considered either did not have any influence on results (depth) or information on them was not available across our sampling sites (water velocity; but see Lohrer et al. (2018)).
Considering the lack of post-settlement snapper associated with biogenic and other structure within the sheltered Kawau Bay region, the question of where Hauraki Gulf snapper come from remains. We see three main possibilities: (a) nursery habitats in other parts of the Hauraki Gulf supply the post-settlement recruits required to sustain the snapper population. It is important to emphasise that our survey was spatially constrained to just one coastal part of the Hauraki Gulf, so this is entirely possible. Hauraki Gulf estuaries could potentially fulfil this nursery function, but we see this as unlikely. For example, the Mahurangi Harbour, one of the Hauraki Gulf's largest estuaries, was included in the present study with a mean count of post-settlement snapper of 0.2. In addition, a previous study using the same sampling method conducted across five sites within the Mahurangi also had a mean count of <0.5 post-settlement snapper (Lohrer et al., 2018). Sheltered coastal Hauraki Gulf sites (such as the islands and harbours on the west coast of Great Barrier Island and the Coromandel Peninsula) are potential candidates as nursery locations, and a broader survey of the Gulf is clearly required to establish if these locations might be important. (b) Nursery habitats outside of the Hauraki Gulf supply recruits to the Hauraki Gulf snapper population. As snapper fisheries in other areas have been shown to receive the majority of recruitment via the movement of juveniles from spawning or nursery grounds hundreds of kilometres away (Hamer, Acevedo, Jenkins, & Newman, 2011), this seems unlikely to be occurring in the Hauraki Gulf. There are two snapper stocks adjacent to the Hauraki Gulf stock. Of those, the east Northland stock has a unique annual recruitment strength signal compared to the Hauraki Gulf, suggesting separate sources of recruitment (Francis & McKenzie, 2015a). The other adjacent snapper stock, the Bay of Plenty, does have a similar annual recruitment strength signal to the Hauraki Gulf (Francis & McKenzie, 2015b). Many Bay of Plenty snapper, however, migrate into the Hauraki Gulf at the time of spawning (Ministry for Primary Industries, 2017), suggesting that the Bay of Plenty is unlikely to serve as the source of snapper recruits to the Hauraki Gulf; it is more likely that the Hauraki Gulf is one of the main spawning areas and a major source of snapper recruits to the Bay of Plenty. Furthermore, snapper larvae have a relatively short pelagic duration (c. 30 days, Parsons et al., 2014) and are understood to disperse over 10's of km during this time (Le Port, Montgomery, & Croucher, 2014; Zeldis, Oldman, Ballara, & Richards, 2005), not the >100 km distance that would be required to transition into the Hauraki Gulf. (c) Post-settlement snapper habitat occupancy in the Hauraki Gulf does not conform to the nursery habitat concept, where certain habitats make a disproportionate per-unit-area contribution to the population (Beck et al., 2001). Instead, the Effective Juvenile Habitat concept may explain our observations (Dahlgren et al., 2006). Effective Juvenile Habitats make a greater than average overall contribution to the adult population by virtue of their large overall area (so do not have a high per-unit-area abundance) (Dahlgren et al., 2006). It is possible that the entire Hauraki Gulf may serve as Effective Juvenile Habitat, due to its semi-sheltered and shallow nature. If this is true, Effective Juvenile Habitat may be far less limiting in the expansive Hauraki Gulf compared to the west coast and northern east coast of the North Island, where the transition to an open coastal environment is more abrupt, and sheltered shallow areas are constrained to harbours.
If post-settlement snapper do follow the nursery versus Effective Juvenile Habitat concepts in different areas, this could potentially be driven by the large area of the semi-sheltered and shallow waters of the Hauraki Gulf relative to the enclosed waters in other parts of northern New Zealand. To put this into perspective, the area of the Hauraki Gulf (<30 m deep) alone is c. 2,475 km2, which is significantly larger than the area of all of the large enclosed water bodies outside of the Hauraki Gulf in northern New Zealand (i.e. the combined area of the Manukau, Kaipara, Hokianga, Herekino, Whangape, Parengarenga, Houhora, Rangaunu, Mangonui, Whangaroa and Whangarei harbours, the Bay of Islands, and Whangaruru Harbour, Whananaki, Ngunguru, Pataua North and South, and Taiharuru estuaries which is c. 2,100 km2). Others have found juvenile habitat availability is a key determinant of adult biomass (Sundblad, Bergström, Sandström, and Eklöv 2014), and the Hauraki Gulf appears to be consistent with this hypothesis, hosting New Zealand's largest adult snapper population and potentially serving as a large area of Effective Juvenile Habitat.
A potential explanation as to why the nursery or Effective Juvenile Habitat concept may operate in a particular area could be related to how predation risk varies across these areas. In other systems, predation risk is known to be highly influential on habitat selection of juvenile fish as they grow through different life stages (Dahlgren & Eggleston, 2000; Grol, Rypel, & Nagelkerken, 2014). In the present study, potential predators (large fish capable of eating post-settlement snapper such as, e.g. eagle ray Myliobatis tenuicaudatus, John dory Zeus faber, trevally Pseudocaranx georgianus, and larger snapper) were rarely observed in Kawau Bay. Whether elevated predator abundance occurs in potential snapper nursery locations outside of the Hauraki Gulf is unknown. Alternatively, a particular area may not have always functioned as an Effective Juvenile Habitat, if anthropogenic effects have degraded and homogenised habitats. As such, the lower densities of post-settlement snapper in the Hauraki Gulf could simply be related to the general degradation of habitats in this area (Inglis, 2003; Lowe et al., 2015).
As this study highlights the low abundance of post-settlement snapper in Kawau Bay, the implications for habitat limitation may vary with year–class–strength, which can be highly variable. As we do not yet know the strength of the year–class surveyed here, we do know that year–class–strength is positively influenced by water temperature (>0.9 correlation) (Francis, 1993; Francis, Langley, & Gilbert, 1997). The slightly higher than average water temperature experienced during March 2017 (20.9°C vs. long-term average of 20.2°C, Leigh Marine Laboratory unpublished data) would suggest that we surveyed a year–class of average strength. Furthermore, 5 years of annual monitoring of post-settlement snapper abundance within nearby Whangarei Harbour does not suggest that 2017 was a low recruitment year (D. M. Parsons unpub. data).
5 CONCLUSIONS
Overall our results suggest that while post-settlement snapper in Kawau Bay are associated with structured non-reef habitats (such as horse mussels) as they are in other areas, their abundance was relatively low. While Hauraki Gulf locations other than Kawau Bay may serve as nursery habitats with abundant populations of post-settlement snapper, another possibility is that shallow and semi-sheltered areas of the Hauraki Gulf function as Effective Juvenile Habitat, potentially supplying adequate recruits to the large Hauraki Gulf snapper population by virtue of the large area of the Gulf. Prudent next steps in investigating the importance of post-settlement snapper habitat in the Hauraki Gulf include a more comprehensive survey of potential post-settlement snapper habitats, and an explicit attempt to link habitat availability, post-settlement snapper abundance and subsequent adult population size. Another important aspect is considering habitat productivity beyond abundance, specifically, understanding whether differential survival and growth of post-settlement snapper occurs across habitat types (Beck et al., 2001). Differential productivity between habitats would be especially important when considering the large areas of these habitats that were lost historically (Morrison et al., 2014, 2009; Powell, 1937; Thrush et al., 1998; Tuck, Hewitt, Handley, & Lundquist, 2017). The significance of other regulating factors, such as larval supply and competition for resources amongst adult snapper, cannot be discounted. Others have demonstrated that the benefit of additional juvenile (here post-settlement) habitat on adult biomass diminishes as juvenile habitat becomes more prevalent, which consequently means that other regulating mechanisms become limiting (Halpern, Gaines, & Warner, 2005; Sundblad et al., 2014; Wilson et al., 2016). This might be a relevant consideration for the Hauraki Gulf if a broader Hauraki Gulf survey did not identify habitats or areas with high post-settlement snapper abundance, suggesting that the Effective Juvenile Habitat concept might apply to Hauraki Gulf snapper. If this were the case, the benefit of habitat protection or restoration efforts within the Gulf, from the singular perspective of supporting post-settlement snapper, would also need to be considered.
ACKNOWLEDGEMENTS
The authors thank Andrew Jeffs, Jeremy McKenzie and Bruce Hartill for project advice and/or comments on the manuscript, and John-Mark Woolley for assistance with GIS. Funding was provided by NIWA's Coasts and Oceans research programme 3 (2016/17 Statement of Corporate Intent).




