Diet of mussels Mytilus trossulus and Dreissena polymorpha in a brackish nontidal environment
Abstract
Benthic suspension feeders have traditionally been considered an important link between pelagic and benthic food webs in shallow seas. Their ecological impact depends highly on the removal rate of pelagic production from the water column. Besides phytoplankton, benthic suspension feeders can feed on microphytobenthos and detritus suspended in the water column by wind-waves or tidal forces. The share of the pelagic component in the diet of benthic suspension feeders is poorly studied in the non-tidal environment. We examined the diatom food items of two bivalve species, Mytilus trossulus Gould and Dreissena polymorpha Pallas, at four shallow locations in the Gulf of Riga basin, in the Baltic Sea, differing in hydrographic characteristics. The share of pennate benthic diatoms was always higher in mussel food than in ambient water, whereas pelagic species were under-represented. Mussels never consumed the dominant genera that were in the water column, Cylindrotheca and Leptocylindrus. There were no differences in the main food source between the two mussel species. Our results suggest that the impact of benthic suspension feeders on pelagic foodwebs and their role in promoting benthic–pelagic coupling is of a minor importance in the study area, whereas the impact on benthic food webs may be mainly by the re-arrangement of energy within benthic pathways.
Introduction
Benthic suspension feeders are an important functional guild in aquatic ecosystems (e.g. Cloern 1982; Higgins & Vander Zanden 2010). Suspension feeding bivalves can reach extremely high biomasses and, in favourable conditions, often dominate communities of the shallow coastal sea (e.g. Kotta & Witman 2009). The energy fluxes in coastal sea ecosystems cannot be understood without a sufficient knowledge of the food sources that benthic suspension feeders exploit (Tomczak et al. 2009). The classical model of a coastal foodweb includes pelagic primary producers as the main source of energy for both the pelagic and benthic suspension feeders, while the latter constitute an important energy pump from the water column to the sea floor. Benthic–pelagic coupling occurs when benthic animals remove planktonic production from the water column and move it to the sea floor in the form of faeces and pseudofaeces (Newell 2004).
However, this point of view has been undermined by several recent studies from tidal systems, which have suggested that microphytobenthos is an important, or even the main, component of the food available to benthic suspension feeders (Kang et al. 1999; Machas et al. 2003; Kasim & Mukai 2006). Microphytobenthos can exceed estuarine phytoplankton both in production and biomass (Shaffer & Sullivan 1988; De Jonge & van Beusekom 1992). Also, it attains the highest biomass in the top few millimetres of the sediment, which can be easily resuspended by hydrodynamic forces (MacIntyre et al. 1996; Lucas et al. 2000; Guarini et al. 2008). However, studies mostly using stable isotope analyses have found fundamental differences in the origin of organic matter in suspension-feeding pathways among different estuarine systems and even within the same estuarine system (Ruckelshaus et al. 1993; Yokoyama et al. 2005, 2009; Kanaya et al. 2008; Dang et al. 2009). Benthic suspension feeders have been found to rely on both benthic and pelagic microalgae and may at times rely almost entirely on one or the other food source (Kamermans 1994; Riera & Richard 1996; Herman et al. 2000; Kang et al. 2006, 2007). Additionally, in some estuaries, detritus of macrophyte, riverine or terrestrial origin is the prevailing food source (Kasai & Nakata 2005; Kanaya et al. 2008). Observed differences among and within estuaries may be attributed to the spatial patchiness of different food sources. This, in turn, is related to physical characteristics of estuaries (riverine inputs and water residence time, marine impact, tidal range, area of tidal flats, turbidity) that determine the amount and spatiotemporal distribution of food, including the biomass and resuspension rates of microphytobenthos. However, no general patterns have been described to date. To complicate the issue even more, isotopic signatures of different suspension feeder species have been found to differ even at the same place and time, indicating either a reliance of species on different food sources (Dubois et al. 2007) or on some unknown attributes related to the methodology. Small-scale spatial variability in signatures within species also remains to be explained (Dubois et al. 2007). To conclude, the causes of the striking variability in the main food pool of benthic suspension feeders are still not fully understood, and the environmental factors influencing it remain unexplained.
Most of the studies discussed above have been conducted in macrotidal estuaries, and a few studies have been conducted in tidal bays. Benthic suspension feeders also inhabit microtidal and nontidal environments, where they may constitute a large share of zoobenthic biomass and consequently form an important part of the ecosystem (Kautsky & Kautsky 2000; Higgins & Vander Zanden 2010). Microtidal and nontidal waterbodies are often in close contact with extensive human populations, thereby having high economic and aesthetic value, as well as high rates of exploitation and human impact relative to the surface area. It is, therefore, of the utmost importance that we get better understanding of the functioning of such ecosystems.
Benthic suspension feeders are important in non-tidal habitats as a food source for higher trophic levels (Öst & Kilpi 1998; Lappalainen et al. 2005), as autogenic engineers (Ward & Ricciardi 2007), and by removal of phytoplankton from the water column (e.g. Budd et al. 2001; Petersen et al. 2008). Their feeding activity, in turn, also impacts benthic communities (Kotta et al. 2009). Despite their obvious importance, little is known about either the food sources of benthic suspension feeders in nontidal systems or the potential spatial variability of their feeding.
Even if resuspension is controlled by strong tidal forces, the influence of wind on the resuspension of benthic algae can be detectable and significant (Demers et al. 1987). In shallow areas of large non-tidal systems such as the Baltic Sea, the resuspension of microphytobenthos may be almost completely wind-driven. Epibenthic suspension feeders form distinctive populations with high biomasses in a number of coastal areas in the Baltic Sea, offering an excellent nontidal model system for the studies on benthic–pelagic coupling in wind-driven conditions. The roles of benthic suspension feeders in the ecosystem of the Baltic Sea are not very well documented.
Here, we aimed to test the impact of benthic suspension feeders on pelagic food webs and their role in promoting benthic–pelagic coupling in a shallow non-tidal water body. This was done by comparing the different diatom species in the water column with the diets of the mussels Mytilus trossulus Gould and Dreissena polymorpha Pallas in a shallow gulf in the Eastern Baltic Sea. Our main hypotheses were: (i) benthic suspension feeders, by actively feeding on phytoplankton, facilitate benthic–pelagic coupling in a non-tidal ecosystem and (ii) the observed relationship may vary according to spatial variability in environmental conditions. We predicted that the species composition of microalgae in the diets of benthic suspension feeders resembles that of the water column and the proportion of the diet comprising plankton is related to local hydrographic conditions.
Study area
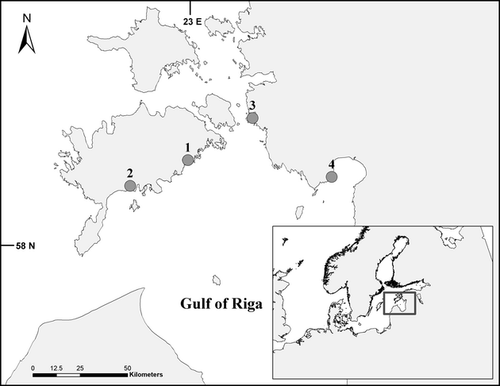
The study area is located in the Gulf of Riga, Southeastern Baltic Sea (Fig. 1) and represents a non-tidal, brackish basin. The study was conducted in the unstratified coastal zone in the northern part of the Gulf with a mean depth of 2 m. Current- and wind-induced water velocities are very low in the area compared with those in oceans, usually falling between 0 and 10 cm s−1 in the summer period (Kotta et al. 2008). Experimental sites had dissimilar hydrographic regimes; three study sites were depositional areas with silty sand and one site was erosional area with a gravel bottom.
Material and Methods
Two epibenthic suspension-feeding bivalves can be found in the Baltic Sea, both being common in the study area. The most widespread mussel species inhabiting the Baltic Sea was formerly known as Mytilus edulis L. and was later renamed Mytilus trossulus Gould. While the naming debate is not over yet, this species will be referred to as Mytilus trossulus in the present study. The bay mussel, M. trossulus, is the dominant suspension feeder on hard bottoms in the whole Baltic Sea, often forming dense banks in shallow coastal waters (Kautsky & Evans 1987). This species is of marine origin, and lower salinity limits both its performance and distribution within the Baltic Sea (Westerbom 2006). The zebra mussel, Dreissena polymorpha Pallas, is an invasive species from the Ponto-Caspian region that has been in the Baltic Sea for almost 200 years (Thienemann 1950). This species, although tolerant to salinity fluctuations, is confined to bays with salinity below 6 and is absent from the northern part of the basin. The species is primarily found in river estuaries, where it can build up remarkable biomasses (Järvekülg 1979; Olenin & Daunys 2005).
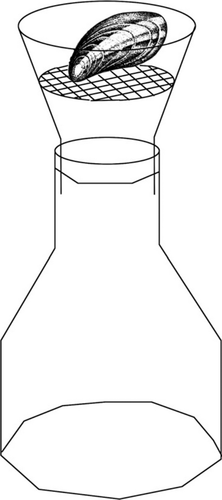
Field experiments were carried out on two dates within the warm season from May to September. The two bivalve species, M. trossulus and D. polymorpha, represented benthic suspension feeders. Mussels were allowed to adapt to the ambient conditions for 12 h before the start of the experiment. Mussels were located at 70 cm depth (30 cm above the sea bottom) at a total depth of 1 m. The height of the incubation unit from the bottom was comparable to the height mussels attached to boulders or macrovegetation commonly inhabited. Three mussels were placed in a net cage on the top of the funnel. Mussel biodeposits fell into the collecting vial below (Fig. 2; Kotta & Møhlenberg 2002). Five or six concurrent replicates were run at each site twice using different mussels. Each incubation lasted 4 h. The average shell length of mussels was 18.3 mm ± 2.5 SE for M. trossulus and 15.0 mm ± 2.3 SE for D. polymorpha, which corresponds to the mean size of mussels in the field. The taxonomic composition of diatoms in the water column and in mussel faeces was used to evaluate the contribution of planktonic food items in the diet of mussels.
At the beginning and end of each experiment, water samples were taken within 5 cm from the funnel containing the mussels to measure chlorophyll a (Chl-a) and planktonic diatom assemblages. The near-bottom temperature and salinity were monitored using CTD (conductivity, temperature, depth) profiling and current velocities with a JFE miniature electromagnetic current recorder. Salinity was measured on the practical salinity scale.
Water samples were immediately filtered through Whatman CF/F filters. The sedimented material in the vials was sorted under a dissecting microscope and faeces were collected with a pipette within 4 h of retrieval. Pseudo-faeces were not produced during the experiments. Filtered material was extracted in the dark with 96% ethanol overnight. Chl-a was quantified spectrophotometrically, correcting for phaeopigments (Pha-a) (Strickland & Parsons 1972). The values of Chl-a equivalent or total Chl-a (Chl-a eq) were calculated as Chl-a eq = Chl-a + 1.52 × Pha-a.
Water samples for diatom species analysis were fixed with acid Lugol's solution. A 50-ml aliquot of each sample was allowed to settle for 24–48 h in a combined chamber and cylinder, and cells were counted with inverted microscope (Olympus IMT-2), following the method of Utermöhl (1958). A magnification of 200× was used for larger cells and cells along one or two diagonals were counted, depending on the density of the sample. The abundance of smaller cells was estimated by examining 20 or 30 objective fields using 400× magnification. Faecal samples for diatom species analysis were fixed with 4% formaldehyde solution. However, formaldehyde was a relatively inconvenient fixative for our purpose, as formalin-fixed samples tended to form lumps; due to this, a few samples were spoiled and were therefore excluded from the analysis. The following procedures were based on the method described by Vilbaste et al. (2000). Samples were treated with HCl to remove carbonates (0.5 ml for a 5-ml sample) and washed carefully by centrifuging with distilled water (three times, 10 min, 1520.5 g). Subsequently, one sample volume of concentrated H2SO4 was added to every sample. Samples were heated at 150–200 °C until all organic substances were removed from the frustules. Samples were then cooled down and bleached by adding tiny amounts of KMnO4 until the solution was coloured light violet. Samples were then heated up again and, if the coloration disappeared, KMnO4 was added again. The process was repeated until the light violet coloration was found to persist. After that, a small amount of crystalline oxalic acid (H2C2O4) was added to the solution to decolour samples. Then, samples were washed with distilled water and centrifuged five times. Samples were allowed to settle in phytoplankton sedimentation chambers and counted, similar to water algal samples. For faecal samples, 400× times magnification was used for counting, and 1000× magnification was occasionally used for identification. On average, 45 objective fields were examined at 400× magnification per sample and, on average, 165 algal cells were counted in every faecal sample (min. 18 cells, max. 760 cells, n = 49 samples). Identification of diatoms was performed to the closest taxon possible – to genus, species or subspecies level – according to the following references: Snoeijs (1993), Snoeijs & Vilbaste (1994), Snoeijs & Potapova (1995), Snoeijs & Kasperoviciene (1996), and Snoeijs & Balashova (1998). Unidentified diatoms were excluded from further analyses. Diatoms were grouped as benthic or planktonic taxa according to Hällfors (2004). To detect any size-related preferences, we measured the lengths of the diatoms. Cell sizes were measured for all counted cells using an ocular scale and volumes were calculated from cell geometry (HELCOM 1988; Hillebrand et al. 1999) or using standard size-classes (Olenina et al. 2006). Wet weight biomasses were calculated for individual taxa and for the total biomass in all samples (http://helcom.fi/Documents/Actionareas/Monitoringandassessment/ManualsandGuidelines/ManualforMarineMonitoringintheCOMBINEProgrammeofHELCOM.pdf). Relative abundances or biomasses of diatoms were used in statistical analyses.
Multivariate data analyses on the relative abundances of diatom taxa were performed using the statistical package PRIMER version 6.1.5 (Clarke & Gorley 2006). Similarities between each pair of samples were calculated using a zero-adjusted Bray–Curtis coefficient. The coefficient is known to outperform most other similarity measures and enables samples containing no organisms at all to be included (Clarke et al. 2006). PERMANOVA (Anderson et al. 2008) was used to test for statistical differences in abundance distributions of taxa in diatom communities between water and faecal samples and among sites. Significant differences in the species composition and dominance structure of diatoms between faecal and water samples indicated the dissimilarity between mussel diet and pelagic microalgal communities. The biomass ratio of benthic algae in faeces was divided using the same ratio in the ambient plankton, further interpreted as an index of independence of mussels from the pelagic food pool. This index was further investigated using ANOVA analysis (StatSoft 2007). A global BEST match permutation test was run to examine the relationship between the biomass ratio of benthic diatoms in faeces and cell lengths of diatoms in ambient water.
Results
The mean values of water salinity, temperature, velocity and Chl-a content are presented in Table 1. A total of 86 diatom taxa was recorded in water and faecal samples, including subspecies. Among these, 26 taxa were present only in water and 55 taxa only in faeces. Based on the literature, 17 taxa were classified as planktonic and 69 as benthic. Diatom communities in the water consisted of both planktonic and benthic taxa and the community composition differed between sites (PERMANOVA pair-wise tests, P < 0.01) and between depositional and erosional areas (PERMANOVA, Tables 2 and 3).
| Site/time | T | S | Velocity | Chl-a |
|---|---|---|---|---|
| 1 (accumulation area) | ||||
| May | 14.7 | 3.8 | 0.0 ± 0.0 | 4 ± 1 |
| July | 25.6 | 4.5 | 6.7 ± 14.4 | 4 ± 1 |
| 2 (accumulation area) | ||||
| May | 13.8 | 2.0 | 2.7 ± 3.0 | 21 ± 5 |
| July | 22.7 | 1.5 | 4.3 ± 10.7 | 8 ± 0 |
| 3 (accumulation area) | ||||
| May | 14.5 | 2.5 | 2.9 ± 2.6 | 9 ± 1 |
| July | 27.2 | 4.9 | 1.0 ± 0.3 | 11 ± 6 |
| 4 (erosion area) | ||||
| May | 14.7 | 3.8 | 10.0 ± 26.3 | 18 ± 1 |
| July | 28.1 | 4.2 | 0.0 ± 0.0 | 21 ± 16 |
- T = temperature (°C); S = salinity (psu); Velocity = water current speed (cm s−1); Chl-a = water chlorophyll a (mg m−3), ‘±’ SE values.
| Factors and interactions | df | SS | Pseudo-F | P-level |
|---|---|---|---|---|
| Origin | 1 | 21,819 | 8.35 | 0.001 |
| Hydrographic regime | 1 | 13,335 | 5.1 | 0.001 |
| Origin × hydrographic regime | 1 | 15,484 | 5.92 | 0.001 |
| Total | 64 |
- SS = sum of squares.
| Pair-wise tests | P-level |
|---|---|
| Water versus faeces in accumulation areas | 0.001 |
| Water versus faeces in erosion area | 0.005 |
| Accumulation versus erosion area in water | 0.001 |
| Accumulation versus erosion area in faeces | 0.001 |
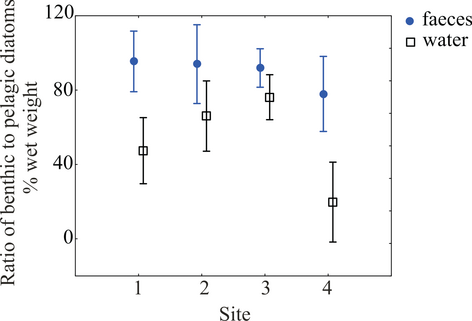
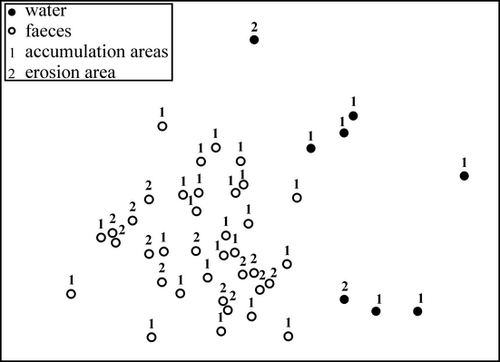
The biomass ratio of benthic to planktonic diatom taxa was always higher in mussel faeces than in ambient planktonic communities (ANOVA, F1,63 = 26.99, P < 0.001; Fig. 3). The ratio of this benthic diatom dominance in faeces to water (i.e. the index of independence of mussels from the pelagic food pool) varied between sites (ANOVA, F3,29 = 6.2, P = 0.002). The abundance distributions in diatom communities also distinguished between mussel faeces and ambient plankton (PERMANOVA, Table 2) and this effect was consistent over all studied locations despite sedimentation characteristics (PERMANOVA pair-wise tests for all sites, P < 0.005; Table 3, Fig. 4). The two most common taxa in water, Cylindrotheca and Leptocylindrus, were absent in faecal samples. On the other hand, most of the diatom species consumed by mussels were absent in water samples (Fig. 5).
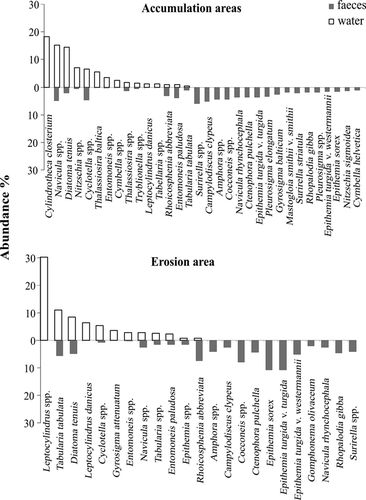
The biomass ratio of benthic algae in mussel faeces was related to site (ANOVA, F3,33 = 16.51, P < 0.001). The abundance dominance of diatoms ingested by mussels differed between all locations (PERMANOVA pair-wise tests, P < 0.02) and between depositional and erosional areas (PERMANOVA, Table 3). The feeding was not related to the bivalve species, as both Mytilus trossulus and Dreissena polymorpha fed on the same diatom species (PERMANOVA, P > 0.05).
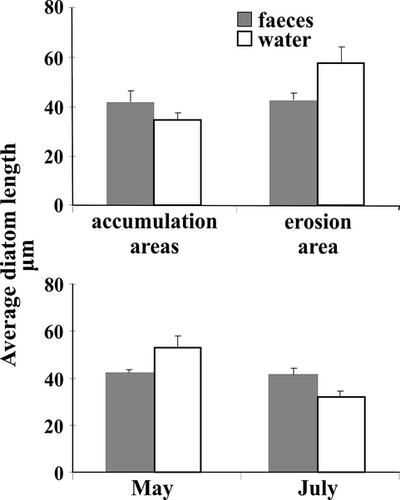
Cell lengths of diatoms were different in faeces and water (PERMANOVA, F1,64 = 25.41, P = 0.001). The average length of diatoms in water varied more than in faeces, indicating a possible preference towards certain size classes (Fig. 6). The share of benthic diatoms in faeces was not related to cell lengths of diatoms in water (BEST, P > 0.05).
Discussion
The main finding of the present study was that the diatom composition in the diet of mussels was significantly different from that in the water column. This indicates a relatively weak linkage between mussels and the pelagic food pool in the study area. Suspension feeding bivalves have been shown to be highly important in coupling pelagic and benthic processes by removing phytoplankton from the water column and depositing faeces on the bottom, which can result in strong effects on both pelagic and benthic foodwebs (Newell 2004). Documented impacts of recent invasions of zebra mussels have shown dramatic re-arrangements of energy pathways in a number of nontidal ecosystems, with losses of energy from pelagic and an increased input to benthic foodwebs (Higgins & Vander Zanden 2010). The impact of bivalve suspension feeders on pelagic foodwebs may occur via both consumptive and competitive pathways (Wong et al. 2003; Prins & Escaravage 2005). However, in some cases no impoverishment of pelagic foodwebs has been observed (Wu & Culver 1991; Noonburg et al. 2003). This has been assumed to be related to poor water exchange between near-bottom and upper water layers in slightly deeper limnic systems (Ackerman et al. 2001). Nevertheless, in a marine, open, sandy sublittoral, with a well-mixed water column, benthic suspension feeders still appeared to be uncoupled from the pelagic primary production (Sasaki et al. 2004). Impacts of suspension-feeding zebra mussels on pelagic ecosystems were found to be substantial across large gradients of ecosystem size and trophic status, and generally larger in shallow, nonstratified ecosystems (Higgins & Vander Zanden 2010). MacIsaac et al. (1999) suggested that zebra mussels strongly enhance benthic–pelagic coupling in shallow lakes. Similarly, our study was conducted in a shallow and nonstratified ecosystem, inhabited by widespread benthic suspension feeder communities. Still, no causal relationships between the biomass of benthic suspension feeders and the status of adjacent pelagic communities have been described in this ecosystem to date. On the contrary, as demonstrated here, benthic suspension feeders are not likely to exert a strong impact on pelagic foodwebs in the studied ecosystem.
Commonly, removal of energy from pelagic foodwebs has been coupled with energetic inputs to the benthic system. Benthic macroalgae have been shown to profit from the presence of benthic suspension feeders both in tidal areas (Pfister 2007; Aquilino et al. 2009) and in our study area (Kotta et al. 2009). However, as suggested by the results of the present study, this phenomenon may not necessarily indicate the removal of energy from the pelagic and input to the benthic system. In our study area, the enhancement of benthic vegetation by benthic suspension feeders may rather indicate a re-arrangement of energy within the benthic system. Such a concept might elucidate a potential, yet rarely considered, role of benthic suspension feeders in modifying competitive interactions between different phytobenthic groups, e.g. by favouring macrovegetation over microphytobenthos.
Studies in tidal systems have revealed a large variability in food sources of benthic suspension feeders occurring at various scales (Riera & Richard 1996; Yokoyama et al. 2005, 2009; Riera 2007; Dang et al. 2009). Local hydrographic conditions may influence the resuspension and lateral advection of sediment and, therefore, generate spatial variability in the food source of benthic suspension feeders. The extreme importance of hydrodynamic processes on benthic suspension feeders was demonstrated in two shallow micro- and non-tidal waterbodies of opposite hydrographic regimes (Sarà 2006). Resuspended sediments were present in the water column and constituted the main energetic pathway in a closed and completely wind-driven pond, but in the lagoon, which was open to substantial horizontal water movement, phytoplankton was the main suspended organic matter and also the main energetic pathway for benthic suspension feeders. Differences between locations based on sedimentation characteristics were much less extreme in the present study, and all locations were open to horizontal flow. Flow regime depended mainly on fetches and wind conditions, and three of our study locations fell in the range of accumulation areas, and only one location represented an erosion area. Accordingly, we cannot draw major conclusions about the generalizability of our results along the natural sedimentation gradient. Nevertheless, we would like to point out that the observed uncoupling between mussels and the pelagic food pool was consistent over all studied locations. Therefore, our results may hint that the main source of energy for mussels does not vary spatially within the range of variability in hydrographic characteristics recorded in the present study.
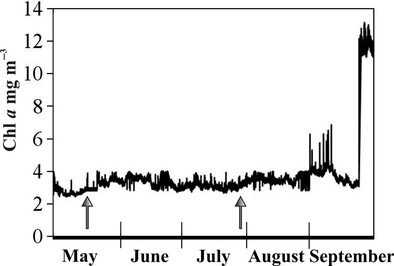
Temporal patterns in the availability of different food sources may also be of importance, as benthic suspension feeders in tidal systems have been found to use extensively pelagic food as a source during periods of lush pelagic food connected to seasonal phytoplankton blooms, especially the spring bloom, and to rely on other food sources during the rest of the year (Page & Lastra 2003; Lefebvre et al. 2009). Seasonal phytoplankton blooms in the present study area usually take place in early spring and late autumn when seawater temperatures are low at 2–7 °C. The feeding activity of studied mussels is low under 5–6 °C (Kotta et al. 2005). Summer blooms of cyanobacteria mainly form in the open areas of the Baltic Sea basin and are not found in the shallow coastal sea where the present study was conducted. The amount of phytoplankton in the water column is, therefore, relatively stable during the warm season in the study area (Fig. 7; Lauringson et al. 2009). Pervasive differences between ingested and water column diatom composition indicate that the extent of the reliance of mussels on the pelagic food source may have been unrelated to the recorded variability in water Chl-a content.
The abundance of certain benthic diatom species in the water column may be explained by their possible tychopelagic life mode in the study area. However, several benthic species were absent from water samples while still being abundant in mussel faeces. This leads to an intriguing question about the process that enabled mussels to feed on algae seemingly absent from the suspension. The questionable food source mainly comprised pennate benthic diatom species, which could have been resuspended by wind–wave action. Wind–waves reaching the bottom cause shear stress that peels off the top layer of the sediment together with the highly nutritious microalgal cover (Miller et al. 1996). Thereby, the shallow-water benthos is characterized by a thin near-bottom water layer with very high turbidity (Muschenheim 1987). This near-bottom ‘layer’ probably represents a highly dynamic system, where the wave action induces periodic resuspension events followed by resedimentation events. The thickness of the layer can possibly also display high spatial variability caused by bottom roughness, where any kind of obstructions like boulders can cause turbulent events and modify the strength of wave-induced stress to the bottom. Such variability in turbulence and in microalgal resuspension conditions has a direct effect on benthic suspension feeders. The amount of particles in water has been shown to decrease rapidly with increasing distance from the bottom, especially heavy particles such as sand grains and big algal cells (Muschenheim 1987). Microalgal particles have also been shown to settle extremely rapidly after resuspension events if the flow is switched down (Lucas et al. 2000, 2001), which may happen in a similar manner between wind-induced pulses of flow. Mussels have been shown to use up to a 7-cm-thick bottom water layer for feeding (Muschenheim & Newell 1992); however, mussels located on boulders, plants and other higher physical structures can feed at much higher positions and can reach upper water layers. Mussels in the present study were located at a height of 30 cm from the bottom. Thereby, the supply of particle-rich near-bottom water layer may have been facilitated by turbulent forces around experimental units similar to the forces around boulders and other protruding structures on the sea floor. Periodic resuspension events may have been occurred at a regular basis as a result of wind–waves. Bivalves may be expected to have higher consumption rates at the pulses of resuspended sediment. These differences may have been further accentuated by the selective capacities of bivalves (Ward & Shumway 2004). For unknown reasons, certain taxa commonly occurring in the water column were completely absent in the diet of mussels in the present study, despite being within the size range of edible particles. The most important of these taxa, Leptocylindrus spp. and Cylindrotheca closterium (Ehrenberg) Reimann & Lewin, have a similar morphology with high length-to-width ratio (common dimensions of cells were 5–10 × 32–84 μm for Leptocylindrus and 1.5–5 × 32–105 μm for C. closterium in our samples). Suspension-feeding bivalves have been shown to select particles based on size (Ten Winkel & Davids 1982; Ward & Shumway 2004) and epicellular chemical substances may also be important selection cues (Ward & Targett 1989; Beninger & Decottignies 2005; Espinosa et al. 2010). Cells of Cylindrotheca spp. have been found to be selectively rejected by cockles fed on a mixed diet of diatoms (Rueda & Smaal 2002), and Rouillon et al. (2005) found Leptocylindrus danicus Cleve and C. closterium to be absent in stomach contents of blue mussels at two contrasting locations, while both species were important planktonic taxa in these habitats. Nonetheless, behind the apparent absence may have also stood a possible independence of mussels from the pelagic food pool and a use of another food source, instead of selective rejection. Indeed, our results may not necessarily suggest selective feeding. Any conclusions related to the selectivity of feeding by mussels in the present context would presume detailed information about the composition of all available sources of food and perhaps further experimental manipulations. To clarify the mechanism of feeding of mussels on items not represented in our water samples, measurements of temporal variability in near-bottom water Chl-a content, with a resolution similar to the scale of wave frequency, might be beneficial.
In wind-driven systems, predominant resuspension events follow much finer temporal scale compared with macrotidal estuaries. The significance of wind–wave-generated resuspension for benthic suspension feeders may be masked by the resuspension or flushing due to tidal forces in tidal systems, and is perhaps more easily detectable in nontidal systems. Wind-driven resuspension processes of microphytobenthos may also be significant in macrotidal systems (Demers et al. 1987; De Jonge & van Beusekom 1995) and it is possible that such wind-driven processes may contribute to the variability in food sources of benthic suspension feeders in tidal systems as well.
The current study was carried out in an area with a monotonously flat topography where bottom slopes rarely exceed a few degrees at scales from tens to hundreds of metres. Bottom slope may strongly impact the hydrodynamic conditions of the location, including water exchange in the near-bottom water layer (Slinn & Riley 1996). Due to its flat topography, the study area is presumed to have a relatively weak supply of fresh phytoplankton from the open sea to near-coastal areas that can be reached by benthic suspension feeders. The consequent temporal scarcity of lush planktonic food may, in turn, decrease the reliance of benthic suspension feeders on the pelagic food source. Observed feeding habits probably reflect the utilization of the richest food source available. To test the generalizability of our finding and the possible variability in food sources of benthic suspension feeders within the studied ecosystem, it seems essential to cover larger gradients in topographic conditions, including steep bottom slopes inhabited by extensive mussel beds in other parts of the Baltic Sea.
Conclusions
Feeding strategies of benthic suspension feeders in non-tidal systems are generally poorly investigated. It still remains to be shown how benthic suspension feeders exploit various local food pools available in such environments. We found that benthic suspension feeders were poorly utilizing the pelagic food pool in a flat shallow non-stratified, non-tidal coastal area. The diets of zebra and blue mussels were quite similar and clearly distinguished from diatom communities in the water column in both May and July. This effect was consistent within the four studied locations with different hydrographic regimes. Most diatom taxa consumed by mussels tended to be absent in the water samples taken, indicating an unknown mechanism for food supply, perhaps in short pulses related to wind–wave action. Our results suggest a need to re-evaluate the role of benthic suspension feeders as consumers of phytoplankton in shallow nontidal ecosystems.
Acknowledgements
Funding for this research was provided by institutional research funding IUT02-20 of the Estonian Research Council and by the Estonian Science Foundation grants 7813, 8807 and 8254. The study has been partly supported by the project ‘The status of marine biodiversity and its potential futures in the Estonian coastal sea' no. 3.2.0801.11-0029 of the Environmental protection and technology programme of the European Regional Fund.




