Cell migration during growth and morphogenesis in thecate hydroids
Abstract
Tissues of cnidarian polyps have no permanent cell composition. During growth and development of the organism different types of the cells continually migrate from the places of their proliferation towards the location of their final functioning. Here we studied the role and mode of cell migration during development of the shoot internode in the colonial hydroid Gonothyraea loveni (Thecata, Campanulariidae). The internode formation starts with emergence of a new growing tip and ends by differentiation of a hydranth. We experimentally placed a ligature proximal to the growing tip to prevent tissue and cell migration towards the tip. A normal-shaped and viable hydranth was formed. However, the ‘experimental hydranths’ had problems opening the hydrotheca and lacked nematocytes. During the life of the ‘experimental hydranths’ no nematocytes appeared in their tentacles. When the ligature was removed, numerous nematoblasts and morular gland cells migrated towards the ‘experimental hydranth’ from the proximal part of the shoot coenosarc. Within a short time, the nematocytes appeared in the tentacles and the gland cells occupied the gastric region of the hydranth. This suggests that a subset of the cells migrate into the hydranth, during or after the final steps of its differentiation, from the proximal region of the shoot or from the stolon coenosarc. Our results also confirm the absence of cell proliferation in the hydranths of the thecate hydroid.
Introduction
The body of colonial hydroids is composed of many similar elements (modules) which can be organized differently in space and time during colony ontogeny (Kosevich 2006a). A hydroid colony starts its development from the primary polyp after the metamorphosis of the larva and can grow continuously, increasing its size, and changing form by adding new modules (Berking 1998; Bouillon et al. 2006). This process results in similar elements being formed due to the reiterative morphogenetic processes. Cyclic morphogenesis repeated continuously in adult animals is a characteristic and unique feature of colonial hydroids (Larwood & Rosen 1979; Rosen 1979).
In general, a hydroid colony comprises two parts: a net of tubes (stolons or hydrorhiza) generally fixed to a substratum, and shoots (hydrocauli) emerging vertically from these stolons in a more or less regular pattern. The shoots bear polyps (hydranths) used to catch prey. All tissues of the colony (the coenosarc) are covered with the perisarc. In thecates (Cnidaria, Hydroidomedusa, Leptomedusae) the polyp expands out of the tube-like endings of the perisarc covering (Fig. 1A).
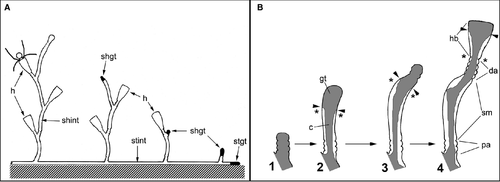
The shoots in Gonothyraea loveni and Laomedea flexuosa, used in this research, display a sympodial mode of growth (Kuhn 1914; Berking et al. 2002; Berking 2006). Each element of the shoot – the internode – consists of two sequences of annuli separated by a smooth, slightly bent tube and followed by the finely structured housing (hydrotheca) of the polyp. The sequence, the number, and the size of the pattern elements are almost invariant and species-specific in thecate hydroids (Kosevich 1990).
In general, morphogenesis employs several cell behaviors, namely cell division and death, cell growth and changes in shape, migration and matrix secretion (Gilbert 2003). In thecate hydroids, colony elements cannot be shaped without the perisarc being secreted and hardened; this forms the outer skeleton (Kosevich 2006b; Kosevich & Fedosov 2008).
In hydroids, the morphogenetic activity resides in the growing tips – termini of the branched colonial body – in the form of growth pulsations (Beloussov & Dorfman 1974; Beloussov et al. 1980). Shoot internode development starts with the emergence of the growing tip that forms the definite internode zones – proximal annulated, central smooth, distal annulated (=hydranth pedicel) – and finally changes shape to form the hydrotheca and hydranth (Kosevich 1990; Marfenin & Kosevich 1984a; Fig. 1B). The growing tip has a relatively constant cell composition (Kossevitch 1999; Marfenin et al. 1999; Kosevich 2006b). The epidermal layer of the tip includes epithelio-muscular (supportive) cells, i-cells plus wandering nematoblasts and tanning cells. The tip gastrodermis consists of highly vacuolated epithelio-muscular (supportive) cells. An intriguing point is that it appears that muscular processes of supportive cells in both epi- and gastrodermis of the growing tip are absent, as they have never been seen in macerates or in electron microscopy studies (Fig. 2A and E).
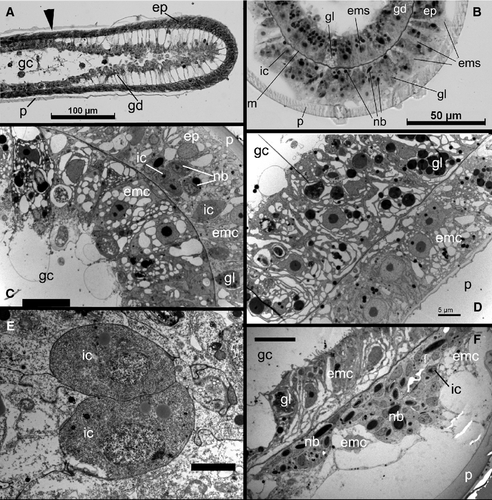
The coenosarc shows more cell types: the epidermis consists of epithelio-muscular and gland cells (predominantly tanning or morular cells), small i-cells, nematoblasts and nerve cells. The gastrodermis is more homogeneous and includes epithelio-muscular and a few gland cells. The interstitial space is occupied by large i-cells (Aizenshtadt & Polteva 1981; Thomas & Edwards 1991; Fig. 2).
The hydranth has more diverse cell composition compared with the coenosarc and the growing tip. The hydranth's epidermis comprises epithelio-muscular (supportive) cells, battery cells, nematocytes, nematoblasts and a few tanning cells, nerve cells and desmocytes connecting mesoglea with the hydrotheca diaphragm. The hydranth gastrodermis is composed of digestive epithelio-muscular (supportive) cells, gland cells in the hypostome (foamy gland and glandular mucous cells) and stomach regions (glandular mucous cells), and vacuolated cells forming rods of the tentacles (Thomas & Edwards 1991). During hydranth differentiation, the i-cells give rise to specialized cell types: nematoblasts, nerve cells, gastroderm mucous and zymogene gland cells. The fully differentiated hydranths lack i-cells or any blast-type cells (Polteva & Tokin 1975; Polteva & Aizenshtadt 1980).
Thus, during hydranth differentiation, the new cell types have to appear in the hydranth rudiment either due to final differentiation from the precursor cells present within the growing tip or as a result of cell migration from the coenosarc regions proximal to the growing tip; or, as is more likely, both processes contribute to the hydranth differentiation.
To distinguish between these possibilities – cell differentiation from the precursors present in the growing tip or differentiation from those migrating from the proximal region after the onset of rudiment differentiation – we conducted experiments to dissociate places of cell multiplication and differentiation during development of hydranths. We did this by applying a ligature at the base of the shoot growing tip: this did not limit growth but prevented cell migration from the coenosarc towards the growing tip. Our results provide evidence that at least some of the cells migrate into the hydranth during or after final stages of its differentiation from the proximal region of the shoot or stolon coenosarc.
Material and Methods
The experimental work was done at the N.A. Pertsov White Sea Biological Station (M.V. Lomonosov Moscow State University). The cultures of animals were started from the material collected in the intertidal zone, kept in the laboratory in filtered natural seawater at 16–18 °C and fed daily with Artemia salina nauplii.
Experimental animals
Two species of colonial hydroids belonging to Fam. Campanulariidae were used as model animals: Gonothyraea loveni Allman, 1859 and Laomedea flexuosa Hincks, 1861. Both species have similar colony organization and mode of colony growth and development, but they differ in some morphological characters (dimensions of hydrothecae and shoot internodes, curvature of shoot internode, etc.) and mode of sexual reproduction (Naumov 1969; Bouillon et al. 2006). The shoots of the colonies display a sympodial type of growth and are composed of equal modules – shoot internodes bearing hydrothecae with hydranths (Naumov 1969; Marfenin & Kosevich 1984b; Marfenin 1993). Both species used in the experiments (G. loveni and L. flexuosa) showed very similar responses to our experimental treatments, and results are therefore presented without reference to species identity.
The next growing tip that continues elongation of the shoot axis emerges at the base of the hydranth pedicel (Fig. 1A). During internode formation, the shoot tip passes through proximal annulation formation, then follows the smooth central part development, distal annulation and hydranth formation (Fig. 1B; Kosevich 1990). Under laboratory temperatures of 16–18 °C the whole cycle of internode formation takes about 30–36 h. The final stage – hydranth bud development, hydranth differentiation and opening of the hydrotheca lid – takes about 20–24 h (Marfenin & Kosevich 1984a,b). At each stage the growing tip can be distinguished from the proximal tissues (the coenosarc): its cells are in permanent contact with the surrounding perisarc and the layers are extremely well organized (Kosevich 2006b; Fig. 2A). Thus, it is easy to identify the tip borders.
Ligature
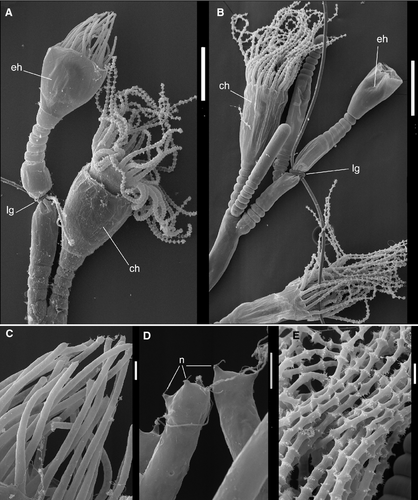
For the experiments, we used the shoots that had 6–10 internodes in the stem and a growing tip at the distal end, separated from the colony. According to previous results, this does not affect the mode of distal internode development (Kosevich 1990, 1991). To separate the places of cell proliferation and final differentiation during the shoot internode development, we put the ligature made of synthetic thread on the shoot internode. In successful cases the perisarc and coenosarc at the place of ligature application were squeezed but not damaged. The most important point is the integrity of the coenosarc – the ligature prevented tissues and cells from passing through the clamped area but did not disrupt the coenosarc itself (Fig. 3A and B). This was the prerequisite for the final stages of the experiment.
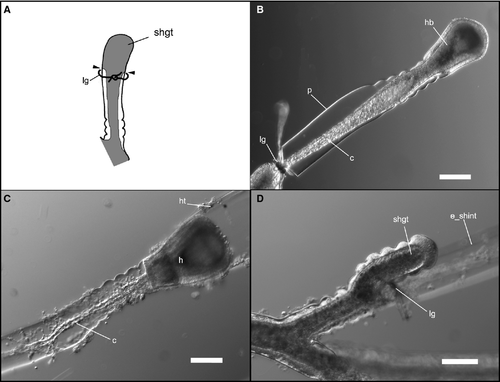
Application of the ligature even below the shoot tip had no obvious effect upon growth of the tip; even when the tip became separated it completed its development (Kosevich 1990, 1991). We conducted a series of experiments with ligatures placed just below the growing tip at different stages of internode formation (Fig. 1B) and at different distances from the growing tip.
Light and electron microscopy
For light and electron microscopy, the distal parts of the shoot, including three to five internodes, were fixed in 2.5% glutaraldehyde in phosphate buffer (pH 7.4; 0.83 Osmol) (Millonig 1964) for 1 h at 4 °C, and postfixed in 1% OsO4 in the same buffer (1 h, room temperature, RT) and stored in 70% ethanol at 4 °C until further processing. Specimens for light microscopy and TEM were embedded in the standard mixture of Araldite and Epon (Electron Microscopy Sciences, Munich, Germany). Semi-thin sections 1–2 mm in thickness were stained with a mixture of toluidine blue and methylene blue (Mironov et al. 1994) and viewed with an Axioplan 2 imaging microscope (Zeiss, Oberkochen, Germany). Ultra-thin sections were stained with uranyl acetate and lead citrate and examined with a JEOL JEM 100B (JEOL, Tokyo, Japan) electron transmission microscope. Specimens for SEM were dehydrated in ethanol series and acetone, critical point-dried, mounted on stubs, sputter coated with platinum and palladium, and viewed with CAM SCAN-S2 (Cambridge Instruments, Cambridgeshire, UK) and SCAN JSM 63 LA (JEOL, Tokyo, Japan) electron scanning microscopes.
Immuno-cytochemistry
For inspection of the nerve system within the hydranths, the latter were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS; Fluka, Buchs, Germany). The nerve cells were detected using primary mouse anti-tyrosinated tubulin antibodies (T9028, Sigma, Munich, Germany) and FITC-conjugated secondary anti-mouse antibodies (Chemicon, Millipore, Schwalbach am Taunus, Germany) and visualized with a confocal microscope Leica TCS SPE (Leica Camera AG, Solms, Germany).
Results
Ligature at the base of the tip
In the first set of experiments we placed the ligature directly at the proximal end of the growing tip during the formation of the central smooth zone of the terminal shoot internode (Figs 1B and 3A). In this way the entire growing tip was separated from coenosarc tissue. Ligature application had no effect on the timing of the development of the internode distal part in comparison with controls. At the end of internode development, the hydranth was formed (Fig. 3B and C).
The first noticeable difference was the attenuated tissues of the internode parts distal to the ligature (Figs 3B–C and 4A–C). The tissue appears translucent, with no visible canal in the coenosarc (Supporting Information Video S1). The other difference is the high proportion of developed hydranths that failed to open the lid of the hydrotheca, which is the final event of the hydranth differentiation (Knight 1965). Within 1–2 days these hydranths disintegrated.
The hydrothecae of the experimental hydranths were smaller (diameter and height), and had fewer and shorter tentacles (Table 1, Fig. 5A and B). Most experimental hydranths also had defective diaphragms of the hydrothecae. Application of the ligature closer to the tip and later in the internode development cycle resulted in a more defective diaphragm.
| Experimental | Control | |||||
|---|---|---|---|---|---|---|
| Hydrotheca | Hydrotheca | |||||
| Diameter (mm) | Height (mm) | Tentacles length (mm) | Diameter (mm) | Height (mm) | Tentacles length (mm) | |
| Gonothyraea loveni | ||||||
| Mean | 0.246 | 0.468 | 0.503 | 0.281 | 0.517 | 1.120 |
| SD | 0.011 | 0.032 | 0.036 | 0.041 | 0.026 | 0.099 |
| N | 14 | 14 | 8 | 13 | 13 | 5 |
| Laomedea flexuosa | ||||||
| Mean | 0.422 | 0.519 | 0.75–0.875 | 0.483 | 0.572 | 1.5–1.75 |
| SD | 0.044 | 0.039 | 0.018 | 0.040 | ||
| N | 9 | 9 | 9 | 9 | ||
- Mean, mean value; SD, standard deviation; N, sample size.
The behavior of those hydranths that managed to open the hydrotheca lid did not differ greatly from the normal control hydranth, but it appeared that co-ordinated behavior was impaired. The hypostome functioned in the same manner as it did in the control hydranths, but the tentacles moved slower and mostly one at a time.
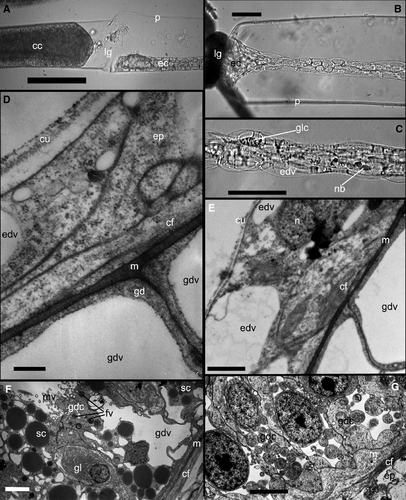
The main and the most conspicuous difference was the absence of nematocytes on the tentacles of the experimental hydranth (Figs 5A,C–E and 6A–B). A few of the nematocytes were present at the very tip of the tentacles. Their number correlated with the dimensions of the tip, isolated from the rest of shoot coenosarc. If the isolated part included a portion of the coenosarc in addition to the growing tip, the number of nematocytes in the experimental hydranth increased. Later on, the nematocysts never appeared in the tentacles of experimental hydranth.

The experimental hydranths were able to catch and engulf the prey (Artemia salina nauplii) when they could bring it to hypostome. They were able to engulf no more than one or two nauplii that were partly digested, as it is the case in normal hydranths. Most of the food was later ejected, as it was not possible to press it out into the colony. Without food or under a moderate feeding regime (half to one nauplii per day) the experimental hydranths had the same life-span time as did the controls (about a week; Marfenin & Kosevich 1984a).
The processes of the experimental hydranth regression (reabsorption, disintegration) proceeded slower in experimental animals. In control colonies, hydranth regression was complete within 15–20 h (at 16–18 °C) but remained incomplete in experimental hydranths: because disintegrated tissue could not be transferred to the gastro-vascular canal of the colony, hydrothecae still contained the lump of cells equal in volume to the shrunken hydranth after 2 days.
Further elongation of the shoot axis in animals with ligatures was delayed. However, most new shoot tips emerged just proximal to ligature after 1 or 2 days (Figs 3D and 5B), restoring growth of the shoot. The next growing tip that should continue shoot axis growth never appeared distal to ligature.
For comparison we applied the ligature just below the growing tip at the later stages of the internode development (Fig. 1B – stages 3 and 4). The results showed significant differences from those mentioned above. The later the stage of the internode development used for the tip separation, the larger the amount of the nematocytes that occupied the developed hydranths. At stage 3, the dimensions of the experimental hydrotheca, hydranth morphology and its behavior were comparable to those of hydranths developed after separation at stage 2. However, there were more nematocytes in the tentacles. Application of the ligature at stage 4 (at the base of the hydranth bud of approximate final size, just before diaphragm formation) resulted in development of the hydranth of normal size and morphology, but with fewer nematocytes. Moreover, the behavior of such experimental hydranths did not differ significantly from that of the control ones: they reacted quickly to any stimuli, easily caught and engulfed prey, and digested it to a greater extent compared with experiment hydranths formed after tip isolation at earlier stages of internode development.
Histology
Histologically, the experimental hydranths from the first set of experiments with ligature application differed from the controls in the relative number of cells that constitute the epithelium of the coenosarc distal of the ligature (Fig. 4A) and the hydranth body itself. The cells of both epithelia layers were much flattened, thin and highly vacuolated, especially the epidermis. Only a few characteristic gland cells (‘tanning cells’, Knight 1968, 1970) were distinguishable in the ectoderm of the coenosarc on histological sections or videos (Fig. 4B and C). The gastrodermis of experimental hydranths looked more homogeneous: most cells are digestive epithelio-muscular cells with relatively translucent cytoplasm with few inclusions (Fig. 4F and G). Tentacles have less prominent mesoglea and a vacuolated epidermal layer (Fig. 4D and E).
Immuno-cytochemical staining with anti-tubulin antibodies revealed a smaller number of nerve elements in the experimental hydranths. The general organization of the nerve system looked similar (Fig. 7). Microscopic inspection of the coenosarc tissue proximal to the ligature revealed an increased amount of the gland cells (‘tanning cells’, morular cells) and nematoblasts – motile cells normally wandering in the coenosarc of stolons and shoots (Supporting Information Data S1; Kossevitch 1999). When the ligature was removed, these motile cells rushed towards the hydranth (Fig. 6C and D) (Supporting Information Data S3). This movement was more unidirectional compared with the migration of such cells within a normal (control) coenosarc: most of these cells migrate predominantly distally within first 2–4 h following removal of the ligature. Nematoblasts constituted the major part of cells migrating through the pedicel into the experimental hydranth (Supporting Information Data S4). Comparison with the control hydranths (Supporting Information Data S6) revealed not only the differences in the number of nematoblasts migrating into the hydranth, but also the observation that in experimental hydranths nearly all the nematoblasts moved towards the hydranth, whereas in control ones, a significant number migrated in the opposite direction.
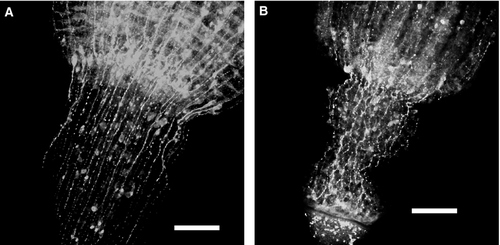
In the 2–4 h following ligature removal (depending on the distance from the ligature to the hydranth) migrating nematoblasts could be recognized in the walls of the gastric region of the hydranths and its tentacles (Fig. 6E and F; Supporting Information Data S5). After reaching the tentacles, nematoblasts took up their position in the battery cells (Fig. 6G). Thus, within 4–5 h the tentacles appeared completely equipped with nematocytes and were almost undistinguishable from the control hydranth in this aspect (Fig. 6A and H).
The gland cells moved slower and there were fewer of them. According to the time-lapse video, these cells enter the hydranth body. It was impossible to follow their path within the hydranth but we can assume that they entered the gastrodermis and differentiated into the gland cells, participating in food digestion.
Ligatures at different distances from the growing tip
These experiments aimed to determine the amount of coenosarc tissues proximal to the growing tip that is sufficient to supply the complete development of fully functional hydranth and continuous growth of the shoot (stem or branch) axis. The ligatures were applied at the nodes of the shoot different distances from the developing terminal internode (Fig. 8A). We did not calculate the exact number of nematocytes within the tentacles of the experimental hydranths – their quantity was estimated by visual comparisons with control hydranths. The effect upon elongation of the shoot axis was determined as the time between the completion of the hydranth formation of the maternal internode and the emergence of the next growing tip.
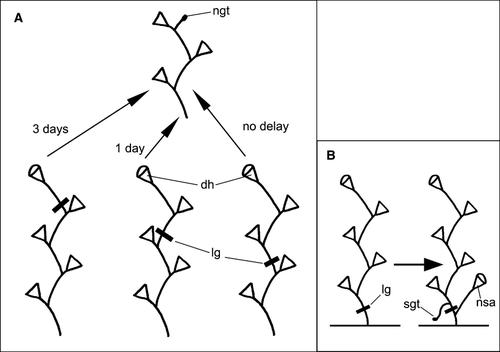
Under favorable temperatures (16–18 °C) and nutrition, the next growing tip of the shoot emerged at the end of the hydranth's differentiation upon the maternal internod) after formation of the diaphragm within the hydrothecae. If the ligature was put at the base of the internode at the final stages of its development (growth of the hydranth bud but before the diaphragm formation), the next growing tip appeared after only 3 days. The formed hydranths had visibly fewer nematocytes than the control ones, but more than those differentiated from the separated growing tip in the first set of experiments. Application of the ligature at the base of the second (from the shoot apex) internode caused a short delay in the next growing tip emergence. It most commonly appeared the day after completion of the hydranth differentiation upon maternal internode. In these cases, in the next developed hydranths, there were no visible differences in the number of the nematocytes in the tentacles.
Application of the ligature at a greater distance from the shoot apex – at the base of the third and beyond internode had no effect: the number of nematocytes in the experimental hydrant was the same, as was the number of tentacles and the mode of shoot axis elongation in control hydranths. Moreover, if the ligature was put close to the shoot base across the fifth to sixth internode from the apex of the shoot – within a day after ligature application the stolon tip appeared upon the internode part distal to the ligature, whereas the shoot tip emerged proximal to the ligature (Fig. 8B).
Discussion
As is well known from experiments on Hydra and other hydroids, discontinuous cell proliferation and migration are typical of this cnidarian taxon (Campbell 1967; Heimfeld & Bode 1986; Schmidt & David 1986; Kroiher et al. 1990; Schaller et al. 1990; Bosch et al. 1991; Zhang & Sarras 1994; Teragawa & Bode 1995; Kossevitch 1999). Regardless of the fact that tissue has no permanent cell composition, not only due to the renewal of the cells but also due to their constant displacement, the shape and size of the soft tissues is not highly variable. At the same time, growth of the organism and its asexual multiplication require active cell proliferation.
Colonial hydroids grow by forming additional colony elements – stolon and shoot internodes, hydranth, etc. In thecate hydroids there are special morphogenetic elements of the colony, growing tips, that shape the new elements (Crowell & Rusk 1950; Crowell 1957; Wyttenbach et al. 1965; Kosevich 1991). The growing tips have a specific organization that is different from the rest of the coenosarc (Beloussov 1973; Beloussov & Dorfman 1974; Zaraisky et al. 1984; Kosevich 2006b) and has a relatively constant cell composition, and it is generally accepted that the growing pits lack cell proliferation (Hale 1964; Crowell et al. 1965; Wyttenbach 1965; Kossevitch 1999; Marfenin et al. 1999). That means that cell multiplication takes place within coenosarc proximal to the growing tip and that tissue extension occurs somewhere there, too.
In thecate hydroids with a sympodial type of shoot growth (including Gonothyraea loveni and Laomedea flexuosa) the development of a new shoot element, the shoot internode, starts with the emergence of a next growing tip at the base of the hydranths of the maternal internode. After formation of the regular pattern of the shoot internode, the growing tip shapes the hydranth bud and finally its tissues differentiate into the hydranth (Berrill 1949a,b; Marfenin & Kosevich 1984a; Kosevich 1990). However, the tissue volume of the tip is much smaller than the tissue of the hydranth and is sufficient only for development of the hydranth tentacles and hypostome (Crowell et al. 1961; personal observations). This means that the rest of the hydranth (its gastric region and the basal part) differentiates from the tissue adjacent to the growing tip during the final stages of the hydranth development. It is probable that the cell composition of the coenosarc and the lower part of the hydranth differ due to functional differences. This interpretation implies that development and differentiation of the hydranth depend upon a definite sequence of events: cell multiplication, cell differentiation and perhaps cell migration. The purpose of the present study was to understand, at least in part, the role of and correlation between these processes. This is particularly relevant as different approaches showed lack of cell multiplication in differentiated hydranths of thecate hydroids (Donakov 1989; Makarenkova 1989).
- It confirms the relative independence of function of the growing tip. As shown by Kosevich (1991), even separation of the tip from the colony does not alter its morphogenetic program. Application of the ligature also has no strong effect on the developmental program of the shoot tip: it completes the formation of the internode and hydranth. The timing of the experimental hydranth development did not differ from that of control ones and was much shorter than the known average time of cell cycle in Hydra – 3–4 days (David & Campbell 1972; Takano et al. 1980). Therefore, the morphogenetic activity of the shoot growing tip is independent of the cell proliferation and cell migration.
- Hydranth development (differentiation) is not strictly determined but rather displays some kind of size and spatial regulation. In unaltered conditions, the tissue of the shoot growing tip transforms into the distalmost part of the hydranth – the hypostome and tentacles. In experiments with ligature application (Fig. 2) the tissue of the growing tip forms both part of the coenosarc and the entire hydranth. The hydranth has slightly smaller dimensions but is not dramatically different from the controls (Table 1). The histological examination of the experimental hydranths revealed that it is achieved mostly by cell vacuolization and flattening. Moreover, the experimental procedure had no effect on the timing of the hydranth development. This testifies to the negligible role of cell proliferation – the average time of the cell cycle in Hydra, for example, is about 3–4 days (David & Campbell 1972; Takano et al. 1980). Thus the same tissue of the growing tip, which in natural conditions occupies the most distal position within the developing hydranth bud differentiating into the hypostome and the tentacles, differentiates in experimental situations according to the relative position within the colony element formed.
- The experimental hydranths (isolation of the growing tip only) lack nematoblasts and certain gland cells. This is evidence that at least part of the cells migrate into the hydranth during or after the final steps of its differentiation. The few nematocytes present in the apexes of the tentacles differentiate from a few nematoblasts that are always wandering, even within the growing tip. The tip isolated at stage 4 (Fig. 1B) already includes most of the tissue involved in the experimental hydranth development, which explains only a slight difference from control hydranths.
- The experiments confirm the absence of cell proliferation in the hydranths of thecate hydroids. There is no multiplication of the supporting cells (epithelio-muscular epidermal and gastrodermal cells) even after a week of functioning. Thus the experimental internodes with hydranths do not give rise to the growing tip. The presence of the nerve system, even poorly developed, compared with control hydranths (Fig. 7), shows that the few i-cells found in the growing tip in histological preparations differentiate predominantly into nerve cells, but not into nematocytes. Hence those i-cells present in the growing tip are already committed to differentiate into nerve cells and do not multiply. This explains why no additional nematocytes appear in the experimental hydranth even after a week, time sufficient for i-cell multiplication and differentiation (Bosch et al. 1991; David et al. 1991), and agrees with the findings that there are no i-cells in differentiated hydranths of thecate hydroids (Polteva & Tokin 1975; Polteva & Aizenshtadt 1980).
- The continuous growth of the shoot axis under favorable conditions requires ‘support’ from at least three adjacent shoot or stolon internodes. This amount of tissue is necessary to supply the growing tip with sufficient cell material to construct new colony elements. Such a correlation between the mode of the shoot axis growth and volume of adjacent coenosarc tissues can explain the previously determined ratio between the number of the growing tips and the coenosarc length (Marfenin 1973, 1977; Marfenin & Kosevich 1984b; Kosevich & Marfenin 1986). Moreover, data from this experiment complement earlier findings, using tissue labeling, about the mode of cell migration (Kossevitch 1999).
As in all multicellular animals, the growth of the thecate hydroid colonies depends on the cell proliferation and differentiation and shaping of the new elements. The data of this work indicate that cell multiplication and differentiation spatially and temporally dissociated from the morphogenetic processes. Active continuous cell migration is the connecting link between these two constituents of colony growth.
Acknowledgements
The work was supported by the Russian Foundation for Basic Research, grant no. 05-04-48662-a and 07-04-00736-a; Russian Ministry of Education and Science, Grant Council of the President of Russia and done within the limits of FTP project ‘Scientific and scientific and pedagogical staff of innovative Russia’ in 2009–2013. The publication of this paper is supported by CONISMA, the Italian National Interuniversity Consortium for Marine Sciences.
The author is grateful to the Staff of White Sea Biological Station and Laboratory of Electron Microscopy (Faculty of Biology, Moscow State University), Prof. N. Dulin (Chicago University) and Optical Research Group (Institute of Developmental Biology RAS, Moscow) for technical assistance, and would like to thank two anonymous reviews for their constructive comments and Prof. P. Cartwright (University of Kansas) for improvement of the English.
Conflicts of Interest
The author has no potential conflicts of interest.




