Liver Involvement in Celiac Disease and Immune-Mediated Diseases of the Small Bowel
Handling Editor: Luca Valenti
Funding: The authors received no specific funding for this work.
ABSTRACT
Disorders of the hepatobiliary system are commonly associated with gastrointestinal (GI) diseases. The GI and hepatobiliary systems interact through the portal vein system and enterohepatic circulation, creating a gut–liver axis that allows for a complex multidirectional interplay between immune, hormonal, dietary and environmental luminal factors that include the gut microbiota. This interaction may underlie the liver affection in autoimmune and immune-mediated small bowel diseases through a variety of pathways that include autoimmune, metabolic, immune-mediated, and/or iatrogenic mechanisms. Despite evidence of a gut–liver axis and co-morbid liver associations with small bowel diseases, the clinical implications and how these conditions should be clinically managed remain unclear. In this narrative review, we describe the hepato-biliary manifestations associated with chronic immune-mediated enteropathies of the small bowel in adults, with particular focus on CeD and Crohn's disease.
Abbreviations
-
- AEA
-
- anti-enterocytes antibodies
-
- AIE
-
- autoimmune enteropathy
-
- aIEls
-
- aberrant intraepithelial lymphocytes
-
- AIH
-
- autoimmune hepatitis
-
- AMA
-
- antimitochondrial antibody
-
- ANA
-
- anti-nuclear antibody
-
- ASMA
-
- anti-smooth muscle antibody
-
- BMI
-
- body mass index
-
- CD
-
- Crohn's disease
-
- CeD
-
- celiac disease
-
- CT
-
- computed tomography
-
- DPG
-
- deaminated gliadin
-
- EATL
-
- enteropathy-associated T-cell lymphoma
-
- GFD
-
- gluten-free diet
-
- GI
-
- gastrointestinal
-
- HBV
-
- hepatitis B virus
-
- HCV
-
- hepatitis C virus
-
- IBD
-
- inflammatory bowel disease
-
- JAK
-
- Janus kinase
-
- MALFD
-
- metabolic-associated fatty liver disease
-
- MASH
-
- metabolic dysfunction-associated steatohepatitis
-
- MASLD
-
- metabolic dysfunction-associated steatotic liver disease
-
- MRCP
-
- magnetic resonance cholangio-pancreatography
-
- NAFLD
-
- non-alcoholic fatty liver disease
-
- NASH
-
- non-alcoholic steatohepatitis
-
- PBC
-
- primary biliary cholangitis
-
- PSC
-
- primary sclerosing cholangitis
-
- RCeD
-
- refractory celiac disease
-
- TCR γ
-
- T-cell receptor γ
-
- TNF
-
- tumour necrosis factor
-
- tTG
-
- tissue transglutaminase
-
- UC
-
- ulcerative colitis
-
- UDCA
-
- ursodeoxycholic acid
-
- ULN
-
- upper limit of normal
Summary
- The gut–liver axis allows for a complex multidirectional interplay between immune, hormonal and environmental factors. This interaction may underlie the liver affection in autoimmune and immune-mediated small bowel diseases; however, their clinical implications and management is still unclear.
- In celiac disease (CeD), hypertransaminasemia is the most common cause of liver enzyme alterations at diagnosis. Other reported conditions include viral hepatitis, associated autoimmune disorders and steatotic liver disease.
- About one-third of IBD patients report abnormal liver tests during the course of disease. Hepatobiliary disorders associated with Crohn's disease (CD) can be divided into diseases with an immune-mediated aetiology or secondary to CD-related inflammation or metabolic imbalances.
1 Introduction
In the intricate landscape of autoimmune and immune-mediated disorders, the complex interplay between distinct diseases often reveals shared pathways and associated conditions. Focusing on the gastrointestinal (GI) system, celiac disease (CeD), Crohn's disease (CD) and inflammatory conditions of the small bowel stand as prime examples of chronic immune-mediated conditions, each characterised by its specific and sometimes overlapping gastrointestinal manifestations [1, 2]. Although the exact pathogenic mechanisms remain elusive, both CeD and CD share autoimmune–immune dysregulation and impaired mucosal barrier function [3, 4]. Moreover, large-scale cohort studies and a recent meta-analysis [2] demonstrate increased risk of inflammatory bowel disease (IBD) in CeD patients and vice versa, persisting over a decade after initial diagnosis and suggesting some sort of shared pathways and triggers [2].
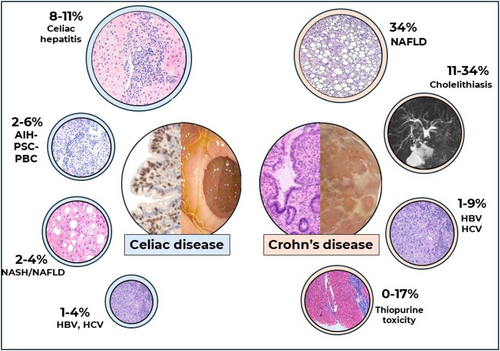
At the same time, several GI diseases, including CD and CeD, are associated with liver conditions encompassing metabolic, immune-mediated and/or iatrogenic pathogenic mechanisms [5, 6] (Figure 1). The GI and hepatobiliary systems interact through the portal vein system and enterohepatic circulation, creating a gut–liver axis that allows for a complex multidirectional interplay between immune, hormonal, dietary and environmental luminal factors that include the gut microbiota [7, 8].
Despite evidence of a gut–liver axis and co-morbid liver associations with small bowel diseases, the clinical implications and how these conditions should be managed remain unclear. In this review, we describe the literature on the hepato-biliary manifestations associated with chronic immune-mediated pathologies of the small bowel in adults, primarily focusing on CeD and CD as well as in autoimmune enteropathy and refractory CeD (RCeD).
2 Liver Pathology in Celiac Disease
CeD is an autoimmune condition affecting primarily the small bowel and induced by gluten ingestion in genetically susceptible individuals (carrying the HLA DQ2 and/or DQ8 haplotypes) [9]. CeD is the most common enteropathy, affecting about 1% of the worldwide population and the incidence is increasing over time [10]. The clinical presentation of CeD can be extremely variable, ranging from typical malabsorptive symptoms to asymptomatic or paucisymptomatic with an increase in transaminases [11]. The diagnosis is on the basis of the presence of CeD-specific serology (anti-tissue transglutaminase IgA) and, in adults, requires confirmation of the villous atrophy at duodenal histology [12, 13]. The only available treatment currently is adopting a strict gluten-free diet (GFD), which can lead to regression of symptoms and histological-serological alterations [9, 14]. Because it is a systemic disease, it has many clinical manifestations and associations [15, 16].
Liver abnormalities in CeD include the common finding of hypertransaminasemia, found in up to 40% of patients at the time of diagnosis [17], the so-called celiac hepatitis, viral hepatitis, associated autoimmune disorders, hepatic hemangioma [18] or non-alcoholic fatty liver disease [19, 20] (NAFLD), now included in the category of metabolic dysfunction-associated steatotic liver disease (MASLD) [21]. Vascular liver disease as porto-sinusoidal vascular disease (PSVD) has been rarely described in CeD [21], although CeD has been reported in 2.4% of patients with PSVD [22].
Celiac hepatitis is defined as abnormal liver function tests and/or histological changes on liver biopsy, in the absence of other causes, which resolves with a GFD [20]. In addition, autoimmune diseases such as autoimmune hepatitis (AIH), primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) can coexist with CeD and are non-responsive to the GFD [19, 20]. MASLD, the most common liver disease with a worldwide prevalence of 25% [23], presents hepatic steatosis or fat deposition in more than 5% of hepatocytes, mainly associated with a diet high in calories and fat, and has at least one of the five cardiometabolic risk factors [24-26]. MASLD [23] can manifest with non-alcoholic steatohepatitis (NASH) (now called metabolic dysfunction-associated steatohepatitis, MASH [27]) in 7% to 30% of the US population [28]. CeD can be present in patients with MASH [29], and therefore, international guidelines recommend investigating CeD in MASLD/MASH of unclear origin [30, 31] (as in patients with unexplained hypertransaminasemia) [32, 33]. The prevalence of CeD in patients with MASLD has been reported between 3.4% and 13% in the Italian population [33] and 7% in the Egyptian population [34], whereas the presence of positive tissue transglutaminase antibody (anti-tTG) in chronic liver disease was even higher, 26.8% in an Italian study that included 19 patients [35]. In a systematic review, the prevalence of CeD in AIH was 4% [36]. Other conditions, such as cholestatic autoimmune diseases, have been reported increased among patients with CeD. In a Swedish cohort of 13 818 CeD patients, the CeD population had a four-fold increased risk of PSC compared to non CeD [37]. Furthermore, 10% to 15% of patients have overlap syndrome, which is a combination of AIH and PBC with CeD [36]. Mechanistically, the association of liver pathology in CeD has been attributed to a dysregulated liver–gut axis, which may include signalling by the intestinal microbiota [38]. Increased intestinal permeability to luminal antigens such as immunogenic peptides, and bacterial products or metabolites [39] may reach the liver through the portal circulation triggering a pro-inflammatory immune response [40], which ranges from fatty infiltration to chronic active hepatitis and cirrhosis [41]. Once the GFD is initiated, most of these changes have been reported to revert to normal [5].
Adopting a strict GFD has been shown to effectively revert celiac hepatitis [20]. However, other factors such as age, sex and body mass index could contribute to the persistent elevation of liver enzymes in CeD and the development of MASLD, especially in CeD patients with body mass index (BMI) over 25, overweight and obese [42, 43]. It is also important to consider that a GFD on the basis of gluten-free counterparts that are rich in sugars and fat can promote metabolic alterations such as hepatic steatosis, hyperinsulinism, diabetes mellitus, hypertension and dyslipidemia [44-48]. Indeed, in 2005, a prospective Italian study of 221 newly diagnosed CeD patients identified NAFLD in 29.4% and MASLD in 14.5% of patients. After 2 years of follow-up, the prevalence of both NAFLD and MASLD increased to 46 and 32%, respectively [49]. The study highlights the need to monitor the nutritional adequacy of GFD, particularly by encouraging naturally gluten-free foods, to minimise highly refined carbohydrates and saturated fat and promote healthy lifestyle habits such as increasing physical activity [50, 51].
2.1 Liver Pathology in Refractory Celiac Disease
Refractory celiac disease (RCeD) is a rare complication of CeD, defined as the persistence or recurrence of malabsorption and villous atrophy despite adherence to GFD for at least 12 months, once other causes of villous atrophy and malignancy have been excluded [52-55]. The estimated cumulative incidence of RCeD is about 1%, affecting around 0.01% of the population [56, 57].
There are two subtypes of RCeD, type I and type II, according to duodenal histological, molecular (clonal T-cell receptor-γ, TCRγ, rearrangements) and flow cytometry (detection of aberrant phenotype intraepithelial lymphocytes, aIELs) features [56, 58]. Particularly, RCeD type II is now considered a pre-T-Cell lymphoma with an “in situ” clonal expansion of aIELs and a 33% to 53% risk of developing enteropathy-associated T-cell lymphoma (EATL) at 5 years from the diagnosis [56, 59, 60]. RCeD type I has a better prognosis, typically characterised by a less severe intestinal insufficiency and lower risk of malignant complications [57]. No curative treatment is currently available for RCeD. To date, therapy is based on immunosuppressive (steroids, thiopurines, infliximab, anti-Janus Kinase) or ablative treatments (cladribine, high-dose chemotherapy followed by stem cell transplantation) and nutritional support [15].
The frequency of liver affection in RCeD is unknown because of the rarity of the condition. Most data on RCeD and its complications come from retrospective studies, case series and case reports. Similarly to responsive CeD [61, 62], in RCeD, there is a higher incidence of autoimmune diseases. For instance, Weber et al. [63] have observed in their cohort of 46 RCeD that 20 (43.5%) of them had another AI disease, including 1 patient with AIH (2.3%). A similar finding was presented in an Italian retrospective study [64] with 13 out of 37 RCeD patients (35%) with an autoimmune disease, of which 3 (8%) had autoimmune liver disease. However, the nature of these studies and their sample size pose major limitations, and further studies are required to confirm these findings. Another form of liver involvement in this group of patients, specifically in RCeD type II, could be related to liver enzymes' alterations due to aIELs migration to the liver [65]. Indeed, in RCeD type II, aIELs have been observed also in the duodenal lamina propria [66-69]. They are not only disseminated in the small intestine, but they can also have extra-intestinal localisations. Lymphocytes presenting the same aberrant phenotype of duodenal IELs have been detected in blood flow cytometry [66, 69], as well as at the level of the skin, lungs and liver [70]. Specifically, in a case report, the authors described aIELs parenchymal infiltrate with the same phenotype as those found in the duodenal mucosa of a patient with a recent diagnosis of RCeD type II [71]. Therefore, altered liver function tests in RCeD with suspected EATL should raise the suspicion of a possible hepatic disease localisation. This rare T-cell lymphoma is diagnosed half of the time in an emergency setting because of perforation, occlusion or haemorrhage and at a late stage, stage III/IV, with liver metastasis in 40%–60% of cases [72-74]. The primary site of EATL location is the small bowel in 90%–95% of cases, often with multifocal intestinal involvement. However, in a small portion of patients, EATL can present on extraintestinal sites, most frequently from RCeD type II skin lesions [75].
2.2 Management of Liver Function Test Alterations in Celiac Disease and Refractory Celiac Disease
The initial approach to patients with incidental elevation of transaminases (> 2–3 times the upper limit of normal, ULN) of unclear origin includes CeD-specific serology and the exclusion of the most frequent causes such as medications, hepatitis B and C (HBsAg and anti-HCV), hemochromatosis (serum ferritin and transferrin saturation), AIH (anti-nuclear antibodies, ANA, and anti-smooth muscle antibodies, ASMA) and PBC (antimitochondrial antibodies, AMA) [19]. Table 1 reports the etiologies of the liver disease in CD patients with hepatic involvement. An abdominal ultrasound to investigate the hepatic morphology and rule out fatty liver or changes highly suggestive of chronic liver disease cirrhosis such as hepatic surface nodularity or a coarsened echotexture is recommended [49]. The clinical assessment should evaluate other factors and conditions promoting liver disease such as alcohol intake and metabolic diseases including Wilson's disease (ceruloplasmin) and alpha-1 antitrypsin deficit [77].
| Liver disease | Etiologic prevalence (%) among celiac patients with a liver disease | Screening tests for diagnosis of liver disorder in celiac disease |
|---|---|---|
| Celiac hepatitis | 49 | Improvement on GFD |
| Hepatitis B | 22 | HBsAg |
| Hepatitis C | 21 | Anti HCV antibodies |
| Autoimmune hepatitis | 17 | ANA, ASMA |
| Alcoholic steatohepatitis | 16 | History of alcohol intake |
| Primary sclerosing cholangitis | 9 | ANCA, ANA, ASMA, anti-endothelial, anti-cardiolipin, MRCP if positive |
| Primary biliary cholangitis | 7 | AMA, MRCP if positive |
| MASLD/MASH | 6 | Liver ultrasound |
| Budd Chiari syndrome | 4 | Liver ultrasound with doppler |
- Abbreviations: ANA, anti-nuclear antibody; ANCA, anti-neutrophil cytoplasmic antibody; ASMA, anti-smooth muscle antibody; MRCP, magnetic resonance biliary tract and pancreas.
The initial monitoring of CeD patients with hepatic involvement can be done similarly to patients with other comorbidities. This approach includes the measurement of liver transaminases (ALT and AST), alkaline phosphatase and gamma-glutamyl transpeptidase. In the case of elevation of transaminases up to 3 to 5 times ULN, without evidence of chronic liver disease, this could be considered as a pattern secondary to celiac hepatitis [20]. Once a strict GFD is initiated, liver enzymes should be repeated after 6 to 12 months to assess response to therapy [78, 79]. However, there is a subset of patients in whom liver transaminases will remain elevated after 1 year of GFD. Therefore, a thorough assessment to exclude other hepatic complications such as MASLD, alcoholic liver disease, chronic viral hepatitis, autoimmune liver disease, and drug or toxin-related injury (see Table 1). The management of associated liver disease in patients with celiac hepatitis is like that of patients without CeD and can be supported by the use of non-invasive tests for liver fibrosis, such as transient elastography and serum biomarkers. These methods reduce the need for liver biopsies, providing safer and faster evaluations. Transient elastography has been used in patients with CeD, showing alterations in a subset of patients with increased transaminases and an improvement in patients on a GFD [80]. The presence of portal hypertension, with a normal transient elastography (< 10 Kpa) is suggestive of PVSD; usually, PSVD is also associated with high spleen stiffness [81]. Serum biomarkers can be useful in the early detection of liver fibrosis, which is crucial for managing liver complications, although untested in CeD. In spite of the absence of clear evidence, monitoring with non-invasive tests could improve patient outcomes and treatment decisions in selected cases [82].
The approach to liver enzymes alterations in RCeD is similar to those without CeD. At the time of RCeD diagnosis, patients have already performed most of the first-line tests excluding the most frequent causes of liver disease [15, 83]. In the case of diagnosis of AIH in an RCeD patient, it should be underlined that a multidisciplinary approach and shared strategy are crucial as both conditions are treated with immunosuppressive drugs including corticosteroids, azathioprine and methotrexate [84, 85].
In RCeD patients presenting with severe malnutrition, in particular RCeD type II, the alteration of transaminases and/or cholestasis should raise suspicion for an underlying neoplastic condition with liver involvement [15, 52, 53, 83]. A liver biopsy is required to confirm the diagnosis with an evaluation of liver lymphocyte infiltrate phenotype using immunohistochemistry (or when available, in off-label settings, employing flow-cytometry) [86]. The work-up should also include blood tests with ferritin, triglycerides, lactate dehydrogenase, liver function tests and complete blood count to exclude haemophagocytic lymphohistiocytosis.
3 Liver Pathology in Crohn's Disease
IBD is a chronic and progressive inflammatory disease of the gastrointestinal tract, with increasing incidence worldwide and caused by the interplay between genetics, environmental factors and intestinal microbiota that lead to an altered epithelial barrier function and abnormal mucosal immune response [87]. CD is a main form of IBD affecting the small bowel. CD is characterised by segmental and transmural inflammation, potentially affecting any part of the digestive tract; the small bowel being involved in about 80% of patients [87]. Symptoms can include abdominal pain, diarrhoea, fatigue and weight loss, occurring in relapsing and remitting manner. CD can also lead to complications, such as bowel obstruction or fistulas, requiring surgery. During the course of the disease about one-third of IBD patients will report abnormal liver tests and 5% of them will develop a chronic liver disease [88]. Moreover, mild and transient abnormalities of liver biochemical tests seem to be correlated with a worse long-term prognosis [89]. Hepatobiliary disorders associated with CD can be divided into diseases with an immune-mediated aetiology or conditions caused by inflammation or metabolic imbalances secondary to CD (Table 2).
| Liver disease | Prevalence (%) | Screening tests for diagnosis of liver disorder in Crohn's disease |
|---|---|---|
| MASLD/MASH | 34 | Liver ultrasound |
| Cholelithiasis | 11–34 | Liver ultrasound, MRCP |
| Hepatitis B | 1–9 | HbsAg |
| Hepatitis C | 1–9 | Anti HCV antibodies |
| 5-ASA toxicity | 0–4 | Exclude other causes; resolution after drug withdrawal |
| Thiopurines toxicity | 0–17 | Exclude other causes; resolution after drug withdrawal |
| Primary sclerosing cholangitis | 3–4 | ANCA, ANA, ASMA, anti-endothelial, anti-cardiolipin, MRCP if positive |
| Autoimmune hepatitis | NA | ANA, ASMA |
| Primary biliary cholangitis | NA | AMA, MRCP if positive |
| Portal vein thrombosis | 1 | Liver ultrasound with doppler |
| Liver abscess | NA | Liver ultrasound |
- Abbreviations: ANA, anti-nuclear antibody; ANCA, anti-neutrophil cytoplasmic antibody; ASMA, anti-smooth muscle antibody; CT, computed tomography; LKM, liver kidney microsomal antibodies; MRCP, magnetic resonance biliary tract and pancreas.
3.1 Non-Immune-Mediated Liver Disorders Associated With Crohn's Disease
MASLD is a growing cause of chronic liver disease and is considered the most common liver disease in patients with CD. According to a recent systematic review [90], the pooled prevalence of MASLD in CD patients is 34.4%, with a risk two times higher in IBD patients versus healthy controls. The prevalence of advanced liver fibrosis was reported at 14% [90]. CD patients with MASLD have fewer metabolic risk factors than patients with MASLD alone, suggesting that CD itself is a risk factor for developing MASLD [91]. Indeed, Mancina et al. [92] showed that PNPLA3 148 M allele carriers with IBD had a higher risk for hepatic steatosis. A cross-sectional study conducted by McHenry et al. [93] with a cohort of 311 CD patients compared with 1244 controls have found CD as an independent risk factor of MASLD. Specific risk factors such as disease duration and previous surgery were reported, whereas MASLD development seems not to be affected by the treatment [94].
Prevalence of cholelithiasis in CD ranges from 11% to 34% [88]. A population-based study showed a significantly higher prevalence of cholelithiasis in CD patients than in control subjects [95]. Ileitis and ileal resection in CD seem to be risk factors for cholelithiasis [88], because of bile salt malabsorption leading to alteration in bile composition.
Furthermore, patients with CD are at increased risk of venous thromboembolism and the portal vein is a common site of thrombosis [96]. Many risk factors have been described such as extent of disease, surgery, disease activity, use of steroids and smoking [6]. Thus, thromboprophylaxis with anticoagulants is recommended in hospitalised patients with active CD and after surgery [97].
Liver abscesses are a rare complication of CD. They can result from direct extension of an intra-abdominal abscess or be secondary to bacterial translocation [98]. Persistent trans-mural inflammation, fistulising phenotype, long-term steroid use and malnutrition are the main risk factors reported. Recurrent fever is the most common symptom associated with abdominal pain in about half of patients. Blood tests are usually not specific, and diagnosis is made with ultrasound or computed tomography (CT) scan [99]. The treatment is based on antibiotic therapy and drainage in case of a large abscess [100].
More recently, in a large cohort patients with PSVD, 5.6% presented a concomitant unspecified IBD suggestive a possible rare association [22].
3.2 Immune-Mediated Liver Disorders Associated With Crohn's Disease
Different immunomediated liver diseases are associated with CD. Primary sclerosing cholangitis (PSC) is characterised by chronic inflammation of intra- and/or extra-hepatic bile ducts leading to biliary strictures and may result in end-stage liver disease requiring a liver transplant [101]. PSC patients have a significantly higher risk of developing cholangiocarcinoma and gallbladder cancer [99, 101]. The prevalence of PSC in patients with CD ranges from 1.2% to 3.4% [88]. A milder course of IBD and involvement of the right colon and terminal ileum is often present [102]. The histological findings compatible with PSC are peri-ductal fibrosis, fibro-obliterative cholangitis, ductular reaction, peri-ductal inflammation and ductopenia [103]. Small duct PSC is a variant of PSC characterised by abnormal liver biochemistry with a cholestatic pattern and histological findings of PSC but normal cholangiography [101]. The long-term prognosis of small duct PSC is better with a reduced risk of progression to end-stage liver disease and of developing cholangiocarcinoma [101]. The prevalence of small duct PSC is higher in CD patients than in ‘classic’ PSC [104]. CD and PSC seem to have an independent course, and CD therapies do not influence the progress of PSC.
Only a few case series have described the association between CD and other immune-mediated liver diseases such as AIH and PBC, with some patients affected by overlap syndromes presenting with common features between two entities, AIH-PSC being the most common [104, 105].
3.3 Drug-Induced Liver Injury in Crohn's Disease
Different immunosuppressive drugs are used for CD and are associated with hepatotoxicity. Methotrexate is known to cause liver injury ranging from asymptomatic hypertransaminasemia to fibrosis and cirrhosis, especially in prolonged use and/or high doses [106]. Hepatotoxic effects are thought to be linked to folate depletion and oxidative stress, which disrupts cellular antioxidant defences. Histological changes in the liver due to methotrexate are graded using the Roenigk classification system, which ranges from fatty infiltration to cirrhosis. Folic acid supplementation may reduce the risk of elevated transaminases, although its efficacy in preventing severe hepatotoxicity remains inconclusive [107].
Azathioprine-induced hepatotoxicity is less common but can manifest as hepatitis, cholestasis or mixed patterns of liver injury [108, 109]. The mechanism involves mitochondrial dysfunction and depletion of intracellular glutathione, leading to necrosis and impaired ATP production. Elevated levels of its metabolite, 6-methylmercaptopurine nucleotide (6-MMPN), are associated with hepatotoxicity. Discontinuation of azathioprine typically leads to recovery of liver function. Co-treatment with antioxidants like N-acetyl-L-cysteine has shown protective effects against mitochondrial damage caused by azathioprine [110]. A recent study on a large cohort of patients with PSVD described the presence of an azathioprine treatment in 3.4% of the subjects; most of them had a concomitant unspecified IBD [22].
5-Aminosalicylic acids (5-ASAs) can cause rare (but serious) liver injury. Hepatotoxicity may present as acute hepatitis or cholestatic injury. The mechanism is not fully understood but may involve idiosyncratic reactions or direct toxicity to hepatocytes. Monitoring liver enzymes during treatment is recommended to detect early signs of liver damage [111]. Monoclonal antibody targeting TNF-alpha has been associated with mild elevations in liver enzymes and rare cases of severe hepatotoxicity such as autoimmune hepatitis-like syndromes. The exact mechanism remains unclear but may involve immune-mediated responses. Discontinuation of infliximab and corticosteroid therapy often results in resolution of liver injury [112, 113].
3.4 Management of Liver Function Test Alterations in Crohn's Disease
CD patients should be routinely screened for liver function test abnormalities [100]. If liver test alterations are detected, drug-induced liver injury because of CD treatment (e.g., thiopurines, methotrexate or anti-TNF drugs) and viral hepatitis, especially HBV and HCV related, should be ruled out. These conditions are beyond the scope of our reviews. Most patients with PSC are initially asymptomatic; thus, diagnosis is based on biochemical alterations and radiological features. PSC should be excluded in every CD patient with persistent or fluctuating alteration of liver biochemical tests with a cholestatic pattern [114]. Magnetic resonance cholangiopancreatography (MRCP) has high sensitivity and specificity (86% and 94% respectively) in identifying PSC [115]; therefore, it should be performed in CD patients with suspected PSC [114]. A liver biopsy is usually not required to assess a diagnosis of PSC unless there is a suspicion of small duct PSC [103]. Patients with evidence of small or large-duct PSC should be considered for liver transplant evaluation, not only in the presence of portal hypertensive complications such as ascites or oesophageal varices but also in the presence of two or more episodes of bacterial cholangitis [114, 116]. No specific therapy for PSC is currently available; ursodeoxycholic acid (UDCA) at the dose of 15–20 mg/Kg/daily has a beneficial effect on cholestasis but fails to demonstrate a reduction of mortality or an increase of liver transplant-free survival [117]. Some retrospective studies have evaluated the efficacy of biologic therapies in patients with concomitant IBD. Infliximab, a monoclonal antibody directed against tumour necrosis factor-alpha (TNFα), was effective in the treatment of IBD associated with PSC but has not shown any improvement on liver biochemistry or imaging, considered as a prognostic surrogate marker of PSC [118]. Adalimumab, another monoclonal anti-TNFα agent, has shown a significant decrease of alkaline phosphatase compared to Infliximab and Vedolizumab after 6–8 months of treatment; however, this improvement was not confirmed after 12–14 months [118]. A retrospective study has suggested a potential specific role of Adalimumab in the improvement of cholestasis in PSC [119]. However, for both biologics, there is a lack of data on clinical outcomes and long-term prognosis in PSC. Vedolizumab is a humanised monoclonal antibody directed against the human lymphocyte α4β7 integrin specific to gut-homing lymphocytes. This peculiar mechanism of action was encouraging but failed to show improvement in liver biochemistry. In the largest cohort published by Lynch et al. [120], the mean alkaline phosphatase level was slightly increased after treatment with vedolizumab and 21% of the patients had liver-related events, including cholangitis, cirrhosis decompensation or liver transplantation [120]. Some concerns were raised by the study of Caron et al. that showed the occurrence of digestive neoplasia in 10% of patients during a follow-up of 19 months [121]. However, the retrospective nature of the studies and the lack of a control group make these findings difficult to interpret.
Diagnosis of AIH is based on biochemical tests and histology. The treatment is based on immunosuppression and the response to treatment is not affected by the presence of CD [122].
Anti-mitochondrial antibodies are usually detected for PBC diagnosis. Moreover, the evidence of elevated immunoglobulin M is also helpful in identifying this group of patients. The treatment is based on the use of ursodeoxycholic acid with a high rate of response [123].
4 Liver Pathology in Autoimmune Enteropathy (AIE)
AIE is a rare form of chronic atrophic enteropathy. Firstly, it was described in children affected by immune dysregulation syndromes [124]; more recently, it has been reported also in adults [125-129].
It is characterised by severe malabsorption-related symptoms, not responsive to any dietary restriction, and specific histological features [129, 130]. The presence of autoantibodies, namely, anti-enterocytes IgA/IgG and anti-goblet cells IgA/IgG, as necessary or highly supportive AIE diagnostic criteria is still debated [1, 129]. Treatment of AIE can be challenging and requires a multidisciplinary approach. Medical therapy is based on nutritional support, fundamental as most of times parenteral nutrition is necessary, and immunosuppressive treatment. Specifically, first-line treatments are usually corticosteroids [131], either systemic or open capsule budesonide, followed by calcineurin inhibitors in case of failure to respond [130, 131]. Recently, there have been case reports of successful use of infliximab in refractory AIE, both in children and adults [132, 133].
Because of its rarity and relatively recent recognition in adults, knowledge of AIE in this setting of patients is scarce and on the basis of case series or retrospective studies (Table 3). AIE can be associated with other AI conditions including AI liver diseases [130, 134, 136, 135]. Ahmed et al. [135] report that 30 out of their 41 adult AIE patients cohort had at least another AI disease, with 2 of them having an autoimmune liver disease (1 autoimmune hepatitis, 1 primary sclerosing cholangitis). Also, Sharma et al. [130] in their case–control retrospective study observed that among their 30 AIE patients, 13 had another AI disease, in particular 3 autoimmune hepatitis and 2 primary sclerosing cholangitis. A slightly lower incidence rate was described by Villanacci et al.'s [136] retrospective study, with 3 out of 17 AIE patients having a coexistent AI disease, of which 1 had PSC. Finally, there are also few case reports on patients with AIE and autoimmune hepatitis diagnosis. In particular, Iaquinto et al. [134] described a case of AIE arising in a patient with a previous diagnosis of autoimmune hepatitis with both ANA and anti-enterocytes autoantibody (AEA) positivity. Another similar case was described by Bishu et al. [136] of AIE associated with autoimmune hepatitis, but without either AEA or ANA positivity. For what concerns other liver diseases in AIE, there are no data in the published literature.
| Study | Sharma et al., 2018 [130] | Iaquito et al., 2021 [134] | Ahmed et al., 2019 [135] | Villanacci et al., 2019 [136] | van Wanrooij et al., 2021 [128] | Overall |
|---|---|---|---|---|---|---|
| Number of patients | 30 | 56 | 41 | 17 | 13 | 157 |
| Sex (F/M) | 12/18 | 34/22 | 24/17 | 9/8 | 8/5 | 87/70 |
| Age (mean, SD when available) | 44 (18) | F 55 (17); M 50 (17) | NA (range: 21–82) | 43 (NA) | 52 (NA) | |
| AI-associated disease (%) | 13 (43%) | 37 (66%) | 30 (73%) | 3 (18%) | 6/10 (60%) | 89/154 (58%) |
| Liver disease | 5 (38%) (3 AIH, 2 PSC) | 1 (3%) (AIH) | 2 (7%) (1 AIH, 1 PSC) | 1 (33%) (PSC) | 0 | 9/89 (10%) |
| Anti-enterocytes Ab+ | 16/29 | 34/48 | 35/41 | 1/13 | 10/12 | 96/143 |
There are no guidelines or consensus published on the follow-up and long-term management of AIE in adult patients and the modalities and frequency of follow-up tests and visits are not clearly defined [124]. For the same reasons, there are no indications of screening tests for other AI conditions differently from, for example, diabetes mellitus type I [137], CeD patients [15] or paediatric patients with AIE [138, 139]. The approach to liver function tests alterations in AIE is similar to other patients, as described in the previous sections. Being AIE an autoimmune disease, an increase in LFTs in AIE patients should always raise suspicion for AIH, PSC or PBC. In these cases, ANA is the most common antibody in AIE adult patients, being observed in 27%–60% of cases [134]. Unfortunately, most of the studies did not report the antibodies' titre.
5 Conclusions
In this review, we presented a comprehensive and up-to-date overview of the clinical manifestations, diagnosis and management of liver diseases associated with the major autoimmune/immune-mediated small bowel enteropathies. These conditions often coexist, some being directly associated with chronic immune-mediated diseases themselves, such as in the case of hypertransaminasemia related to CeD, or PSC in IBD, whereas others being related to the primary disease treatment or patients' lifestyle, for example, drug-related hepatitis. Thus, in patients with CeD and CD, annual or bi-annual monitoring with liver function tests should be performed to ensure early detection and treatment of liver-related complications. In these patients, a multidisciplinary approach is indispensable, requiring the involvement of gastroenterologists, hepatologists, immunologists, radiologists and pathologists. This integrated approach is crucial for optimising patient outcomes, as it allows for the timely identification and management of complications that may arise from either the liver or the small bowel disease.
Notably, the literature on liver involvement in immune-mediated small bowel diseases, such as autoimmune enteropathy and refractory celiac disease, remains limited. This scarcity hinders a comprehensive understanding of the gut–liver axis and its clinical implications, particularly in managing co-morbid hepatic conditions. Additionally, the reliance on retrospective studies, small sample sizes and tertiary referral centres scenarios introduces potential biases, including selection bias and publication bias, which may distort findings and limit generalisability. Thus, further research is needed to address these gaps and validate existing observations.
Author Contributions
The authors confirm contribution to the paper as follows: conceptualization: Luca Elli, Nicoletta Nandi and Elena F. Verdu. Resources: Nicoletta Nandi, Andres Jose Gomez-Aldana, Francesco Simone Conforti and Marco Maggioni. Writing-original draft: Nicoletta Nandi, Andres Jose Gomez-Aldana, Francesco Simone Conforti, Maria Ines Pinto Sanchez and Elena F. Verdu. Writing – review and editing: Luca Elli, Elena F. Verdu, Detlef Schuppan and Flavio Caprioli. All authors reviewed the results and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.