Shallow-water benthic hydroids from the Maltese Islands (Central Mediterranean)
Abstract
Forty species of benthic hydroids, belonging to 15 families and 21 genera, were found in a collection, obtained by SCUBA diving, from the Maltese Islands. Paradoxically for the well studied Mediterranean region, the hydroid fauna of these islands is virtually unexplored. Of 40 species, 33 were identified to species level, with 28 of them reported for the first time for the study area. Hydractinia monocarpa is recorded for the first time in the Mediterranean Sea, and a presumed new species of the genus Clytia has been found. The most speciose families are Campanulariidae with 10 species (25%), Aglaopheniidae with six species (15%) and Sertulariidae with five species (12%). Aglaophenia and Clytia are the most diverse genera, both with six species (15%). A problem with respect to subspecies of Sertularella ellisi is resolved based on nematocyst observations. In terms of geographic distribution, eight species (24%) are endemic to the Mediterranean Sea, this number increases to 16 species (48%) when including those restricted to Mediterranean and nearby Atlantic waters, whereas the remaining species have wider distributions.
Introduction
The Mediterranean Sea is one of the most studied areas of the world. Often known as the cradle of great civilizations, its waters, flora and fauna have been extensively documented; Hydrozoans are not an exception; throughout the last century, many specialists have studied this group, especially in the vicinity of the main oceanographic stations of the western basin and the Adriatic Sea. This has yielded a large amount of data on the taxonomy and ecology of the Mediterranean Hydrozoa (cf. Bouillon et al. 2004). However, there remain areas where this group is not well sampled, specifically the African coasts and the eastern basin (cf. Morri et al. 1999).
The Maltese archipelago is, in this sense, an undersampled area. Located in the eastern basin of the Mediterranean Sea, the Maltese islands are influenced by flows of Modified Atlantic Water that mixes here with Levantine Intermediate Water (Astraldi et al. 1999; Lermusiaux & Robinson 2001). With the exception of some species of Aglaophenia reported by Svoboda & Cornelius (2007) and a hydromedusa by Evans (1968), most studies of the Maltese fauna have dealt with other marine taxa, such as molluscs (Cachia et al. 1991, 1996, 2001, 2004), thoracican barnacles (Rizzo & Schembri 2009), decapod crustaceans (Schembri & Lanfranco 1959), echinoderms (Tanti & Schembri 1923) and fishes (Lanfranco 1993). This paper aims to improve the knowledge of benthic hydroids from the Maltese islands.
Material and Methods
Sampling took place in April 1992, April and December 1993, and between July 1996 and September 1997. Hydroids were sampled with SCUBA from 12 stations, three from Gozo and nine from Malta (Fig. 1) at depths up to 30 m. Live specimens were anesthetized using a 1:1 mixture of seawater and magnesium chloride and cooled in a refrigerator for 12 h before transferring them to Bouin's fluid. Hydrozoans were finally transferred to 70% ethanol after a short period of washing in water/alcohol.
Inasmuch as the ecology and distribution of most hydroid species collected as part of this study are well known and have recently been reviewed (Peña Cantero & García Carrascosa 2002; Bouillon et al. 2004), we will provide only brief accounts on the autoecology (e.g. bathymetric range, reproductive phenology and primary substrates in Mediterranean waters) and distribution (worldwide and Mediterranean scales) for each species; these are mainly based on our own data and on those of Peña Cantero & García Carrascosa (2002), and only when the information comes from a different source will be indicated in the text. New data and other matters are discussed in each species.
In the general biogeographic discussion, the zoogeographical patterns established by Boero & Bouillon (1993) will be employed, except for the category ‘circumtropical’, which is here treated as circumglobal (present in temperate, subtropical and tropical waters of all oceans).
List of samples
- S-2, Qajjenza (Southeastern Malta), 01.iv.1993, 0–1 m, photophilic algae community: Ventromma halecioides (Alder, 1859).
- S-2, Qajjenza (Southeastern Malta), 08.xii.1993, 0-1 m, photophilic algae community: Coryne pusilla (Gaertner, 1774).
- S-4, Delimara area (Southeastern Malta), 22.xi.1996, 0–1 m, photophilic algae community: Halecium sp., Halecium pusillum (M. Sars, 1856), Aglaophenia octodonta (Heller, 1868), Dynamena disticha (Bosc, 1802), Clytia gracilis (M. Sars, 1850), Clytia noliformis (McCrady, 1859), Clytia paulensis (Vanhöffen, 1910), Clytia sp.
- S-4, Delimara area (Southeastern Malta), 20.vii.1997, 16 m, sciaphilic assemblages: Egmundella amirantensis Millard & Bouillon, 1973, Dynamena disticha (Bosc, 1802), Clytia sp.
- S-13, Mercanti reef (Northeastern Malta), 24.xi.1996, 3–7 m, seagrass Posidonia oceanica community: Aglaophenia picardi Svoboda, 1979, Kirchenpaueria pinnata (Linnaeus, 1758), Monotheca obliqua (Johnston, 1847), Sertularella ornata (Broch, 1933), Sertularia perpusilla Stechow, 1919, Clytia hemisphaerica (Linnaeus, 1767), Clytia linearis (Thornely, 1900), Clytia paulensis (Vanhöffen, 1910), Laomedea sp., Orthopyxis asymmetrica Stechow, 1919.
- S-17, Qalet Marku (North Malta), 13.xii.1996, 3 m, seagrass Cymodocea nodosa community, Hydractinia sp., Laomedea sp.
- S-20, Qawra (North Malta), 22.xii.1996, 6–21 m, seagrass Posidonia oceanica community: Eudendrium sp.1, Aglaophenia harpago von Schenck, 1965, Aglaophenia kirchenpaueri (Heller, 1868), Halopteris liechtensternii (Marktanner-Turneretscher, 1890); Synthecium evansi (Ellis & Solander, 1786), Obelia sp.
- S-27, Cirkewwa (Northwestern Malta), 17.ix.1997, 6-28 m, wreck: Turritopsis dohrnii (Weismann, 1883), Eudendrium sp. 2, Egmundella amirantensis Millard & Bouillon, 1973, Halecium tenellum Hincks, 1861, Clytia linearis (Thornely, 1900), Clytia sp.
- S-30, Migra l-ferha (West Malta), 26.iv.1992, 10 m, photophilic algae community: Aglaophenia octodonta (Heller, 1868).
- S-31, Ghar Lapsi (South Malta), 15.viii.1997, 8–10 m, photophilic algae community: Halecium sp., Aglaophenia kirchenpaueri (Heller, 1868), Aglaophenia tubiformis Marktanner-Turneretscher, 1890, Halopteris liechtensternii (Marktanner-Turneretscher, 1890), Sertularia distans Lamouroux, 1816, Orthopyxis crenata (Hartlaub, 1901).
- S-33, Wied iz Zurrieq (South Malta), 13.vii.1997, 0-1 m, photophilic algae community: Coryne muscoides (Linnaeus, 1761), Halecium sp., Aglaophenia kirchenpaueri (Heller, 1868), Aglaophenia octodonta (Heller, 1868).
- S-33, Wied iz Zurrieq (South Malta), 13.vii.1997, 11 m, sciaphilic assemblages: Dynamena disticha (Bosc, 1802).
- S-33, Wied iz Zurrieq (South Malta), 11.viii.1997, 11 m, sciaphilic assemblages: Eudendrium armatum Tichomiroff, 1890.
- S-33, Wied iz Zurrieq (South Malta), 21.viii.1997, 5 m, sciaphilic assemblages: Clytia linearis (Thornely, 1900), Clytia sp.
- S-43, Reqqa Point (North Gozo), 29.vii.1997, 11-22 m, precoralligenous community: Hydractinia monocarpa Allman, 1847, Filellum sp., Halecium sp., Dynamena disticha (Bosc, 1802), Sertularia distans Lamouroux, 1816, Clytia linearis (Thornely, 1900), Orthopyxis crenata (Hartlaub, 1901).
- S-46, Dwejra (Western Gozo), 27.vii.1996, 10 m, precoralligenous community: Aglaophenia kirchenpaueri (Heller, 1868).
- S-46, Dwejra (Western Gozo), 27.vii.1997, 3 m, photophilic algae community: Dynamena disticha (Bosc, 1802), Clytia linearis (Thornely, 1900).
- S-46, Dwejra (Western Gozo), 28.vii.1997, 3–16 m, photophilic algae community: Coryne muscoides (Linnaeus, 1761), Halopteris liechtensternii (Marktanner-Turneretscher, 1890), Sertularella ornata (Broch, 1933), Laomedea sp.
- S-46, Dwejra (Western Gozo), 31.vii.1997, 18 m, precoralligenous community: Scandia gigas (Pieper, 1884), Halecium sp., Sertularella ellisii (Deshayes & Milne Edwards, 1836), Sertularia distans Lamouroux, 1816.
- S-49, Xatt l-ahmar (South Gozo), 27.vii.1997, 26 m, sciaphilic assemblages: Aglaophenia elongata Meneghini, 1845, Clytia hemisphaerica (Linnaeus, 1767).
Results
Family Oceaniidae Eschscholtz, 1829
Genus Turritopsis McCrady, 1857
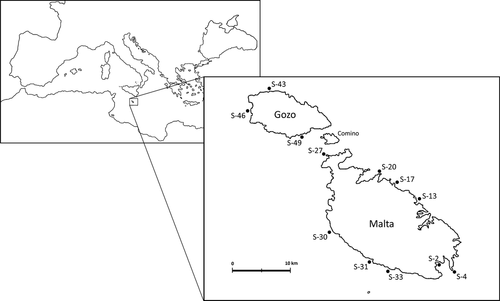
Turritopsis dohrnii (Weismann, 1883)
(Fig. 7)
Material examined: H46 (S27), one stem, c. 15 mm high, on a sunken wooden boat.
Ecology: The bathymetric range of Turritopsis dohrnii remains unknown because it has been widely misidentified as Turritopsis nutricula in Mediterranean waters. Schuchert (1984) found it at a depth of 2–4 m and Puce et al. (2009) at 0–20 m; our material was collected at 27–28 m. It has been reported (as T. nutricula) epibiotic on the echinoid Dorocidaris papillata (cf. Motz-Kossowska 1986), the gorgonian Isidella elongata (cf. Stechow 2008; Riedl 1959) and tubes of Serpulida (Balduzzi et al. 1980); the present material was found on wood of a wreck. In the Mediterranean, fertile colonies were found in July–August (Boero & Fresi 1986 as T. nutricula) and May (Puce et al. 2009).
Distribution: Unlike the cosmopolitan species T. nutricula, Schuchert (1984) considered Turritopsis dohrnii a Mediterranean endemic. Miglietta et al. (2006) demonstrated that T. nutricula and T. dohrnii are distinct valid species. Given the peculiarity of its life cycle (Piraino et al., 1996), it was also demonstrated that T. dohrnii possesses a high invasive potential, explaining its worldwide distribution from Japan to Pacific and Atlantic coasts of Panama (Miglietta & Lessios, 2009). In the mediterranean, it has been reported from the Adriatic Sea and the Ionian Sea (Miglietta et al. 2000 as T. nutricula), the Balearic Islands (Schuchert 1984), the Gulf of Naples (Piraino et al. 1996 as T. nutricula) and the Ligurian Sea (Puce et al. 2009).
Family Eudendriidae L. Agassiz, 1862
Genus Eudendrium Ehrenberg, 1834
Eudendrium armatum Tichomiroff, 1890
(Fig. 2G-I)
Material examined: H37 (S33), one colony, c. 60 mm high, on hard substrate.
Ecology: Eudendrium armatum has been reported at depths from the intertidal to 50 m (Boero & Fresi 1986); the present material was collected at 11 m. It has been reported growing on bio-concretions (Boero & Fresi 1986) and massive sponges (Morri & Bianchi 1999), as well as epilithic (Marques et al. 2000); our material was found on the ceiling of a cave with strong currents. According to this and its greater affinity for caves (Marques et al. 2000), it seems to be a rheophilic and sciaphilic species. In the Mediterranean, fertile colonies were found in February–March and July–October (Marques et al. 2000).
Distribution: Endemic to the Mediterranean Sea, where it has been reported from the eastern and western basins (Bouillon et al. 2004).
Eudendrium sp.1
Material examined: H16 (S20), one stem, c. 25 mm high, on hard substrate.
Remarks: The scarcity of material and the absence of gonophores prevent us from identifying this species properly.
Ecology: Eudendrium sp.1 was found at a depth of 6 m.
Eudendrium sp.2
Material examined: H20 (S27), two stems, up to 20 mm, on metal part of sunken boat.
Remarks: We keep Eudendrium sp.2 separated from the previous one by differences observed in the cnidome. This species resembles Eudendrium capillare or Eudendrium armatum in having a single group of microbasic euryteles (8 × 3 μm in the present material).
Ecology: Eudendrium sp.2 was collected at 28 m depth.
Family Hydractiniidae L. Agassiz, 1862
Genus Hydractinia van Beneden, 1843
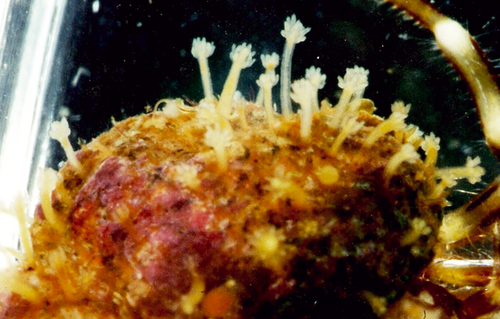
Hydractinia monocarpa Allman, 1847
(Figs 2A–D and 8)
Material examined: H31 (S43), one fertile colony, on gastropod shell occupied by a hermit crab.
Description: Colony growing on gastropod shell. Encrusting hydrorhiza covered by coenosarc; with slender spines covered by tissue except distal part. Colony polymorphic. Gastrozooids up to 1 mm high, with 12–14 tentacles placed in two close whorls, resembling a single one, hypostome dome-shaped. Gonozooids without tentacles when fully developed, sometimes appearing as stumps on gonozooids with non-fully formed gonophores. Usually with two opposite gonophores, one much larger, sometimes with a large single one. Gonophores as sessile sporosacs without radial canals, with about 30–40 eggs.
Remarks: In some cases, Hydractinia aculeata may lack tentacles on its gonozooids (P. Schuchert, pers. comm.), although usually gonophores develop from fully formed polyps that remain otherwise unchanged. Despite this fact, we regard our material as Hydractinia monocarpa on the basis of its close resemblance to the description given by Schuchert (2001), as well as the nematocyst size: Hydractinia aculeata has larger heteronemes (15 × 5 μm) than the present material (8.4 × 3.2 μm).
Ecology: Only found in Mediterranean waters at a depth of 21–22 m (present study). Hydractinia monocarpa is usually found on shells of the gastropod Boreotrophon clathratus (cf. Allman 1876), but it is also reported on the gastropod Bela sp. (Jäderholm 1909) and on sertularian hydroids (Calder 1972); our material was growing on gastropod shell occupied by a hermit crab. Our fertile material was collected in July.
Distribution: Hitherto, Hydractinia monocarpa was considered to have an Arctic distribution (Schuchert 2001). It has been reported from Spitsbergen and Canada (Calder 1972) and the Japanese coasts (Namikawa 1994). This is the first record for the Mediterranean Sea and consequently its distribution can be considered boreal.
Hydractinia sp
(Fig. 2E, F)
Material examined: H12 (S17), one colony, on gastropod shell.
Remarks: The scarcity of material and the lack of gonophores prevent us from identifying this material to species level.
Ecology: Hydractinia sp. at a depth of 3 m.
Family Corynidae Johnston, 1836
Genus Coryne Gaertner, 1774
Coryne muscoides (Linnaeus, 1761)
(Fig. 2J–K)
Material examined: H21 (S33), one fertile stem, c. 15 mm high, on Cystoseira sp.; H28 (S46), some fertile stems, up to 40 mm high, on hard substrate.
Ecology: Coryne muscoides has been reported in the Mediterranean from the intertidal to 20 m depth (Puce et al. 2009); our material comes from zero to 5 m. It has usually been found growing epibiotic on a great range of substrates such as algae, Posidonia oceanica and invertebrates, as well as epilithic; the present specimens were found on Cystoseira sp. and epilithic. Fertile throughout the year; our fertile material was found in July.
Distribution: Mediterranean–Atlantic species. In the Mediterranean it is known from the western basin.
Coryne pusilla Gaertner, 1776
(Fig. 2L)
Material examined: No label (S2), one fertile stem, c. 20 mm high, on Cystoseira sp.
Remarks: As stated by Brinckmann-Voss (1970), the identification of Coryne pusilla has been a difficult task, probably due to its incomplete description. Schuchert (2001) pointed out that the presence of a perisarc collar is not a resolving diagnostic character to distinguish Coryne muscoides and Coryne pusilla. Coryne muscoides usually has a well developed collar, which C. pusilla tends to lack. However, the character may be the reverse, as it is shown here (see Fig. 2J–L and Table 1). According to the literature, the study of the cnidome is key for the proper identification of the species of the genus Coryne. The present data on the size of the large stenotele in Coryne muscoides and C. pusilla, as well as other taxonomic traits, concurs with those obtained by Schuchert (2001) (cf. Table 1).
| Species/trait | Present study | Schuchert (2001) |
|---|---|---|
| Coryne muscoides | ||
| Perisarc collar | Some indistinct | Mostly present |
| Tentacle numbers | 14–16 | 16–22 |
| Size of the colonies (in mm) | 15–40 | 50–120 |
| Size range of large stenotele (in μm) | (25–32) × (13–16) | (25–33) × (14–22) |
| Mean ± SD (n) of large stenotele (in μm) | (25.8 ± 2.4) × (15.6 ± 1.2) (11) | |
| Coryne pusilla | ||
| Perisarc collar | Present | Mostly absent |
| Tentacle numbers | 18–22 | 18–28 |
| Size of the colonies (in mm) | 20 | Up to 30 |
| Size range of large stenotele (in μm) | (18–21) × (12–14) | (18–23) × (11–15) |
| Mean ± SD (n) of large stenotele (in μm) | (19.5 ± 1.3) × (13.1 ± 1.3) (4) | |
Ecology: Coryne pusilla has been usually reported from the tidal level (Boero & Fresi 1986; Schuchert 2001), although it is also found at intermediate depths (5–15 m) (Boero 1981); our material also comes from the tidal level. As stated by Schuchert (2001), Coryne pusilla grows on a variety of substrates, such as leaves of Posidonia oceanica (Boero 1981), algae (Brinckmann-Voss 1970), and hydroids and barnacles (Boero & Fresi 1986); our material was found on Cystoseira sp. In the Mediterranean, fertile colonies were found in May (Brinckmann-Voss 1970) and March and August (Boero & Fresi 1986); the present fertile material in July.
Distribution: Boero & Bouillon (1993) considered C. pusilla a boreal species. However, Schuchert (2001) considered valid the records from the coasts off South Africa, Kerguélen, Seychelles, Korea, Japan, New Zealand and Eastern Canada, and consequently its distribution can be considered to be circumglobal (except for the Western Pacific). In the Mediterranean waters it has been reported from both the eastern and western basins (Bouillon et al. 2004).
Family Campanulinidae Hincks, 1868
Genus Egmundella Stechow, 1921
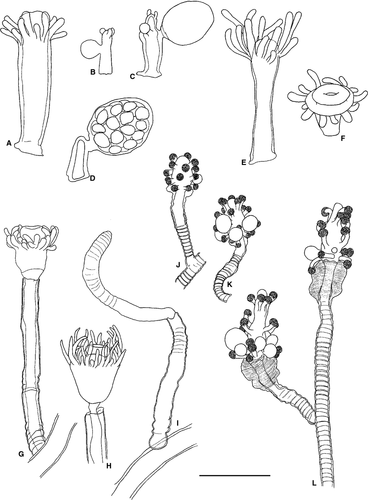
Egmundella amirantensis (Millard & Bouillon, 1973)
(Fig. 3A)
Material examined: H23a (S4), stolonal colony, on Dynamena disticha; H46b (S27), stolonal colony, on Turritopsis dohrnii; H47 (S27), stolonal fertile colony, on Eudendrium sp.2.
Remarks: Some authors (e.g. Calder 1991) considered E. amirantensis conspecific with Lafoeina tenuis. However, due to the presence of a short pedicel and a well marked diaphragm in some hydrothecae, as well as the agreement in the size of both the hydrotheca and the gonotheca with the original description, we keep our material as E. amirantensis.
Ecology: Egmundella amirantensis has been found at depths from 1 to 1062 m; our material comes from 27 to 28 m. Egmundella amirantensis is able to grow on a wide range of organisms such as algae, hydroids and bryozoans; we found it exclusively on other hydroids. In the Mediterranean, the gonothecae have been found in summer; our fertile material was found in September.
Distribution: Boero & Bouillon (1993) considered Egmundella amirantensis an Indo-Pacific species. Nevertheless, it is also known from the Bermudas (Calder 1991), so it probably has a circumglobal distribution. In the Mediterranean it is known from the Adriatic and the western basin.
Family Lafoeidae Hincks, 1868
Genus Filellum Hincks, 1868
Filellum sp
Material examined: H30 (S43), one colony growing on Halimeda tuna.
Remarks: The scarcity of material and the absence of coppinia prevent us from identifying this specimen accurately.
Ecology: Our material was collected at 21–22 m depth, growing on the alga Halimeda tuna.
Family Hebellidae Fraser, 1912
Genus Scandia Fraser, 1912
Scandia gigas (Pieper, 1884)
Material examined: H36b (S46), one stem, c. 10 mm, on Cystoseira sp.
Ecology: In the Mediterranean, Scandia gigas has been found from the tidal level to 89 m depth; our material was collected at 18 m. It is an epibiotic species, frequently growing on algae, Posidonia oceanica and invertebrates, commonly on hydroids and bryozoans, although it can also be found epilithic; our material was collected on Cystoseira sp. In the Mediterranean, fertile colonies have been reported from April to September.
Distribution: Scandia gigas is a boreal species. In Mediterranean waters it has been reported from both the eastern and western Mediterranean.
Family Haleciidae Hincks, 1868
Genus Halecium Oken, 1815
Halecium pusillum (M. Sars, 1857)
(Fig. 3B)
Material examined: H4 (S4), some stems, c. 2 mm high, on Cystoseira sp.
Ecology: Halecium pusillum has been found at depths from the tidal zone to 40 m; our material comes from the tidal level. It has frequently been found epibiotic on algae, seagrasses and some invertebrates such as bryozoans, hydrozoans, cirripeds and molluscs, but also epilithic; our material was found on Cystoseira. In the Mediterranean, fertile colonies have been reported from February to April and October.
Distribution: Halecium pusillum has a circumglobal distribution. In the Mediterranean it is known from the western basin.
Halecium tenellum Hincks, 1861
(Fig. 3C–E)
Material examined: H47 (S27), some shorts stems, c. 1 mm, on Eudendrium sp.2.
Ecology: In the Mediterranean, Halecium tenellum has been found at depths between 0.5 and 200 m; our material was collected at 28 m. It is an epibiotic species commonly reported to grow on algae, hydrozoans and bryozoans, as well as on other invertebrates; our material was found on Eudendrium sp.2. Fertile colonies have been reported in January (Puce et al. 2009) and from April to May.
Distribution: Considered a cosmopolitan species, it has been reported from the Western Mediterranean, the Adriatic and the Black Sea.
Halecium sp
Material examined: H4b (S4), a few hydrothecae, on Dyctiopteris sp.; H21b (S33), a single hydrotheca, on Cystoseira sp., H36c (S46) a single hydrotheca on Dyctiopteris sp.; H42c (S31), a few hydrothecae, on Dyctiopteris sp.; H43b (S43), a single hydrotheca, on Clytia linearis, H46c (S46), a few hydrothecae, on Turritopsis dohrnii.
Remarks: The scarcity of material and the lack of gonothecae prevent us from providing a proper identification.
Ecology: Our material was collected from the tidal level to 18 m depth, epibiotic on algae and hydroids.
Family Aglaopheniidae Marktanner-Turneretscher, 1890
Genus Aglaophenia Lamouroux, 1812
Aglaophenia elongata Meneghini, 1845
(Fig. 3F)
Material examined: H24 (S49), some colonies, up to 160 mm, on hard bottom.
Ecology: Aglaophenia elongata has been found at depths of 10–74 m; our material was collected at 26 m. It has been reported epilithic, as well as epibiotic on algae, Posidonia oceanica and invertebrates such as molluscs and bryozoans; the present material on bedrock. This species seems to be fertile throughout the year, having been reported with corbulae from February to December.
Distribution: Boero & Bouillon (1993) consider A. elongata to have an Atlantic–Mediterranean distribution. Nevertheless, Vervoort (1993) considered the records from outside Mediterranean waters to be doubtful. In the Mediterranean it has been reported from the eastern and western basins.
Aglaophenia harpago von Schenck, 1965
(Fig. 3G)
Material examined: H14 (S20), some colonies, up to 5 mm, growing on Posidonia oceanica leaves.
Ecology: Aglaophenia harpago has been reported from depths of 3–40 m (Svoboda & Cornelius 2007); our material was collected at 7 m. It seems that the presence of this species is strongly influenced by that of its specific substrate, Posidonia oceanica. However, there are a few cases where it grows on other substrates such algae and Cymodocea. Fertile colonies have been reported from March to July (Bouillon et al. 2004).
Distribution: Endemic to the Mediterranean Sea, where it has been reported from both the eastern and western basins (Bouillon et al. 2004).
Aglaophenia kirchenpaueri (Heller, 1868)
(Fig. 3H-I)
Material examined: H18 (S20), one stem, c. 15 mm, on rhizome of Posidonia oceanica; H19 (S33), some stems, up to 10 mm, on Cystoseira sp.; H39 (S31), several stems, up to 44 mm, on hard substrate; Sdf (S46), some fertile stems, up to 20 mm, on hard substrate.
Ecology: Aglaophenia kirchenpaueri has been found from the intertidal to 120 m; our material was collected from the tidal level to 21 m. It has been reported epibiotic on a wide range of substrates such as algae, Posidonia oceanica and invertebrates, although it seems to have a preference for hard substrate; our material was found epibiotic on Cystoseira sp. and P. oceanica as well as epilithic. Fertile colonies have been reported from February to November; our fertile material was collected in July.
Distribution: Mediterranean–Atlantic distribution. In the Mediterranean known from the western basin.
Aglaophenia octodonta (Heller, 1868)
(Fig. 3J)
Material examined: H2 (S4), some stems, up to 20 mm, on Cystoseira sp.; H20 (S33), a short stem, c. 5 mm, on Aglaophenia kirchenpaueri; S5 (S30), numerous stems, up to 25 mm, on hard substrate.
Ecology: Aglaophenia octodonta has been found from the intertidal to 30 m; our material was collected from the tidal level to 10 m. It has usually been reported epibiotic on algae, as well as on Posidonia oceanica and on a wide range of invertebrates and also as epilithic; our material was collected on Cystoseira sp., Aglaophenia kirchenpaueri and epilithic. It has been reported with corbulae throughout the year.
Distribution: Mediterranean–Atlantic species. In the Mediterranean it has been recorded from both basins.
Aglaophenia picardi Svoboda, 1979
(Fig. 3K)
Material examined: H10 (S13), several fertile stems, up to 30 mm, on hard substrate.
Ecology: Aglaophenia picardi has been reported at depths between the tidal level and 45 m; the present material was collected at 5 m depth. It has been reported epibiotic on algae and a wide range of invertebrates, as well as epilithic; our material was found on hard substrate. Fertile colonies have been reported throughout the year; our fertile material was collected in November.
Distribution: Mediterranean–Atlantic species. It has been recorded from both the eastern and western Mediterranean.
Aglaophenia tubiformis Marktanner-Turneretscher, 1890
(Fig. 3L)
Material examined: H42 (S31), some stems, up to 12 mm, on Dyctiopteris sp.
Ecology: Aglaophenia tubiformis has been reported from the tidal level to more than 40 m; our material was collected at 10 m depth. It has been found on algae and invertebrates, but also epilithic; our material was growing on Dyctiopteris sp. Fertile material has been collected from February to October.
Distribution: Mediterranean–Atlantic species. In the Mediterranean it has been collected from the Eastern and Western Mediterranean. It has not been reported from the Black Sea.
Family Halopterididae Millard, 1962
Genus Halopteris Allman, 1877
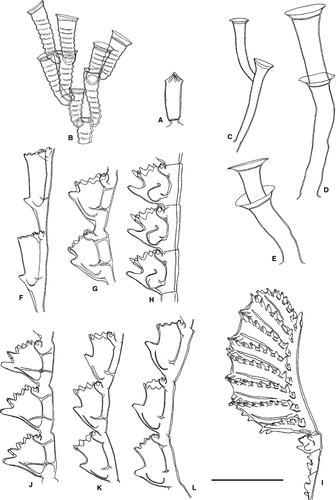
Halopteris liechtensternii (Marktanner-Turneretscher, 1890)
(Fig. 4A,B)
Material examined: H17b (S20), a short stem, c. 5 mm, on Synthecium evansi; H29 (S46), some stems, up to 10 mm, on rhizomes of Posidonia oceanica; H38 (S31), some stems, up to 18 mm, on hard substrate.
Ecology: Halopteris liechtensternii has been collected from the intertidal to 40 m; our material comes from 3–21 m. It has been reported epibiotic on algae and invertebrates, including other hydroids, as well as on abiotic substrates; our material was found on rhizomes of Posidonia oceanica. Fertile colonies of H. liechtensternii have been reported from March to October.
Distribution: Endemic to the Mediterranean Sea, where it has been collected from the Eastern and Western Mediterranean.
Family Kirchenpaueriidae Stechow, 1921
Genus Kirchenpaueria Jickeli, 1883
Kirchenpaueria pinnata (Linnaeus, 1758)
(Fig. 4C,D)
Material examined: H8 (S13), a few stems, up to 3 mm, on Posidonia oceanica leaves.
Ecology: Kirchenpaueria pinnata has been reported from the tidal level to 145 m depth; our material was collected at 7 m. It has been found on algae, Posidonia oceanica and a wide range of invertebrates, but also epilithic; our material was found on P. oceanica leaves. Fertile colonies have been reported throughout the year.
Distribution: Mediterranean–Atlantic species. In the Mediterranean it has been reported from the Eastern and Western Mediterranean.
Genus Ventromma Stechow, 1923b
Ventromma halecioides (Alder, 1859)
(Fig. 4E)
Material examined: S83 (S2), a few colonies, up to 3 mm, on Sargassum sp.
Ecology: In the Mediterranean, Ventromma halecioides has been reported from depths between the tidal level and 50 m; our material comes from the intertidal. The most common substrates are algae, but it is also been found on the seagrass Zostera nana and invertebrates, as well as epilithic; our material was collected growing on Sargassum sp. Fertile colonies have been reported from March to September.
Distribution: Circumglobal species. In the Mediterranean it has been found from both in the eastern and the western basins, including the Black Sea.
Family Plumulariidae McCrady, 1859
Genus Monotheca Nutting, 1900
Monotheca obliqua (Johnston, 1847)
(Fig. 4F)
Material examined: H6b (S13), some stems, up to 2 mm, on leaves of Posidonia oceanica.
Ecology: Monotheca obliqua has been found at depths from the tidal level to 70 m; our material was collected at 7 m. It has been reported epibiotic on hydroids and other invertebrates, as well as epilithic, but the most common substrate is algae and Posidonia oceanica; similarly, our material was found on P. oceanica. It has been reported with gonothecae from February to October.
Distribution: Indo-Pacific species. In the Mediterranean it has been found in the eastern and western basins, including the Black Sea.
Family Syntheciidae Marktanner-Turneretscher, 1890
Genus Synthecium Allman, 1872
Synthecium evansi (Ellis & Solander, 1786)
(Fig. 4G)
Material examined: H17 (S20), a large stem, c. 20 mm, on rhizome of Posidonia oceanica.
Ecology: Synthecium evansi has been found at depths from 2 to 250 m; our material was collected at 21 m. It grows not only epibiotic on invertebrates, but also epilithic; the present material collected growing on rhizome of P. oceanica. It has been reported with gonothecae from July to November.
Distribution: Mediterranean–Atlantic species. In the Mediterranean it has been reported from the Western Mediterranean and the Adriatic.
Family Sertulariidae Lamouroux, 1812
Genus Dynamena Lamouroux, 1812
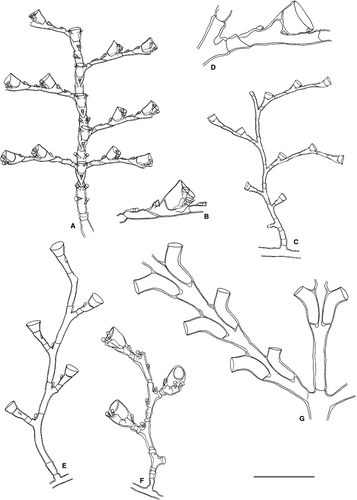
Dynamena disticha (Bosc, 1802)
(Fig. 5A,B)
Material examined: H22 (S33), several fertile stems, up to 40 mm high, on hard substrate; H23 (S4), several stems, up to 30 mm high, on hard substrate; H25 (S46), a few stems, up to 10 mm high, on Dyctiopteris sp.; H33 (S43), some fertile stems, up to 8 mm high, on Halimeda tuna.
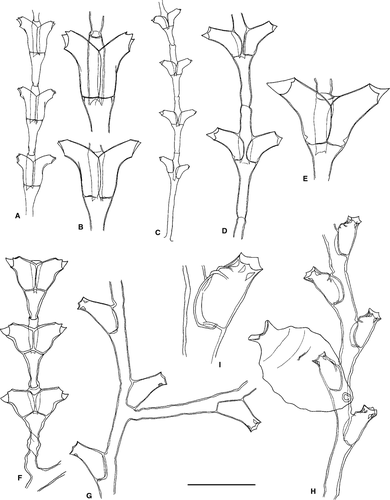
Ecology: Dynamena disticha has been found at depths from the tidal level to 60 m; the present material from 3 to 22 m. It has been reported on a wide range of biotic substrates, including algae, Posidonia oceanica, numerous invertebrates, but also on abiotic substrate; our specimens were found epibiotic on Dyctiopteris sp. and Halimeda tuna, as well as epilithic in a cave bottom with strong currents. In the Mediterranean, fertile material has been reported in February, and from July to October; the present fertile material was collected in July.
Distribution: Circumglobal species, present in both the Eastern and Western Mediterranean.
Genus Sertularella Gray, 1848
Sertularella ellisii (Deshayes & Milne Edwards, 1836)
(Fig. 5G)
Material examined: H36a (S46), some stems, up to 30 mm high, on Cystoseira sp.
Ecology: Sertularella ellisii has been reported from the intertidal to 90 m depth; our material was collected at 18 m. It has been found on a wide range of substrates, such as algae, Cymodocea nodosa, Posidonia oceanica, several invertebrates, as well as epilithic; our material was found on Cystoseira sp. Fertile throughout the year, having been reported with gonothecae from January (Puce et al. 2009) and from February to December.
Distribution: Mediterranean–Atlantic species. In the Mediterranean it has been reported from both the Eastern and Western Mediterranean.
Sertularella ornata (Broch, 1933)
(Fig. 5H,I)
Material examined: H5 (S13), a single, fertile stem, c. 20 mm high, on hard substrate; H28b (S46), a single short stem, on Coryne muscoides.
Ecology: Sertularella ornata has been found at depths of 4–120 m; our material was collected at 3–5 m. This species is epibiotic on algae, Posidonia oceanica and numerous invertebrates, and also on artificial substrates; the present material was found on hard substrate. Fertile material has been reported in May, June and November; our fertile specimen was collected in November.
Distribution: Mediterranean–Atlantic species. In the Mediterranean it has been reported from the Eastern and Western Mediterranean.
Remarks: Sertularella ellisii (Deshayes & Milne Edwards, 1836) and Sertularella ornata (Broch, 1933) have been considered subspecies by some authors (cf. Medel Soteras & Vervoort 1998). The comparative study of the cnidome shows critical differences that allow us to keep them as separate species (see Table 2). Sertularella ornata has two well characterized groups of microbasic mastigophores, whereas S. ellisii has only one, which is bigger than the smallest group of S. ornata. Material from both the Chafarinas islands (ISA 16 and REY 4) and the Maltese islands (present study) has been used to add support, but more morphological and molecular data are needed to shed light on the issue. Note: recently, Galea (2010) proposed a new name for Sertularella ornata Fraser, 1937 – resulting in Sertularella fraseri Galea 2010 – with the aim of avoiding confusion.
| Species/nematocyst group | Sample | n | Length (μm) | Width (μm) | ||
|---|---|---|---|---|---|---|
| Range | Mean ± SD | Range | Mean ± SD | |||
| Sertularella ellisii | ||||||
| Small | H36 (S46) | 18 | 6.5–7 | 6.9 ± 0.2 | 1.5–2 | 1.9 ± 0.2 |
| ISA 16 | 14 | 6.5–7 | 6.8 ± 0.3 | 1.5–2 | 1.9 ± 0.2 | |
| Sertularella ornata | ||||||
| Large | H5 (S13) | 102 | 7–9 | 8.1 ± 0.3 | 3–4 | 3.1 ± 0.3 |
| REY 4 | 23 | 7–8.5 | 7.8 ± 0.4 | 3–3.5 | 3.1 ± 0.2 | |
| Small | H5 (S13) | 20 | 5.5–7 | 6.3 ± 0.3 | 1.5–2 | 2.0 ± 0.2 |
| REY 4 | 11 | 6–6.5 | 6.1 ± 0.2 | 1.5–2 | 1.9 ± 0.2 | |
Genus Sertularia Linnaeus, 1758
Sertularia distans Lamouroux, 1816
(Fig. 5C–E)
Material examined: H32b (S43), numerous stems, up to 5 mm high, on Halimeda tuna; H35 (S46), some fertile stems, up to 11 mm high, on Sargassum vulgare; H41 (S31), some stems, up to 6 mm high, on Dyctiopteris sp.
Ecology: Sertularia distans has been found at depths between 0.5 and 90 m; our material was collected from 10 to 22 m. It is a typically epibiotic species, reported from algae, P. oceanica and a wide range of invertebrates; the present material found on the algae Halimeda tuna, Sargassum vulgare and Dyctiopteris sp. The presence of gonothecae has been reported from March to August; the present fertile material was found in July.
Distribution: Circumglobal species. It has been reported from both the Eastern and Western Mediterranean.
Sertularia perpusilla Stechow, 1919
(Fig. 5F)
Material examined: H9 (S13), several short stems, c. 3 mm high, growing on leaves of Posidonia oceanica.
Ecology: Sertularia perpusilla has been collected at depths of 1–74 m; our material was found at 7 m. Sertularia perpusilla is considered an exclusive epibiont of Posidonia oceanica and our material comes from the same substratum. It has been reported with gonothecae from March to October.
Distribution: Endemic to the Mediterranean Sea, where it has been reported from the eastern and western basin.
Family Campanulariidae Johnston, 1836
Genus Clytia Lamoroux, 1812
Clytia gracilis (M. Sars, 1850)
Material examined: H3 (S4), a few hydrothecae, on Cystoseira sp.
Ecology: Clytia gracilis has been found from the tidal zone to 205 m depth; our material comes from the tidal level. It has been reported to grow on a wide range of substrata, both biotic and abiotic, as for instance some species of algae, invertebrates and rocks. The presence of gonothecae has been reported from June to September.
Distribution: Circumglobal species. It has been reported from the Eastern and Western Mediterranean.
Clytia hemisphaerica (Linnaeus, 1767)
(Fig. 6A–C)
Material examined: H10b (S13), a few hydrothecae, on Aglaophenia picardi; H24b (S49), several fertile colonies, on Aglaophenia elongata.
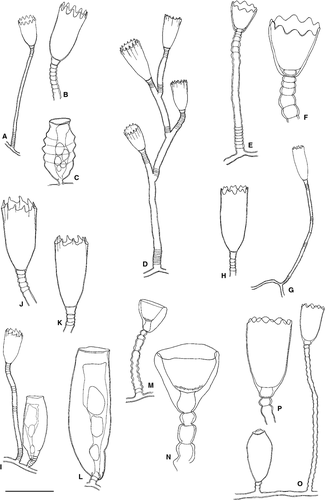
Ecology: Clytia hemisphaerica has been found from the intertidal to 144 m; our material is from 5 to 26 m. It has been found epibiotic on algae, seagrasses and many invertebrates, on floating objects and also on abiotic substrates; the present specimens on Aglaophenia spp. Fertile colonies have been reported throughout the year; our fertile material was collected in July.
Distribution: Circumglobal species. In the Mediterranean it has been found from both the eastern and western basins.
Clytia linearis (Thornely, 1900)
(Fig. 6D)
Material examined: H7 (S13), a few hydrothecae, on leaves of Posidonia oceanica; H26 (S46), stolonal colony, on Dyctiopteris sp.; H32 (S43), fertile stem, c. 10 mm high, on Halimeda tuna; H34 (S43), some fertile colonies, on Dyctiopteris sp.; H43 (S33), few hydrothecae, on hard substrate; H48 (S27) few hydrothecae, on hard substrate.
Ecology: Clytia linearis has been recorded from the intertidal to 200 m; the present material was found from 3 to 22 m. It has been found epibiotic on several types of substrates, including algae, Posidonia oceanica and invertebrates, but also epilithic; our material was found on the algae Halimeda tuna and Dyctiopteris sp., leaves of Posidonia oceanica and epilithic. It seems to be fertile throughout the year; our fertile colonies were found in July.
Distribution: Circumglobal species. It has been reported from the Eastern and Western Mediterranean.
Clytia noliformis (McCrady, 1859)
(Fig. 7E,F)
Material examined: H1 (S4), few hydrothecae, on Cystoseira sp.

Ecology: Clytia noliformis has been found at depths between 0 and 10 m (Boero & Fresi 1986); our material comes from the tidal level to 3 m. It is usually restricted to algae and Posidonia oceanica, although rarely it may also be found on mussels and other hydroids (Puce et al. 2009); our material was collected on Cystoseira sp. It has been reported with gonothecae in August (Boero & Fresi 1986) and January (Puce et al. 2009).
Distribution: Circumglobal species. In the Mediterranean it is known from the western basin and the Adriatic (Bouillon et al. 2004).
Clytia paulensis (Vanhöffen, 1910)
(Fig. 6G,H)
Material examined: H10b (S13), few hydrothecae, on Aglaophenia picardi; H23c (S4), few hydrothecae, on Dynamena disticha.
Ecology: Clytia paulensis has been reported from depths between the tidal level and 250 m; our material was collected from 5 to 26 m. It has been reported epibiotic on algae, Posidonia oceanica and invertebrates, as well as epilithic on rocks; the present material was found on Dynamena disticha and Aglaophenia picardi. It has been found with gonothecae from April to August.
Distribution: Circumglobal species. In the Mediterranean it is known from both the eastern and western basin.
Clytia sp
(Fig. 6I–L)
Material examined: H23b (S4), a few hydrothecae, on Dynamena disticha; H44 (S33), a colony with gonothecae, on Eudendrium sp.; H48 (S27), some hydrothecae, on undetermined hydroid stolon.
Description: Previously recorded from the Chafarinas islands (Peña Cantero & García Carrascosa 2002), this campanulariid forms stolonal colonies rarely branched to second order. Pedicel with basal and distal rings. Hydrotheca delicate, deep and small, with six to nine cusps separated by asymmetrical and wavy embayments. Gonotheca elongate, basally curved, distally truncated, inserted on short pedicels with up to four rings.
Ecology: Our material comes from 5–17 m depth. In addition, Peña Cantero & García Carrascosa (2002) reported this rare species up to 46 m.
Distribution: Probably a Mediterranean endemic. At present it is only known from the Chafarinas and Maltese islands.
Genus Laomedea Lamouroux, 1812
Laomedea sp
Material examined: H11 (S13), few stems, on Aglaophenia picardi; H13 (S13), few stems, on Cymodocea nodosa; H27 (S46), one stem, up to 7 mm high, on Myriapora truncata.
Remarks: Due to the scarcity of available specimens and their infertile state, we cannot allocate them to a species with confidence.
Ecology: Our material was collected at depths between 3 and 16 m. It was growing on algae, hydroids and bryozoans.
Genus Obelia Péron & Lesueur, 1810
Obelia sp
Material examined: H15 (S20), a single hydrotheca, on unknown substrate.
Remarks: Due to the small amount of material and the lack of gonothecae, we are not able to identify this species.
Ecology: Our material was collected at 7 m.
Genus Orthopyxis L. Agassiz, 1862
Orthopyxis asymmetrica Stechow, 1919
(Fig. 6M,N)
Material examined: H6 (S13), several hydrothecae, on leaves of Posidonia oceanica.
Ecology: Orthopyxis asymmetrica has been found at depths from near the tidal level to 25 m; the present material at 7 m. It is an exclusive epibiont of Posidonia oceanica, which our material comes from. It has been reported with gonothecae in May.
Distribution: Endemic to the Mediterranean Sea, where it has been reported from the western basin.
Orthopyxis crenata (Hartlaub, 1901b)
(Fig. 6O,P)
Material examined: H30b (S43), stolonal, fertile colony, on Halimeda tuna; H33b (S43), stolonal, fertile colony, on Halimeda tuna; H40 (S31), very few hydrothecae, on Dyctiopteris sp.; H42b (S31) a few hydrothecae, on Dyctiopteris sp.
Ecology: In Mediterranean waters, Orthopyxis crenata has been reported from near the tidal level to 25 m depth; our material was collected from 10 to 22 m. It is exclusively epibiotic, frequently reported from algae, seagrasses and invertebrates; the present material has been found on the algae Halimeda tuna and Dyctiopteris sp. Fertile colonies are known to occur in March and October; our fertile material was collected in July.
Distribution: Circumglobal species. It has been reported from the Eastern and Western Mediterranean.
Discussion
The 40 species found by us belong to 15 families and 21 genera. Of the total, 32 (80%) species belong to the subclass Leptothecata Cornelius, 1992, while the remaining eight (20%) are of the subclass Anthoathecata Cornelius, 1992. The most speciose family is Campanulariidae with 10 species (25%), followed by Aglaopheniidae with six species (15%) and Sertulariidae with five species (12%). Aglaophenia and Clytia are the predominant genera, both with six species (15%).
Notwithstanding the limitations of these qualitative samples, some interesting conclusions may be drawn from our study. In terms of substrate used and biocenology, most species are ubiquitous facultative epibionts (34 species, 87%), being found mainly on hard substrate (seven species, 18%) and/or algae (17 species, 44%), and mostly inhabiting the photophilic algal community (24 species, 62%). Although some species (e.g. Aglaophenia elongata) were observed only to be epilithic (four species, 10%), they have been reported from other substrate types. Despite being much less represented in our material, the Posidonia oceanica community supports a rich assemblage of hydroids (nine species, 23%), from facultative species (six species, 15%), such as Aglaophenia kirchenpaueri, Clytia linearis and Synthecium evansi, to obligate and specialized epibionts restricted to this seagrass (three species, 8%), namely Aglaophenia harpago, Orthopyxis assymetrica and Sertularia perpusilla. By contrast, Cymodocea nodosa, the other seagrass present in our collection, harbours but a single species. Leaves of Posidonia oceanica grow slower and live longer (cf. Cancemi et al. 2002), which would promote the occurrence of epibionts. Five species (13%) were exclusively found on other hydroids (i.e. Egmundella amirantensis, Clytia hemisphaerica, Clytia paulensis, Clytia sp. and Halecium tenellum), whereas nine species (23%) were found on other substrates, too. Other species have been found on wood or metal of a wreck (Eudendrium sp.2 and Turritopsis dohrnii respectively), as well as on other invertebrates (Hydractinia monocarpa on gastropod shell and Laomedea on bryozoa). Dynamena disticha and Eudendrium armatum seem to have sciaphilic and rheophilic affinities, being found in caves and tunnels with strong currents (cf. Marques et al. 2000). In summary, the wide range of benthic communities available in the Maltese archipelago provides diverse substrate types, promoting regional species richness of benthic hydroids.
As to their ecological role, some species, especially the largest ones (Aglaophenia spp., Eudendrium spp.), serve as substrate for other hydroids and support some invertebrates, such as Caprellida and other small crustaceans and echinoderms. They also serve as a trophic resource for the Caprellida and some species of opisthobranchs, observed during examination of the material. Thus, hydroids can positively influence local levels of biodiversity.
Concerning the general biogeographic accounts, some quantitative differences between the present and other works are shown in Table 3. The hydroid fauna from the Maltese archipelago is dominated by circumglobal species (12 species, 36%). This proportion is similar to that reported by Peña Cantero (1995) from the Chafarinas islands. However it is considerably higher than that obtained by Boero & Bouillon (1993) for the whole Mediterranean Sea. This difference could simply be due to discrepancies in the criteria employed when establishing the zoogeographic patterns. As already mentioned, Boero & Bouillon (1993) considered circumtropical those species present in temperate, subtropical and tropical seas, whereas we have considered them circumglobal. In addition, some species considered cosmopolitan by Boero & Bouillon (1993) are here treated as circumglobal. This could explain the low percentage of cosmopolitan species (one species, 3%) in our classification. We consider unlikely the existence of truly cosmopolitan species, following the argument of Boero & Bouillon (1993): ‘their records in the literature could be due to insufficient possibilities of discrimination in current taxonomy’.
| Mediterranean (whole) (Boero & Bouillon 1993) | Chafarinas islands (Peña Cantero 1995) | Levant Sea (Morri et al. 1999) | Maltese islands (present study) | |
|---|---|---|---|---|
| Mediterranean endemic | 19.6 | 9.6 | 13.75 | 24.2 |
| Mediterranean-Atlantic | 11.8 | 12.3 | 9.3 | 24.2 |
| Tropical Atlantic | 12.1 | 4.1 | – | 0 |
| Boreal | 15.2 | 2.7 | – | 6.1 |
| Indo-Pacific | 8.1 | 11 | 2.9 | 6.1 |
| Circumglobal | 16.9a | 31.5b | 21.4c | 36.4 |
| Cosmopolitan | 12.8 | 12.3 | 50.3d | 3 |
| No. of species | 296 | 86 | 38 | 40 |
- Boero & Bouillon (1993) for the whole Mediterranean region, Peña Cantero (1995) for the Chafarinas islands and Morri et al. (1999) for the Levant Sea. [(a) as circumtropical, (b) as circumtropical and circumglobal, c) as circum-(sub)tropical, (d) as wide distribution] Data from Morri et al. (1999) taken from a pie chart.
Surprisingly, the proportion of species endemic to the Mediterranean (eight species, 24%) is the highest ever reported for a regional hydrozoan fauna (see Table 3). This peculiarity is accentuated if we also consider the Mediterranean–Atlantic species (eight species, 24%), which gives a total of 48%, distinctly higher than the 31% reported by Boero & Bouillon (1993). In this sense, the Maltese islands could be a representative location for the Mediterranean and nearby Atlantic hydroid fauna. This is probably explained by the presence of Modified Atlantic Water (Astraldi et al. 1999; Lermusiaux & Robinson 2001) and the central position of the Maltese Archipelago in the Mediterranean Sea.
In the light of the comparative data (cf. Table 3), Indo-Pacific species are neither confined nor concentrated in the eastern basin (but see Boero & Bouillon 1993). Unexpectedly, the highest proportion of Indo-Pacific species (11%) was reported from the Chafarinas islands (Alboran Sea, western basin) by Peña Cantero (1995), whereas the lowest one (3%) was obtained by Morri et al. (1999) from off Lebanon (Levant Sea, eastern basin). Our data (two species, 6%), as those of Boero & Bouillon (1993), constitute intermediate values between the extremes mentioned above. Possible explanations require the assumption that not all the Indo-Pacific hydroid fauna necessarily have to be a Tethyan relict, inasmuch as the present distribution is the result of multiple past selective pressures and historical events. In other words, some of the present Indo-Pacific fauna could have had their biological origin outside the Tethys Sea, with subsequent establishment and expansion.
On one hand, some species with a wide prior distribution (e.g. circumglobal) could have been posteriorly confined to a smaller area (e.g. Indo-Pacific). On the other hand, the inflow of Atlantic surface waters through the Strait of Gibraltar, with lower salinity and density, and the outflow of Mediterranean deeper, saltier and denser waters, could act as a kind of one-way trapdoor for some Atlantic species, especially those restricted to shallow waters (cf. Boero & Bouillon 1993), and a similar phenomenon could have occurred in the Proto-Atlantic. Whatever the true origin of the species, the long time that the Mediterranean Sea remained isolated from the Indo-Pacific after the closure of the Tethys should have implied vicariant speciation, not the presence of the same species in both the Mediterranean and the Indo-Pacific (cf. Boero & Bouillon 1993).
The Suez Canal was opened in the latter half of the 19th century. Thus, Indo-Pacific species should have had ample time to disperse into the Mediterranean. Under this scenario, all Lessepsian migrants (not only the Indo-Pacific species) would be well represented across the whole Mediterranean. Although the presence of marine alien species is well documented for the Maltese islands (Sciberras & Schembri 2004), we have not found invasive hydroids previously known to be established in the eastern basin (cf. Morri et al. 1999). Unlike the Indo-Pacific species, invasive species appear restricted to the easternmost part of the Mediterranean Sea (Morri et al. 1999). This may be due to either differences in the timing of colonization between these two groups or, as stated before, because the Indo-Pacific species were already present in the Mediterranean before the opening of the Suez Canal (unlike the Lessepsian migrants). Fauna dispersion (within fouling or ballast water) by ships could also contribute to observed patterns (cf. Morri & Boero 1986).
For the above reasons and the data presented in Table 3, the hypothesis of the existence of some areas with high densities of Indo-Pacific species is not supported, and such decreasing gradient from east to west is not as marked, or even may not exist. Also, a Tethyan relict fauna and migration through the Suez Canal may not be the only explanations for the observed distribution patterns.
The nearly complete absence of studies on hydroids in the study area is remarkable. Records are limited to a few species of Aglaophenia (A. harpago, A. octodonta, A. picardi, A. tubiformis) collected by Svoboda & Cornelius (1991), and a medusa referable to Turritopsis found near Malta by Evans (1968), as Hydractinia borealis). Consequently, 28 of the 33 species found in our collection are reported from Maltese waters for the first time. These records thus constitute an important contribution to knowledge of the hydroid fauna of the Eastern Mediterranean Basin inhabiting marine bottoms of the Maltese Archipelago.
Acknowledgements
The authors would like to express their gratitude to Mr Carmel Sammut for collecting and sending us the material, as well as for his photographic work. The publication of this paper is supported by CONISMA, the Italian National Interuniversity Consortium for Marine Sciences.
Conflicts of Interest
None of the authors have any potential conflicts of interest.




