Thermal sensitivity of native and invasive seabreams
Abstract
This study compared the mortality and metabolic response of the Senegal seabream, Diplodus bellottii, an African species recently reported in Southern Europe and the white seabream Diplodus sargus, a native species, across a range of temperatures. The temperatures tested were 18, 26, 28 and 30 °C. Mortality was zero at 18 °C and very low at all other temperatures for both species, with the exception of D. bellottii, which experienced 32% mortality at 30 °C. Metabolic rates increased steadily with increasing temperatures, with a steep increase at 30 °C for D. bellottii. Thermal sensitivity ranged between 2 and 3 for both species and for all thermal intervals, with the exception of the thermal sensitivity between 28 and 30 °C for D. bellottii, which was 7. It was concluded that D. bellottii is under thermal stress at 30 °C. Diplodus bellottii may have expanded its distribution northwards due to an increase in sea surface temperatures. However, further warming may result in habitat loss for the juveniles, since Southern European estuarine systems will reach temperatures that may lead to lower fitness in juveniles of this species.
Introduction
Recent changes in climatic conditions have resulted in altered geographic ranges for several species in ecosystems around the world (Walther et al. 2002, 2009; Parmesan 2006). Theoretical studies have concluded that most of the important elements of climate change, such as environmental warming and altered precipitation patterns, are likely to increase the prevalence and colonization success of biological invaders (Dukes & Mooney 1999). However, these general predictions should be directly tested. Various authors have done that for different taxa; for instance, D'António (1992) showed how invasions by exotic grasses were favoured by global change, and Harris & Tyrrell (2001) reached a similar conclusion for various species of coastal algae, Stachowicz et al. (2002) for ascidians and Broennimann et al. (2007) for Spotted Knapweed.
The Senegal seabream, Diplodus bellottii (Steindachner 1882), is an African sparid fish that occurs along the Northwest African coast. It has been reported as a native species from Senegal to Cabo Blanco (Mauritania), at 15°–20° N (Steindachner 1882; Metzelaar 1919; Fowler 1936; Cadenat 1964; Blache et al. 1970), and has also been reported from the Saharian fishing grounds, 23°–26° N, by Rucabado & LLoris (1977). The absence of records for the Moroccan coast is possibly due to a lack of monitoring of these waters. Recently, this species has been recorded at increasingly higher latitudes along European coastal zones including Southern Spain in the 1980s, at 36° N (Rodríguez & Rodríguez 1984; Reina-Hervás & Serrano 1987), Southern Portugal in 1990, at 37° N (Santos et al. 1998), the Tagus estuary (Portugal), at 38° N, in 1995 (Cabral et al. 2001) and as far north as the Ria de Aveiro, at 40° N, in 2005 (Vinagre et al. 2010).
This species has thus achieved a remarkable expansion of its geographic range, from subtropical latitudes of 20° N to temperate latitudes of 40° N (Fig. 1). According to Walther et al. (2009) the Senegal seabream can be classified as an invasive species, having rapidly increased its range.
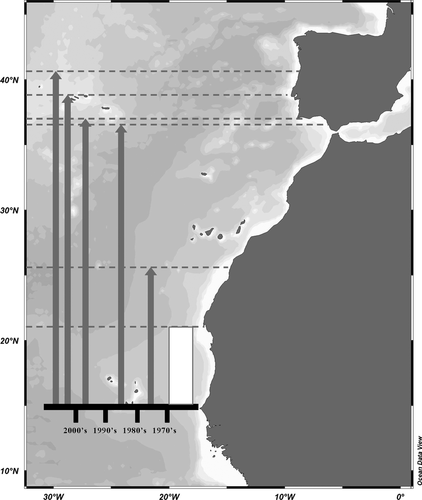
Diplodus bellottii juveniles use estuaries as nursery areas and are now the most abundant Diplodus species in estuaries at 38° N, both in the Tagus and the Sado estuaries, the two largest estuaries in Portugal and among the largest in Europe (Vinagre et al. 2010). The high abundance of this species generated concerns regarding its potential impacts and, in particular, the possibility of competition with other Diplodus species that have high commercial value (Cabral et al. 2001; Horta et al. 2004; Vinagre et al. 2010). One Diplodus species deemed to be at a potential competitive disadvantage is the white seabream, Diplodus sargus (Linnaeus 1758). This species is now the second most abundant Diplodus species in estuaries at 38° N. Its diet overlaps with that of D. bellottii, juveniles use the same estuarine and coastal nurseries, and adults occur in the same coastal areas (Santos et al. 1998; Vinagre et al. 2010).
The rapid northern shift of the range of D. bellottii has been attributed to climate warming (Vinagre et al. 2009). Consequently, it is thought that further warming may favour this species over native Diplodus species (Horta et al. 2004; Vinagre et al. 2010).
This study aims to compare the responses of these species' juveniles to elevated temperatures likely to occur in their estuarine nurseries in the future due to climate warming. Mortality and metabolic rates were investigated in juveniles of the invasive D. bellottii and the native D. sargus at 18, 26, 28 and 30° C.
Material and Methods
Fish (3–8 g) were collected by beach seine in the Tagus estuary in September 2009, and kept in four indoor re-circulating water systems, each comprising five replicate 70-l tanks, supplied with aerated seawater, with a salinity of 35. The number of individuals of each species per tank was five for Diplodus sargus (total n = 100, 25 individuals per system) and four for Diplodus bellottii (total n = 80, 20 individuals per system). Seabream were allowed to acclimate for 3 weeks at 24° C, the water temperature at the field site when the fish were collected. Fish were fed twice a day with shrimps ad libitum, but were starved for 24 h before the respirometry experiments. After 3 weeks, temperature was altered at a rate of 1° C per hour until reaching experimental temperatures of 18, 26, 28 and 30° C. Each of the four indoor re-circulating water systems had a different temperature with five replicate tanks. These temperatures were chosen to reflect: (i) the mean western Atlantic coastal water temperature (38° N) in summer (18° C), because juveniles of both species grow in coastal nurseries in summer, (ii) the mean summer estuarine water temperature 24° C plus 2° C = 26° C (Centro de Oceanografia Database), representing future postulated climate warming in 2100 (Miranda et al. 2002), because juveniles of both species also grow in estuarine nurseries in summer, and (iii) the water temperature registered during summer heat waves in the Tagus estuary nurseries (28° C) and the water temperature during probable heat-waves in the future, 28° C plus 2° C = 30° C, as heat waves are predicted to be more intense, more frequent and to last longer (Miranda et al. 2002).
Oxygen saturation in the water varied between 95 and 100%. Fish were kept at the experimental temperatures for 1 month and were checked daily to monitor mortality. After this period, closed respirometry was carried out on individual fish of each species. Each individual was allowed to deplete the available oxygen in a sealed respirometry chamber of 350 ml (Loligo systems, Tjele, Denmark), filled with filtered seawater containing 50 mg·l−1 streptomycin (Rosa & Seibel 2008). The O2 levels were recorded continuously with a Clarke-type O2 electrode connected to a Strathkelvin Instruments 929 Oxygen Interface (Rosa & Seibel 2008). The chamber was submerged in a flow-through container coupled with a water bath (Lauda Ecoline) that kept experimental temperature stable. Control runs were carried out to quantify background respiration. Before each experiment fish were allowed to acclimate to the chamber (which was not sealed during acclimation) for 2 h. Fish exhibited calm behaviour after a few minutes in the chamber. Experiments were terminated when the fish showed the first signs of equilibrium loss. All experiments were carried out in shaded day light (09L:15D).
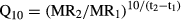
These experiments were designed so that lethal endpoints were not reached and no fish were killed on purpose. Fish were expected to survive the 30 days and the respirometry experience (which they did). These experimental temperatures had previously been used with other temperate and subtropical fishes with minimal effects on survival (Vinagre et al. 2012a,b,c). Based on their distribution ranges and the habitats where they live, our initial expectation was that seabreams would be among the more heat-resistant species at temperate latitudes. Also, other individuals of these species had been previously maintained at our facilities for periods of months and observed for their general health, lack of disease and ~100% survival at control temperatures. This previous experience meant to assure that adverse effects were minimized and that these species could be maintained in captivity without problems, such as the onset of diseases or captivity stress. We believe that incidental deaths occurring during the experiment were due solely to temperature and that all other stress effects that could contribute to the death of these fish were minimized.
One-way ANOVAs as used to test for the effect of temperature on RMR, followed by Tukey post-hoc tests, whenever the null hypothesis was rejected. ANOVA assumptions were tested prior to the analysis.
Results
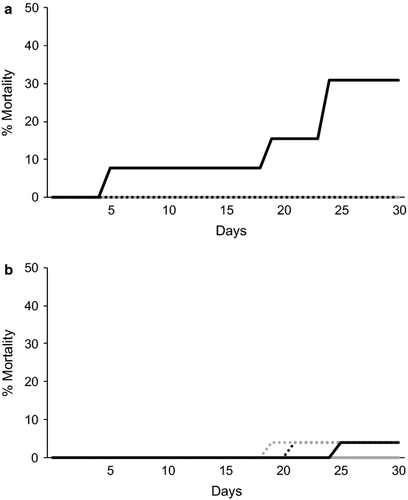
Mortality was zero for both species at 18° C. One Diplodus sargus died after 21 days at 26° C, one died after 19 days at 28° C, and another after 25 days at 30° C (Fig. 2a). For Diplodus bellottii, mortality was zero at 18, 26 and 28° C. However, mortality was 32% at 30° C (Fig. 2b). The fish that died did not show any signs of disease, they responded to food and were not lethargic. We believe that mortality was due solely to the prolonged higher metabolic demands experienced by the fish at the higher temperatures.
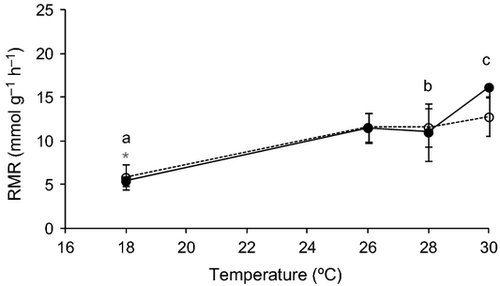
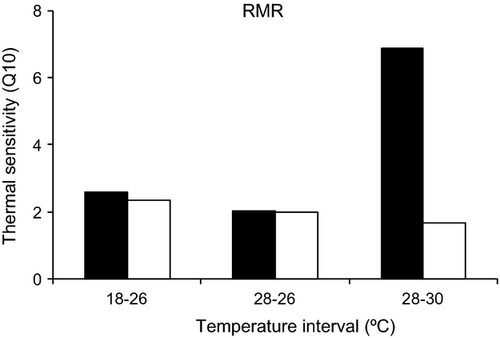
Temperature effects on metabolism (RMR) were significant for both species (Table 1). However, in D. bellotti the temperature effect was more dramatic, with a steep increase at 30° C (Fig. 3). Post-hoc tests showed that whereas for D. bellottii metabolism was significantly different between 18 and 28° C (P < 0.05) and 18 and 30° C (P < 0.05), for D. sargus metabolism was significantly different between 18° C and all other temperatures (P < 0.05). Thermal sensitivity ranged between 2 and 3 for both species and for all thermal intervals, with the exception of the thermal sensitivity between 28 and 30° C for D. bellottii, which was 7 (Fig. 4).
| Diplodus bellottii | Diplodus sargus | |||||||
|---|---|---|---|---|---|---|---|---|
| df | MS | F | P | df | MS | F | P | |
| Routine metabolic rate | 3 | 40.86 | 6.30 | <0.05 | 3 | 35.48 | 14.62 | <0.05 |
- Significant values in bold.
Discussion
A general trend of higher vulnerability of Diplodus bellottii to elevated temperatures was observed. The drastic increase in mortality at 30° C demonstrates that D. bellottii is unable to acclimate to this temperature, unlike Diplodus sargus, which showed very low levels of mortality at all temperatures. Diplodus bellottii experienced a sharp increase in metabolism at 30° C in contrast to D. sargus. Thermal sensitivity was also much higher for D. bellottii at 30° C than at other temperatures, reaching a value of 6, whereas D. sargus maintained a low level of thermal sensitivity, between 2 and 3, at all experimental temperatures. Thermal sensitivity values between 2 and 3 are usual, with higher values being seen in fishes exposed to temperatures outside their normal temperature range (Johnston et al. 1991). The thermal sensitivity values estimated for D. sargus confirm that this species is much more tolerant to 30° C than is D. bellottii, which is clearly not adapted to such high temperatures.
The mean water temperature in the Tagus estuary is c. 24° C, in summer, when juveniles of D. bellottii and D. sargus occupy the same estuarine nursery grounds; however, during heat waves, water temperature has been observed to reach 28° C for more than 1 week. Climate warming projections state that heat waves will be more intense in the future. This means that D. bellottii juveniles could be faced with temperatures very close 30° C, which we have shown to be stressful for these juveniles.
Results of this study raise the interesting question of why the African fish D. bellottii is more sensitive to high temperatures than a native European con-generic. Diplodus bellottii is a native of a subtropical area that is affected by the Western African upwelling zone; this means that although it lives in warmer waters than those found in Southern European coasts, temperatures are lower than at similar latitudes in the tropics that do not have upwelling. The recent increase in geographic range by D. bellottii corresponds to a move from an upwelling system in the tropics to another upwelling system in a temperate/subtropical zone. Temperatures in the Portuguese upwelling system are colder (15–18° C mean temperature) than in the Western African upwelling system (20–25° C mean temperature) (Levitus & Boyer 1994). It is possible that weakening of the Portuguese upwelling system (Lemos & Pires 2004) and a consequent slight temperature increase made it possible for D. bellotti to colonize this zone. As shown by our results, coastal temperatures in the Portuguese coast are well below this species' upper thermal limit and even a 2° C increase due to climate change is not likely to affect the juveniles that occupy coastal areas. Yet, juveniles of D. bellottii that use the Portuguese estuaries will clearly face stressful temperatures in the future associated with climate change.
A second important question is why the European native species D. sargus is so tolerant to high temperatures? D. sargus has a broad geographic distribution, from 48° N to 36° S, which includes tropical and equatorial waters with no upwelling. Diplodus sargus populations native to Portugal maintain tolerance to high temperatures, possibly due to a positive selection pressure on juveniles using estuarine nursery grounds.
It can thus be concluded that juveniles of both Diplodus species will be well within their thermal tolerance envelopes in future climate change scenarios, if growing in coastal nurseries. Juveniles of D. sargus will also face no thermal stress in estuarine nurseries, but juveniles of D. bellottii will face stressful temperatures that may threaten their growth and survival within estuarine nurseries.
Acknowledgements
Authors would like to thank everyone involved in the field work and maintenance of the experimental tanks. This study had the support of the Portuguese Fundação para a Ciência e a Tecnologia through the grant SFRH/BPD/34934/2007 awarded to C. Vinagre and the strategic project PEst-OE/MAR/UI0199/2011.




