Novel Emerging Mechanisms in Acetaminophen (APAP) Hepatotoxicity
Alejandro Hionides-Gutierrez, Naroa Goikoetxea-Usandizaga authors contributed equally to this work as first.
María L Martínez-Chantar, Francisco Javier Cubero authors contributed equally to this work as senior.
Funding: This work was supported by grants from Ministerio de Ciencia, Innovación y Universidades MICINN: PID2020-117116RB-I00, PID2020-113299-I00, CEX2021-001136-S integrado en el Plan Estatal de Investigación Científica y Técnica e Innovación, cofinanciado con Fondos FEDER (for M.L.M-C); Ministerio de Ciencia e Innovación, Programa Retos-Colaboración RTC2019-007125-1 (for M.L.M-C); Instituto de Salud Carlos III, Proyectos Investigación en Salud DTS20/00138 (for M.L.M-C); La Caixa Scientific Foundation (HR17-00601) (for M.L.M-C); La Caixa Consortium (for M.L.M-C); Ayudas Fundación Científica AECC para proyectos coordinados (IGTP-AECC_2022–042) (for MLM-C); Transferencia tecnológica 2022 (6/12/TT/2022/00001) (for M.L.M-C); Desarrollo Tecnológico en Salud (DTS20/00138) (for MLM-C); Ayudas a proyectos de investigación y desarrollo en salud (2023333041) (for M.L.M-C); Health Research 2017 (HR17-00601) (for M.L.M-C); Caixa Impulse Innovation 2023 (CI23-20155) (for M.L.M-C). MICINN Retos PID2020-11782RB-I00 MICIU/AEI/ 10.13039/501100011033 all of which were co-financed with Fondos FEDER (for F.J.C.), EXOHEP2-CM (S2022/BMD-7409) (for F.J.C.), and the HORIZON-HLTH-2022-STAYHLTH-02 under agreement No 101095679 (for F.J.C.). CSG is an Atracción de Talento (CAM) 2019 2019-T1/BMD-13313. AH is a recipient of a UCM Real Colegio Complutense (RCC) Harvard—Santander scholarships CT15/23.
Handling Editor: Luca Valenti
ABSTRACT
Background
Drug-induced liver injury represents a critical public health issue, marked by unpredictable and potentially severe adverse reactions to medications, herbal products or dietary supplements.
Aims
Acetaminophen is notably a leading cause of hepatotoxicity, impacting over one million individuals worldwide.
Materials & Methods
Extensive research has elucidated the intricate mechanisms driving APAP-induced liver injury, emphasising the significant roles of endoplasmic reticulum stress, oxidative stress, mitochondrial dysfunction and cell death.
Results
These insights pave the way for innovative therapeutic strategies, including the use of magnesium, bile acids, microbiota modulation and mesenchymal stem cells.
Discussion & Conclusion
This review explores into these pathological mechanisms, proposing viable therapeutic interventions for patients suffering from APAP-induced liver injury.
Summary
- Given the limited therapeutic window of N-acetylcysteine (NAC), there is an urgent need for treatments targeting both the early and late stages of AILI.
- Specific Mg2+ transporters, modulators of bile acid transporters and modification of gut microbiota are emerging as interesting therapeutic targets in APAP hepatotoxicity.
- In addition, mesenchymal stem cells (MSCs) represent a promising avenue for treating severe liver injury.
- The modulation of endoplasmic reticulum (ER) and oxidative stress, mitochondrial dysfunction via the activation of mitochondrial fussion and mitophagy, as well as cell death are emerging pathological mechanisms that will give yield to novel therapeutic avenues for patients with DILI.
Abbreviations
-
- ACLF
-
- acute-on-chronic liver failure
-
- ACSL4
-
- acyl-CoA synthetase long chain family member 4
-
- AILI
-
- APAP-induced liver injury
-
- AKT
-
- protein kinase B
-
- ALF
-
- acute liver failure
-
- ALT
-
- alanine aminotransferase
-
- AMPK
-
- AMP-activated protein kinase
-
- APAP
-
- acetaminophen
-
- APC
-
- antigen presenting cell
-
- AST
-
- aspartate aminotransferase
-
- ATG
-
- autophagy-related Parkin or autophagy-related
-
- BA
-
- bile acids
-
- CAR
-
- constitutive androstane receptors
-
- CHOP
-
- CCAAT enhancer-binding protein homologous protein
-
- CXCR
-
- CXCL10-C-X-C chemokine
-
- CYP
-
- cytochrome
-
- DAMPS
-
- danger-associated molecular patterns
-
- DCs
-
- dendritic cells
-
- DILI
-
- drug induced liver injury
-
- ER
-
- endoplasmic reticulum
-
- ETC
-
- electron transport chain
-
- FAO
-
- fatty acid oxidation
-
- FGF21
-
- fibroblast growth factor-21
-
- FPR1
-
- formyl peptide receptor-1
-
- GPx
-
- glutathione peroxidase
-
- GSH
-
- glutathione
-
- GSK3β
-
- glycogen synthase kinase 3
-
- HBV
-
- hepatitis B virus
-
- HGF
-
- hepatocyte growth factor
-
- HLA
-
- human leukocyte antigens
-
- HMGB1
-
- high-mobility group protein B1
-
- HO-1
-
- heme oxigenase-1
-
- H2O2
-
- hydrogen peroxide
-
- HSP70
-
- heat-shock protein-70
-
- iDILI
-
- idiosyncratic drug-induced liver injury
-
- ILCs
-
- innate lymphoid cells
-
- IM
-
- intestinal microbiota
-
- JNK
-
- C-Jun N terminal kinase
-
- KC
-
- kupffer cells
-
- LXR
-
- liver X receptors
-
- MAPKs
-
- mitogen-activated protein kinases
-
- MASLD
-
- metabolic dysfunction-associated steatotic liver disease
-
- MCFU
-
- mitochondrial Fe+2 uniporter
-
- MFN
-
- mitofusin
-
- MLKL
-
- mixed lineage kinase domain like pseudokinase
-
- MnSOD
-
- manganese superoxide dismutase
-
- MOMP
-
- mitochondrial outer membrane permeabilisation
-
- MPO
-
- myeloperoxidase
-
- MPT
-
- mitochondrial permeability transition
-
- MSCs
-
- mesenchymal stem cells
-
- mtFAO
-
- mitochondrial fatty acid oxidation
-
- mTOR
-
- mechanistic target of rapamycin kinase
-
- NAC
-
- N-acetylcysteine
-
- NAPQI
-
- N-acetyl-p benzoquinone imine
-
- NK
-
- natural killer cells
-
- NMDA
-
- N-methyl-D-aspartate
-
- NO
-
- nitric oxide
-
- NPC
-
- non-parenchymal cells
-
- NQO1
-
- quinone oxidoreductase-1
-
- NRF2
-
- nuclear factor erythroid 2-related factor
-
- ONOO−
-
- peroxynitrite radicals
-
- OXPHOS
-
- oxidative phosphorylation
-
- PGC1α
-
- proliferator-activated receptor coactivator-1α
-
- PI3K
-
- phospho-inositol 3 kinase
-
- PINK1
-
- PTEN induced kinase-1
-
- PRX
-
- peroxiredoxin
-
- PTP1B
-
- protein tyrosine phosphatase-1B
-
- PXR
-
- pregnane X Receptor
-
- RIPK
-
- receptor interacting serine/threonine kinase
-
- ROS
-
- reactive oxygen species
-
- SHK
-
- shikimic acid
-
- Th
-
- T helper cell
-
- TLR
-
- toll like receptor
-
- TNF
-
- tumour necrosis factor
-
- Treg
-
- T regulatory cell
-
- UC MSCs
-
- umbilical cord-derived MSCs
-
- UPR
-
- unfolded protein response
-
- VEGF
-
- vascular endothelial growth factor
-
- XBP-1
-
- X box binding protein 1
-
- 5-LO
-
- 5-lipoxygenase
1 Introduction
Drug-induced liver injury (DILI) is a significant public health concern, characterised by an unpredictable and potentially severe adverse reaction to medications, herbal products or dietary supplements. The liver is the responsible organ for metabolising and eliminating xenobiotics, and, thus, the prime target for medication-induced damage. Despite its relative safety, drug hepatotoxicity accounts for over 45% of all acute liver failure (ALF) cases in the United States and affects between 1 to 1.5 million people globally, as recently reported [1-3].
DILI can exhibit different ways including hepatocellular, cholestatic and mixed liver injury, each with distinct clinical and pathological characteristics [4]. Hepatocellular liver injury is the most prevalent type and it is characterised by hepatocyte necrosis, hepatic inflammation and significant elevation of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels [4]. Cholestatic liver injury is characterised by cholestasis in the capillary bile ducts, often accompanied by jaundice, bile duct hyperplasia or injury, and portal phlebitis. Mixed liver injury shows qualities of both hepatocellular and cholestatic liver injury [5].
Whereas the mechanisms underlying APAP-induced hepatotoxicity have been extensively studied, revealing a complex interplay of factors influencing the development and severity of liver injury, the pathomechanisms remain elusive and the identification of new signalling pathways is continuously emerging which might give way to novel therapeutic avenues for DILI patients.
2 Pathomechanisms in APAP-Induced Liver Injury (AILI)
2.1 Current Drug Metabolism Pathways
When APAP is taken at therapeutic doses, approximately 85%–90% is metabolised by phase II conjugating enzymes and excreted in the urine. Specifically, about 50% of APAP is converted to APAP-glucuronide by UDP-glucuronosyltransferase, and around 30% is converted to APAP-sulphate by sulphotransferase [6, 7]. About 2% of APAP is excreted unchanged in the urine. Only 5%–9% of APAP is metabolised by cytochrome P450 (CYP) enzymes (phase I), primarily CYP2E1, to form the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) [8]. NAPQI is rapidly detoxified by conjugation with glutathione (GSH), which is abundant in the liver [9]. The resulting NAPQI-GSH conjugate is excreted into bile and further degraded in other organs such as the kidneys, with the final products excreted in urine [10].
However, in case of APAP overdose, excessive NAPQI is produced, depleting GSH storage in cytoplasm and alters the electron transport chain (ETC) in the mitochondria leading to endoplasmic reticulum (ER) and oxidative stress, ultimately causing hepatocyte death [11, 12].
Pharmacokinetic studies suggest that the induction of phase II detoxification enzymes can accelerate the inactivation of APAP, reducing its metabolism by cytochrome P450 enzymes to NAPQI. This indicates that enhancing phase II enzyme activity could alleviate APAP hepatotoxicity. Activation of liver X receptors (LXR) has been shown to prevent APAP-induced liver injury by inducing phase II conjugating enzymes and suppressing phase I P450 enzymes [13].
Nuclear receptors and transcription factors such as pregnane X receptor (PXR), constitutive androstane receptor (CAR), and retinoid X receptor α (RXRα) also regulate the expression and activity of cytochrome P450 enzymes [14]. Changes in the expression of these receptors in response to lipids, cholesterol, bile acids and xenobiotics can alter APAP-induced toxicity by modulating NAPQI generation. Activation of antiviral responses by polyinosinic-polycytidylic acid has been shown to attenuate APAP-induced hepatotoxicity by downregulating RXRα and PXR and their downstream CYP enzymes [15]. Similarly, genetic deletion or pharmacological suppression of 5-lipoxygenase (5-LO) in mice induced the phase II detoxification enzyme SULT2A1 and inhibited the pro-toxic phase I enzyme CYP3A11, thereby reducing NAPQI formation and ameliorating AILI [16].
2.2 Emerging Drug Metabolic Pathways in AILI
2.2.1 Function of the Endoplasmic Reticulum (ER) Stress
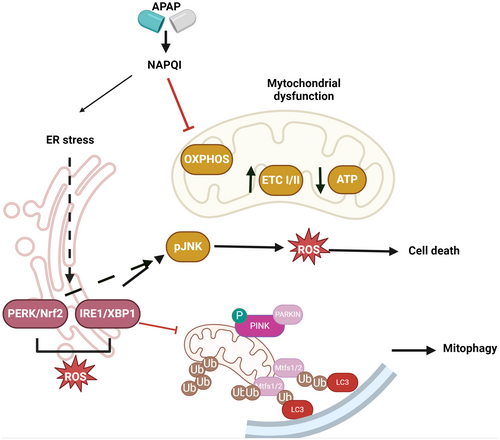
ER stress is a common feature in various hepatic injury models, including alcoholic liver disease, nonalcoholic fatty liver disease, ischaemia–reperfusion injury and cholestatic liver disease [17]. Therefore, it is not surprising that the ER stress also plays a role in APAP hepatotoxicity. This stress response is initiated by an impaired protein folding process triggered by the covalent binding of NAPQI to ER proteins [18].
An important component of the unfolded protein response (UPR) is the transcription factor X-box binding protein 1 (XBP1), which plays a critical role in the pathogenesis of APAP-induced liver injury [19]. APAP overdose triggers the splicing and activation of XBP1, leading to its translocation to the nucleus. Activated XBP1 then upregulates genes involved in the UPR, contributing to the severity of APAP-induced liver damage. Studies have shown that the cytoplasmic expression of XBP1 correlates with the degree of liver injury in both human patients and animal models of APAP overdose [19]. Importantly, inhibition of XBP1, either genetically or pharmacologically, was found to protect against APAP hepatotoxicity. Xbp1-deficient mouse livers exhibited decreased UPR and c-Jun N-terminal kinase (JNK) activation, but enhanced autophagy in response to high-dose APAP. This enhanced autophagy modulated CYP2E1 activity. Additionally, XBP1 inhibition led to decreased expression of CYP2E1, further reducing APAP metabolism and toxicity. These findings highlighted the central role of XBP1 in the pathogenesis of APAP-induced liver injury and suggested that targeting XBP1 may be a promising therapeutic approach for the treatment of APAP overdose-related AILI.
A key UPR target gene of XBP1 is CCAAT-enhancer-binding protein homologous protein (CHOP), which was shown to play a deleterious role in APAP hepatotoxicity by inhibiting liver regeneration. This finding has helped to clarify the role of the UPR/ER stress in mediating APAP-induced hepatotoxicity [20]. Given these data, interventions to inhibit CHOP may offer potential therapeutic strategies for treating APAP poisoning in patients with evident liver damage.
2.2.2 Linking ER Stress With Mitochondria Dysfunction: Role of Redox Homeostasis in AILI
Importantly, NAPQI, the toxic reactive consequence of APAP metabolism, can also directly activate the glycogen synthase kinase 3 (GSK3β)/nuclear factor erythroid 2-related factor 2 (Nrf2) signalling pathway. This activation of Nrf2 by NAPQI is a key adaptive mechanism that helps protect the liver against the oxidative stress and mitochondrial dysfunction induced by APAP overdose. In the context of DILI, this pathway is particularly important for understanding the mechanisms underlying AILI [21].
Activated Nrf2 promotes the transcription of antioxidant enzymes such as quinone oxidoreductase 1 (NQO1), heme oxygenase 1 (HO-1), and microsomal epoxide hydrolase, and enhances GSH synthesis. These enzymes play a defensive role by detoxifying NAPQI. For instance, fibroblast growth factor 21 (FGF21) overexpression due to APAP excess induces peroxisome proliferator-activated receptor coactivator-1α (PGC-1α) expression, increasing Nrf2 abundance in the liver as a compensatory protective mechanism against APAP hepatotoxicity. Moreover, protein tyrosine phosphatase 1B (PTP1B) acts as a negative regulator of tyrosine kinase growth factor signalling, and its deficiency in mice enhances the hepatic Nrf2 system, protecting hepatocytes from APAP-induced cell death [22].
A study revealed that shikimic acid (SHK) enhanced Nrf2 expression through the Phospho-inositol 3 kinase (PI3K)/protein kinase B (Akt)/GSK-3β pathway in hepatocytes, leading to improved Nrf2 stability. By stimulating the PI3K/Akt pathway, SHK prevented Nrf2 degradation mediated by GSK-3β phosphorylation at Ser 9 in APAP-treated hepatocytes, suggesting a PI3K/Akt/GSK-3β-dependent regulation of Nrf2 stability [23]. Using a mouse model of APAP overdose, the study demonstrated the hepatoprotective effects of SHK in vivo. Treatment with SHK reduced APAP-induced liver toxicity in mice, evidenced by decreased serum ALT and AST levels, reduced hepatocyte necrosis and improved GSH production, indicative of enhanced antioxidant capacity. Additionally, SHK attenuated ROS production and decreased myeloperoxidase (MPO) levels, indicating reduced liver inflammation. Immunoblot analysis of liver tissues revealed increased levels of p-Akt, p-GSK3β and Nrf2 following SHK treatment, suggesting the involvement of the Akt/GSK3β/Nrf2 pathway in the hepatoprotective effects of SHK against APAP-induced liver injury in mice [23].
Additionally, JNK, a mitogen-activated protein kinases (MAPK), was reported to regulate the activity of the transcription factor Nrf2 [1, 4]. Specifically, JNK can interact with and phosphorylate Nrf2, primarily at Ser residues in the Neh6 domain, by impairing the ability of Nrf2 to induce the expression of its target genes involved in antioxidant defence and detoxification, such as NQO1 [24, 25]. Moreover, JNK translocated into the mitochondria, causing dysfunction in the mitochondrial ETC and increased ROS. This accumulation of ROS perpetuates JNK phosphorylation, creating a feedback loop of sustained JNK activation and ROS amplification. The activated JNK induces the opening of the mitochondrial permeability transition (MPT) pore, increasing mitochondrial permeability and transition pore activity, which ultimately leads to DNA breaks and cell necrosis. Notably, the effect of JNK on mitochondria is dose-dependent; at low APAP doses, JNK activation is transient and the induced increase in mitochondrial permeability is reversible [25].
APAP overdose leads to mitochondrial dysfunction primarily through the covalent binding of NAPQI to mitochondrial proteins. This interaction depresses mitochondrial respiration and ATP synthesis, inducing mitochondrial oxidative stress with increased production of ONOO−, a potent oxidant and nitrating agent, and inhibiting enzymes involved in mitochondrial fatty acid oxidation (mtFAO). This impairs the liver's ability to use fatty acids for energy production, leading to the accumulation of triglycerides and hepatic steatosis. This cascade results in altered membrane permeability, collapse of mitochondrial membrane potential, disruption of ATP synthesis, release of mitochondrial proteins into the cytoplasm and hepatocyte cell death [5].
In the context of AILI, mitochondrial proteins are major targets of NAPQI. Key mitochondrial targets include housekeeping proteins, glutathione peroxidase (GPx) and ATP synthase. NAPQI also disrupts the mitochondrial ETC at complexes I and II, causing electron leakage and formation of superoxide radicals. These radicals are converted to hydrogen peroxide (H2O2) by manganese superoxide dismutase (MnSOD) or react with nitric oxide (NO) to form peroxynitrite (ONOO−) [26]. H2O2 is detoxified by glutathione (GSH) and various antioxidant enzymes, including catalase, GPx and peroxiredoxin (Prx) [27]. ONOO− is also detoxified by reacting with GSH. However, excessive free radicals deplete GSH, leading to accumulation of ONOO−, formation of nitrotyrosine protein adducts and mitochondrial DNA damage [28].
NAPQI can also uncouple oxidative phosphorylation (OXPHOS) by disrupting the mitochondrial membrane potential, preventing efficient ATP synthesis. Additionally, APAP causes depletion and damage of mitochondrial DNA, further impairing OXPHOS [29]. The resulting energy deficit and oxidative stress contribute to hepatocyte dysfunction and death. Specifically, the mitochondrial dysfunction caused by APAP leads to increased production of ROS. This can cause lipid peroxidation, further damaging mitochondrial membranes and impairing their function (Figure 1).
Interestingly, the severity of AILI appears to be influenced by underlying metabolic conditions. For example, individuals with metabolic dysfunction-associated steatotic liver disease (MASLD) may be more susceptible to APAP hepatotoxicity due to pre-existing mitochondrial dysfunction and impaired fatty acid oxidation (FAO) [30]. The combination of APAP-induced mitochondrial impairment and the metabolic stress of MASLD can overwhelm the liver's adaptive capacity, leading to more severe injury.
2.2.3 Cell Death: What Happens After Mitochondrial Disturbance?
Every drug or toxin is associated with a specific mechanisms of cell death in liver injury, the extent of liver damage and the specific cell death pathways activated. In hepatocytes, direct toxicity and stress in intracellular organelles (such as ER or mitochondrial dysfunction) can trigger apoptosis via mitochondrial outer membrane permeabilisation (MOMP) or lead to necrosis through MPT. Apoptosis involves caspases, chromatin fragmentation and phagocytosis of cell bodies, with a low inflammation profile. In contrast, necrosis is characterised by cell swelling and plasma membrane rupture (oncosis), promoting a severe inflammatory reaction. While hepatocyte loss is the primary contributor to liver injury, bile duct epithelial cells and sinusoidal cells may also be affected [31].
During APAP overdose, necrosis is the primary form of cell death in hepatocytes. This process involves cell rupture and the release of intracellular molecules, activating an immune response. Necroptosis, a regulated form of necrosis initiated by tumour necrosis factor (TNF) receptor activation in the presence of caspase inhibitors like Z-VAD-FMK, involves the activation of the pseudokinase mixed lineage kinase domain like pseudokinase (MLKL) by receptor interacting serine/threonine kinase 1 and 3 (RIPK1 and RIPK3) interaction. Necroptosis requires RIPK1 activity and can be inhibited by necrostatin. The release of intracellular components during necrosis causes ion imbalance, mitochondrial dysfunction, ATP depletion and elicits an inflammatory response [32].
Importantly, in response to APAP overdose, autophagy is activated as a survival mechanism to remove damaged mitochondria and other harmful cellular components. This selective degradation of mitochondria by autophagy is known as mitophagy. The PTEN-induced kinase 1 (PINK1)/Parkin pathway is a key regulator of mitophagy in AILI. PINK1 accumulates on the outer membrane of depolarised mitochondria and recruits the E3 ubiquitin ligase Parkin, which ubiquitinates mitochondrial proteins to mark them for degradation by the autophagosome. Impairment of autophagy exacerbates APAP-induced liver injury. Mice deficient in the autophagy genes Parkin or autophagy-related 5 (Atg)5 showed increased susceptibility to APAP hepatotoxicity, with greater mitochondrial dysfunction, oxidative stress, and liver necrosis. Conversely, pharmacological induction of autophagy by the mechanistic target of rapamycin kinase (mTOR) inhibitor rapamycin or the AMP-activated protein kinase (AMPK) activator metformin protected against APAP-induced liver injury in mice [33, 34].
Autophagy also plays a role in the resolution of AILI. As the liver regenerates after APAP overdose, autophagy helps remove damaged mitochondria and promotes the recovery of normal mitochondrial function [33, 34]. Mice with impaired autophagy due to Atg7 deletion showed delayed liver regeneration after APAP treatment [33].
In addition to mitophagy, APAP exposure was observed to decreased the expression of the mitochondrial fusion-associated proteins mitofusin (MFN)1, MFN2 and OPA1. This loss of mitochondrial fusion proteins promoted mitochondrial fragmentation and dysfunction, contributing to APAP-induced liver injury [35, 36]. In fact, MFN2 is a key substrate targeted for ubiquitination and degradation by the PINK1/Parkin mitophagy pathway. Decreased MFN2 levels can impair mitophagy, leading to the accumulation of damaged mitochondria and exacerbating APAP-induced liver injury.
Pharmacological inhibition of JNK, which can target MFN2 for proteasomal degradation, was found to prevent the loss of MFN2 and mitochondrial fragmentation in APAP-treated hepatocytes. Maintaining MFN2 levels and mitochondrial fusion appears to be a protective mechanism against APAP-induced mitochondrial dysfunction and liver injury [36].
Last but not least, ferroptosis, another form of regulated cell death, has been suggested to occur in APAP-induced liver injury. Ferrostatin-1, a specific ferroptosis inhibitor, has been shown to increase cell viability in primary mouse hepatocytes treated with APAP, suggesting a role for ferroptosis in this context [37]. Moreover, knockdown of acyl-CoA synthetase long-chain family member 4 (ACSL4), a key enzyme in ferroptosis, also alleviated APAP hepatotoxicity and lipid peroxidation [38]. Moreover, the regulation of redox homeostasis specifically activating the Nrf2 pathway protected against APAP-induced liver injury via the inhibition of ferroptosis [39, 40].
3 Mechanism-Based Treatment
The pathogenesis of AILI comprises several key stages (APAP absorption, phase I and phase II metabolism, oxidative stress, hepatocyte death, hepatic inflammation and liver regeneration), highlighting the complexity of the disease and offering a wide range of therapeutic targets. To date, however, the only approved pharmacological treatment for APAP overdose and early stages of idiosyncratic DILI is NAC, an antioxidant that increases the hepatic levels of glutathione, facilitating the scavenging of reactive metabolites and ROS [41, 42]. Nevertheless, NAC treatment is only effective within 20 h of the overdose [35] and standard doses of NAC in patients with advanced liver injury may not be sufficient [43]. Therefore, new therapeutic approaches are needed in this field.
Calmangafodipir (an SOD mimetic that avoids ROS production) and Fomepizole (an inhibitor of Cyp2e1 that avoids NAPQI formation) have shown positive phase I results with a good safety profiles and reduced biomarkers of paracetamol toxicity (NCT03177395), and patients are being recruited for a phase II clinical trial to test Fomepizole in the treatment of APAP overdose (NCT05517668). Although both drugs have a rational potential to become effective DILI treatments, they target the early stages of AILI; fomepizole indeed has a very narrow therapeutic window, restricted to very early APAP metabolism phase [44]. Thus, treatments that address both early and late stages of AILI are very much needed.
3.1 Magnesium (Mg2+) Homeostasis Regulators
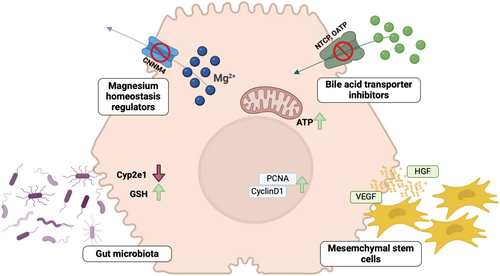
González-Recio et al. [45] recently highlighted the relevance of magnesium homeostasis during AILI. Indeed, restoration of intracellular magnesium levels alleviated main AILI hallmarks; mitochondrial dysfunction, ER stress, cell death, inflammation and enhanced liver regeneration [45].
Magnesium, or Mg2+ in its ionised form, is one of the most abundant divalent cations in the cell, involved in major cellular and physiological processes, and indispensable for health and life because it is an essential cofactor for ATP, the cellular source of energy [46]. Magnesium regulates glucose, lipid, and protein metabolism, and it is involved in the control of neuromuscular function, regulation of cardiac rhythm, modulation of vascular tone, hormone secretion, and N-methyl-D-aspartate (NMDA) release in the central nervous system [47, 48]. The largest amount of magnesium is found in the skeleton, with the remaining amount distributed in muscles and soft tissues to support metabolic, muscle function and enzymatic activity [49]. In mammals, the total cellular magnesium concentration is maintained in the mid-millimolar range, [50] and the amount present in the blood and other extracellular fluids is quite small, but this is most important for the balancing of physiological activity and emphasises the significance of maintaining an operative level of magnesium in the body for its integrity and health [49].
Due to the fundamental nature of magnesium in the regulation of cell function and signalling, intracellular magnesium levels need to be tightly controlled. The flux of magnesium across cell membranes is controlled by the activity and interplay of several magnesium transporters and channels. The transporters can, in general, be divided into two groups: those responsible for magnesium uptake to cells and those involved in its efflux [51]. Among the well-characterised transporters, TRPM6/7 underlie magnesium entry at the plasma membrane. TRPM6 predominately expresses in the intestines and kidneys, mediating the entrance of dietary magnesium and its reabsorption within the kidneys. On the other hand, TRPM7 displays ubiquitous expression and an absolutely different role: it is engaged with more general cellular magnesium homeostasis and signalling. MagT1 and SLC41A1 also play a key role maintaining cellular magnesium homeostasis [51].
Members of the CNNM family, but mainly CNNM2 and CNNM4, take part in magnesium homeostasis through the mediation of magnesium efflux and the handling of magnesium in the kidney [52]. CNNM2 is expressed in the kidney as well as in the brain, playing an important role in tubular reabsorption of magnesium that is important to systemic magnesium balance. CNNM4 is an apical membrane protein predominantly expressed in the intestine, where it is important for magnesium import across the apical cell membrane from the gut lumen in the uptake of dietary magnesium [53]. CNNM4 is expressed in many other tissues, however, including the brain and teeth, and its mutants are associated with the inherited syndrome Jalili Syndrome [54].
The essential role that magnesium plays in mediating biochemical reactions at a cellular level explains why alterations in its homeostasis in the body lead to various disorders. Magnesium dysregulation, often resulting in deficiency, can be caused by various factors including inadequate dietary intake, chronic diseases, gastrointestinal disorders and certain medications. Symptoms of magnesium deficiency include muscle cramps, fatigue, irregular heartbeat and mental disturbances. Prolonged magnesium deficiency can impair bodily functions, contributing to chronic conditions such as hypertension, cardiovascular diseases and metabolic disorders [47].
Particularly in the liver, magnesium deficiency has been associated with the risk of developing MASLD and MASH, [55] cirrhosis, alcoholic-derived liver damage and hepatocellular carcinoma [56]. Following an exhaustive analysis of all magnesium transporters in the liver, Simón et al. observed that CNNM4 levels were significantly increased in MASLD patients and in various animal models that resemble the human pathology [57]. CNNM4 acted as an extruder of magnesium, reducing the levels of this cation in the cytoplasm and mitochondria, therefore resulting in functional perturbations in both cellular compartments. Indeed, one-third of cellular magnesium content is localised within the mitochondria and its deficiency compromises energy production and heightens susceptibility to oxidative damage. Magnesium deficiency also exacerbates ER stress by disrupting calcium homeostasis, as it modulates both the influx and efflux of this cation, leading to protein misfolding and apoptosis [56, 58]. Interestingly, silencing CNNM4 demonstrated to reduce lipid accumulation, oxidative stress and ER stress response, while increasing microsomal transfer protein activity and restoring VLDL secretion, thereby improving liver health [57].
Focusing on the role of CNNM4 in magnesium homeostasis and its therapeutic potential in AILI, this disease is characterised by the dysregulation of mitochondrial function and ER stress. Previous studies have demonstrated that mitochondria act as major intracellular reservoirs of magnesium, which is released upon mitochondrial depolarisation, perturbing cellular energy metabolism, impairing ATP production, and sensitising the cell to stress responses [56, 58]. The study of magnesium transporters identified significant upregulation of CNNM4 in patients with acute liver failure due to APAP toxicity, in animal models mimicking human pathology, and in primary hepatocytes treated with APAP overdose [45]. As happened in patients with MASLD, similar extruder activity was observed in experimental APAP models, with patients exhibiting perturbed serum magnesium levels compared to healthy populations. These findings served as proof of concept for analysing the role of magnesium and the modulation of CNNM4 in preclinical models of APAP-induced liver damage [45].
In primary hepatocytes, silencing CNNM4 restored magnesium homeostasis in the mitochondria and cytoplasm, significantly reducing ER stress and restoring mitochondrial activity. Notably, magnesium supplementation alone did not show any effect, suggesting that CNNM4's role as an extruder is fundamental for maintaining magnesium homeostasis in hepatocytes. In vivo experiments showed that silencing CNNM4 in mice resulted in a significant reduction of the main hallmarks of AILI, namely necrosis and inflammation. Furthermore, mitochondrial and ER functions were restored, as well as magnesium content in the serum and hepatocytes. Importantly, a regenerative response was observed in APAP overdose scenarios, potentially due to increased ATP levels in the liver mediated by CNNM4 silencing through a mitochondrial activity-dependent process [45, 59].
Targeting CNNM4 could therefore represent a novel therapeutic approach for treating APAP-induced liver damage, especially in cases where traditional treatments such as NAC are ineffective [45]. Further research is warranted to explore the clinical applications of CNNM4 modulation in liver diseases and other conditions associated with dysregulated magnesium transport.
3.2 Inhibition of BA Transporters
Altered bile acid (BA) homeostasis is a common feature in AILI, though the exact pathophysiological role of BAs remains unclear. Recent observations by Ghallabh et al. indicate that an APAP overdose disrupts the blood-bile barrier, resulting in BA leakage from the bile canaliculi. These leaked BAs are then reabsorbed by the liver in a process described as a ‘futile BA cycle’, leading to elevated intracellular BA concentrations sufficient to cause hepatocyte death. This transient cholestasis precedes hepatocyte death following APAP overdose [60].
Interestingly, the study also demonstrates that this reuptake can be interrupted as a therapeutic intervention. In mice, bile acids are absorbed by NTCP and OATPs (SLC01B1 and SLC01B3). While blocking NTCP alone has proven beneficial in certain cholestatic conditions, [61] inhibiting both uptake carriers is crucial to effectively block BA absorption. Ghallabh et al. showed that pharmacological blockage of NTCP using Myrcludex B, combined with Oatp knockout, significantly reduced APAP-induced hepatotoxicity [60].
Overall, this study identifies futile BA cycling as a mechanism that follows APAP-induced oxidative stress and exacerbates hepatocyte death, paving the way for novel therapeutic approaches. Importantly, the authors suggest that the therapeutic window for interrupting futile BA cycling extends beyond the 8-h window within which NAC is effective. Nevertheless, further studies are needed to test this potential therapeutic intervention. Specifically, NTCP and OATPs should be blocked after APAP overdose and the effect compared to NAC. Additionally, since BAs are predominantly taken up by NTCP in humans, the effect of Myrcludex B should be tested independently in studies with human hepatocytes.
3.3 Microbiota
The gastrointestinal tract and the liver are closely related. Bile from the liver influences the microbial environment of the intestine, while venous blood containing products of the microbiota and immune reactions flows from the intestine to the liver via the portal vein. Normally, the immune cells of the liver counteract small bacterial translocations with immune tolerance. However, dysbiosis can increase intestinal permeability and microbial translocation to the liver, exposing hepatic immune cells to exogenous antigens and triggering a protective immune response [62].
Recent research has shown that the intestinal microbiota (IM) is an important modulator in AILI. The gut microbiota has been shown to be involved in all pathological stages of AILI, from drug absorption and metabolism to liver regeneration. Malfatti et al. observed that antibiotic mediated IM depletion decreases APAP bioavailability in mice, what suggests that bacteria influence APAP metabolism and transportation, both directly and indirectly. Absorbed APAP can be then metabolised by phase I enzymes (cytochrome P450) into toxic NAPQI when phase II pathways (sulfation and glucuronidation) are saturated due to overdose. IM derived products can regulate the transformation step since they modulate the expression and activity of cytochrome P450 enzymes. Indole-3-carboxylic acid and supplementation with oral magnesium (which increases I3C) mediate CYP2E1 inhibition. GSH is the defence that neutralises harmful NAPQI effects and bacteria such as Akkermansia muciniphila or Lactobacillus rhamnosus enhance the host antioxidant capacity [63]. Likewise, IM-derived 2-hydroxybutyric acid and hypaphorine exert hepatoprotective effects and attenuate AILI in mice partly by maintaining the GSH level (Qin). Recent research has shown that ferroptosis is associated with AILI and in this line, IM-derived daidzein and butyrate have shown a protective effect against APAP-induced ferroptosis in mice. Cell death elicits inflammation increasing liver injury, and on top, APAP-mediated intestinal dysbiosis and increased intestinal permeability directly impact liver inflammation, sensitising to AILI. Finally, through the gut-liver axis, IM-bacteria and IM-derived microbial products play a key role during liver regeneration, hinting that the efficiency of liver tissue repair and regeneration is at least partially controlled by the composition of the individual microbiota, [63] Yin et al. [64] have recently observed that partial hepatectomy in mice pretreated with antibiotics is associated with delayed liver regeneration and increased mortality, mainly due to impaired lipogenesis, highlighting the relevance of IM-derived short chain fatty acids. On the other hand, IM-derived trimethylamine N-oxide has been observed to suppress liver regeneration, exacerbating AILI. Overall, IM and IM-derived substances potentially target multiple steps during AILI, even simultaneously, exerting beneficial or harmful effects [63].
In this line, manipulation of IM–through probiotics, prebiotics or postbiotic-has been widely considered for the treatment of many chronic diseases. Since AILI progresses so rapidly, some patients even suffer fulminant liver failure, targeting the IM once the liver is damaged seems quite a challenge. On a recent review, Chen hypothesised about combining NAC with IM-derived bioactive compounds able to improve liver inflammation and enhance regeneration, or with a ‘cocktail’ of multiple bioactive compounds that could target different pathological stages of AILI. However, the identification of those beneficial compounds is still limited, and the dosage has not been still determined. As Chen highlights, prevention strategies by manipulating the IM in those individuals at high risk of AILI might be a good approach. Dietary interventions with metabolites from identified probiotics and nutrients that enrich ‘protective’ microbes may be a good choice to reduce AILI susceptibility. In this sense, oral magnesium has been shown to increase the availability of Bifidobacterium and prevent AILI, even mice who received a faecal transplant from human who had received oral magnesium intake showed alleviated AILI [63]. Overall, it is clear that AILI progression involves not only the liver but also IM, and although a variety of challenges need to be addressed, modulating IM might become a promising therapeutic approach to prevent or alleviate AILI, at both early and late stages.
3.4 Improving Regeneration Through Mesenchymal Stem Cells
Regarding the last step of AILI pathophysiology, the use of mesenchymal stem cells has been at the forefront of regenerative medicine therapies for many years, including for the treatment of liver disease. Multipotent mesenchymal stromal cells, also referred to as mesenchymal stem cells (MSCs), are a population of self-renewable and undifferentiated cells with significant potential for the regeneration of damaged tissues. These cells are present in the bone marrow, adipose tissue, dental pulp and various other mesenchymal adult tissues [65].
Systemically injected MSCs travel through the bloodstream to reach damaged organs, establishing themselves in the tissue parenchyma through a process known as ‘homing’. MSCs contribute to the regeneration of injured tissue through at least three mechanisms: (1) differentiation into parenchymal cells; studies have shown that MSCs derived from different sources can differentiate into a variety of cells, including hepatocyte-like cells. (2) Secretion of Trophic Factors; MSCs secrete factors such as hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF), which promote tissue regeneration. And (3) Modulation of the immune system; MSCs can modulate the immune response, either by suppressing or stimulating it, through the secretion of soluble factors and direct contact with immune cells, depending on the context [65].
Interestingly, clinical studies have demonstrated the efficacy and feasibility of bone marrow derived MSCs therapy in patients with liver conditions. For instance, a study by Suk et al. involved 72 patients with liver cirrhosis who received either one-time or two-time administrations of 50 million autologous BMSCs. Results showed a significant reduction in fibrosis and improvement in Child–Pugh scores without serious adverse events over 12 months. Wang et al. found that intravenous injection of umbilical cord-derived MSCs (UC-MSCs) was well tolerated in patients with primary biliary cirrhosis, significantly decreasing ALP and GGT levels. Similarly, Zhang et al. [66] demonstrated the safety and efficacy of UC-MSCs for patients with ischemic-type biliary lesions post-liver transplantation, noting reduced ALP, GGT, and total bilirubin levels. In a phase I-II study involving patients with acute-on-chronic liver failure (ACLF), BMSC therapy significantly improved clinical scores without transplant-related adverse events. Another study by Lin et al. on hepatitis B virus (HBV)-related ACLF showed that allogeneic BMSC injections significantly improved clinical measurements and reduced mortality and severe infection rates.
Regarding AILI, the use of MSCs in animal models of APAP-induced liver injury have shown promising results. MSCs have been demonstrated to home to the injured liver, reduce oxidative stress, decrease inflammation, and enhance liver regeneration [67]. However, specific trials for APAP overdose are currently lacking.
Overall, MSC therapy has been shown to ameliorate clinical manifestations, reduce liver fibrosis, and inhibit disease progression in liver disease patients, providing a basis for the potential efficacy of MSCs in APAP-induced liver injury.
4 Conclusions
DILI is a significant public health concern, particularly due to the liver's crucial role in metabolising and detoxifying xenobiotics. Despite its relative safety, APAP remains a leading cause of ALF because of its widespread use and the risk of overdose. Several studies have illustrated the complex mechanisms underlying AILI, highlighting the critical roles of mitochondrial dysfunction, ROS, and ER stress. Given the limited therapeutic window of N-acetylcysteine, there is an urgent need for treatments targeting both the early and late stages of APAP-induced liver injury. Future research should prioritise the identification and development of agents that can regulate mitochondrial function and alleviate ER and oxidative stress. Such therapies could provide dual benefits by protecting hepatocytes from damage and promoting liver regeneration.
The study of Mg2+ homeostasis offers promising new therapeutic approaches. Specific Mg2+ transporters are emerging as interesting therapeutic targets because restoring intracellular magnesium levels not only improves mitochondrial and ER function but also enhances hepatic regenerative capacity following APAP overdose in mice. Additionally, the discovery that altered bile acid metabolism precedes hepatocyte death suggests the possibility of alleviating liver damage through inhibitors of bile acid transporters. Although challenging, due to limited knowledge about beneficial bacteria and their derived products, manipulating the gut microbiota could become a preventive strategy for high-risk patients.
Harnessing the regenerative potential of MSCs presents a promising avenue for treating severe liver injury. Expanding clinical and preclinical studies to evaluate MSCs specifically for APAP-induced liver injury could revolutionise the treatment landscape for AILI (Figure 2).
In addition, there is a need for novel animal models that better mimic the human liver response to hepatotoxicity in order to study the mechanisms of DILI and test new therapies. Managing polypharmacy in patients with impaired liver function or those on immunosuppressive therapy is particularly challenging because of the increased risk of DILI from drug interactions. Future research should investigate these interactions and develop risk mitigation strategies. Finally, prioritising personalised medicine to identify individuals at increased risk of DILI will enable tailored treatments. Focusing on these areas may lead to innovative treatments and improved management of DILI, ultimately enhancing patient care.
5 Conflicts of Interest
The authors declare no conflicts of interest.