Portal hypertension and hepatocellular carcinoma: Des liaisons dangereuses…
Handling Editor: Alejandro Forner
Abstract
Background and Aims
Portal hypertension (PHT) and hepatocellular carcinoma (HCC) are major complication of cirrhosis which significantly contribute to morbidity and mortality. In this review, we aim to describe the consequences of both angiogenesis and inflammation in the pathogenesis of PHT and HCC, but also the difficulty to propose adapted treatment when PHT and HCC coexist in the same patients.
Methods
Studies for review in this article were retrieved from the PubMed database using literature published in English until March 2021.
Results
Portal hypertension occurs secondary to an increase of intrahepatic vascular resistances, the opening of portosystemic collateral vessels and the formation of neovessels, related to vascular endothelial growth factor (VEGF). Recently, bacterial translocation-mediated inflammation was also identified as a major contributor to PHT. Interestingly, VEGF and chronic inflammation also contribute to HCC occurrence. As PHT and HCC often coexist in the same patient, management of PHT and its related complications as well as HCC treatment appear more complex. Indeed, PHT-related complications such as significant ascites may hamper the access to HCC treatment and the presence of HCC is also independently associated with poor prognosis in patients with acute variceal bleeding related to PHT. Due to their respective mechanism of action, the combination of Atezolizumab and Bevacizumab for advanced HCC may impact the level of PHT and its related complications and to date, no real-life data are available.
Conslusions
Appropriate evaluation and treatment of PHT remains a major issue in order to improve the outcome of HCC patients.
Abbreviations
-
- AVB
-
- acute variceal bleeding
-
- CSPH
-
- clinically significant portal hypertension
-
- DAMP
-
- damage-associated molecular pattern
-
- HCC
-
- hepatocellular carcinoma
-
- HSC
-
- hepatic stellate cell
-
- HVPG
-
- hepatic venous pressure gradient
-
- IL
-
- interleukin
-
- LSEC
-
- liver sinusoidal endothelial cell
-
- NO
-
- nitric oxide
-
- PAMP
-
- pathogen-associated molecular pattern
-
- PDGF
-
- platelet-derived growth factor
-
- PHT
-
- portal hypertension
-
- PPR
-
- pattern recognition receptors
-
- RPS
-
- reactive oxygen species
-
- TKIs
-
- tyrosine kinase inhibitors
-
- TLR
-
- toll like receptor
-
- VEGF
-
- vascular endothelial growth factor
1 INTRODUCTION
Portal hypertension (PHT) is a major complication of cirrhosis and is responsible for variceal bleeding, ascites and hepatorenal syndrome, which significantly contribute to morbidity and mortality. In the case of cirrhosis, PHT occurs secondary to an increase of intrahepatic vascular resistances due to sinusoidal alterations and the presence of fibrosis that cause distortion of the vascular architecture within the liver. The opening of portosystemic collateral vessels, and the formation of neovessels, related to vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) production (so called angiogenic process),1-3 as well as a subsequent increase of the splanchnic blood flow contribute and perpetuate PHT. Recently, bacterial translocation-mediated inflammation has also been identified as a major contributor to PHT occurrence.4, 5 In clinical practice, PHT is defined by an increase in portal pressure above 12 mm Hg or by a pressure difference between portal vein pressure and hepatic venous pressure higher than 5 mm Hg. Different methods to measure the pressure in the portal vein exist, but the most used technique in patients is the measurement of the hepatic venous pressure gradient (HVPG) through a transjugular approach. A HVPG higher than 10 mm Hg is associated with ascites and variceal occurrence, so-called clinically significant portal hypertension (CSPH) and a value higher than 12 mm Hg exposes the patient to a risk of variceal bleeding.
Hepatocellular carcinoma (HCC) is also a major complication of cirrhosis. Similar to PHT, an increased level of VEGF and chronic inflammation also contributes to HCC occurrence.6 Moreover, patients with PHT present higher risk to develop HCC, and HCC through changes in the hepatic architecture and vascular invasion also contributes to PHT occurrence.7, 8 As PHT and HCC often coexist in the same patient, management of PHT and its related complications as well as HCC treatment appear more complex. Indeed, CSPH may hamper the access to curative treatment for HCC such as liver resection/ablation but also to locoregional therapies or systemic treatment in case of significant ascites.6 Ascites and oesophageal varices have been identified as predictive factors of death in patients with HCC independently of the severity of the underlying liver disease and the HCC stage.9, 10 Conversely, the presence of HCC is also independently associated with poor prognosis in patients with acute variceal bleeding (AVB) related to PHT.8, 9, 11, 12 Thus, understanding the interaction between HCC and PTH is a major issue in order to improve the management of such complications and the outcome of these patients.
Recently, the positive results of the Imbrave 150 study, a randomized study comparing Atezolizumab (anti PD-L1) in combination with Bevacizumab (anti VEGF) versus Sorafenib (tyrosine kinase inhibitor), have prompted us to redefine our management strategy for advanced HCC by proposing the combination of Atezolizumab and Bevacizumab as a first-line treatment.13 Patients were well selected, and few complications related to PHT were noticed in this phase 3 study. However, due to their respective mechanism of action, the combination of Atezolizumab and Bevacizumab may potentially impact the level of PHT and its related complications, and to date, no real-life data are available.
In this review, we will focus (i) on the consequences of both angiogenesis and inflammation in the pathogenesis of PHT and HCC and (ii) on the link between PHT and HCC, and (iii) we will discuss the potential impact of the combination Atezolizumab and Bevacizumab on PHT.
Studies for review in this article were retrieved from the PubMed database using the search terms ‘hepatocellular carcinoma’, ‘liver cancer’ and ‘primary liver carcinoma’, both individually and in combination with the terms ‘portal hypertension’, ‘acute variceal bleeding’, ‘ascites’ and ‘TIPS’. The search included literature published in English until March 2021.
2 ANGIOGENESIS AND CHRONIC INFLAMMATION CONTRIBUTE TO PORTAL HYPERTENSION PATHOGENESIS
In case of cirrhosis, PHT is initiated by an increased hepatic resistance caused by the distortion of liver vascular architecture due to fibrosis and regenerative nodules but also to an increase of vasoconstrictor stimuli. PHT is further perpetuated by opening of collateral vessels/formation of neovessels and changes in the systemic circulation that culminate in an increased portal-tributary inflow.1, 14 The demonstration that angiogenesis is a main actor in the pathophysiology of PHT is relatively recent.3 During decades, the formation of portosystemic collateral vessels in PHT has been related to the opening of pre-existing vascular channels secondary to the increased portal pressure. Recently, a neovascularization in the mesenteric vascular bed and in the portosystemic collateral vessels has been described and attributed to VEGF and PDGF production.1 VEGF and VEGF receptor type 2 expression were observed in the mesentery from portal vein-stenosed rats, and the inhibition of VEGF receptor 2 signalling was associated with a 50% decrease in the formation of portal-systemic collateral blood vessels in rodent models with portal vein stenosis.15, 16 VEGF increases vascular permeability and induces the migration and survival of endothelial cells favouring angiogenesis process.17, 18 In addition to promoting neovessels formation in the mesentery and portosystemic system, VEGF also plays a role within the liver parenchyma. Increased hypoxia, inflammatory cytokines (IL-6, IL-1α), growth factors (epidermal growth factor, transforming growth factor-α and -β, fibroblast growth factor, PDGF), shear and oxidative stress within the liver lead to increase VEGF production by hepatocytes but also Hepatic Stellate Cells (HSCs).19 HSCs are activated through its VEGF receptor leading to fibrosis occurrence and also VEGF production that interacts with adjacent liver sinusoidal endothelial cells (LSECs).3 HSC-signalling pathways involving PDGF regulate microvascular structure and function in liver and PHT.20 Portal myofibroblasts also appear to be critical in pathological angiogenesis though the production of collagen that stabilizes the newly formed vessels.21, 22 Interestingly, sinusoidal angiogenesis depends on the liver stiffness, and mechanotransduction appears as an important pathway in liver fibrosis progression.23-25 LSECs exhibit pro-angiogenic properties and promote fibrosis through HSCs activation on low stiffness substrates at early disease stage. On contrary, LSECs showed random migration and leaky sinusoids in high stiffness substrates.24 The newly formed intrahepatic neovessels present varying diameter and flow pattern leading to the impairment of oxygen and nutrient supply to hepatocytes and to the recruitment of inflammatory cells.3 Thus, angiogenesis mediated by VEGF promotes an extensive network of portosystemic collateral vessels, contribute to increased splanchnic and portal venous inflow that perpetuates and exacerbates PHT and favours liver fibrosis progression and inflammation (Figure 1). The use of anti-VEGF such as Sorafenib was associated with PTH reduction and targeting angiogenesis at early stage of the liver disease may appear as a good strategy.26-29 However, VEGF also plays a role in hepatic tissue repair and fibrosis resolution.30
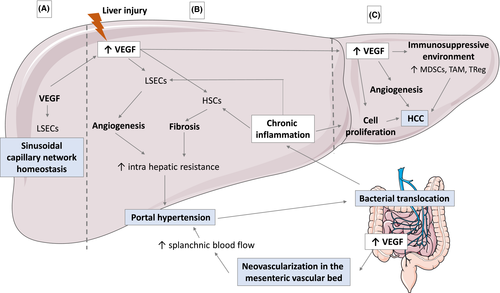
Chronic inflammation and PHT are also linked since bacterial infection increases portal pressure and PHT favours bacterial translocation.31, 32 PHT increases intestinal permeability, thus favouring bacterial translocation. Dysbiosis is associated with cirrhosis and increases with the severity of liver disease.33, 34 Bacterial translocation corresponds to the passage of microbial products through the mucosa into mesenteric lymph nodes and then to the liver. In the liver, the innate immune system recognizes damage-associated molecular pattern (DAMPs) and pathogen-associated molecular pattern (PAMPs) through pattern recognition receptors, such as Toll-like receptor (TLRs).35 Kupffer cells are the first cells to respond to PAMPs through TLR4 and consequently adopt a pro-inflammatory phenotype leading to the production of proinflammatory cytokines such as TNF, IL-6 and IL-12 and to the generation of reactive oxygen species (ROS).36 ROS production will conduct to a decrease in nitric oxide (NO) secretion, which usually regulates intrahepatic vascular tone by maintaining HSCs in a quiescent phenotype, and thus promote fibrosis process.37 ROS will also contribute to local tissue damage and propagating innate immune signalling through DAMPs and promote VEGF production.38 In addition, bacterial translocation will conduct to stimulation of TLR4-mediated signalling in HSCs and LSECs directly promoting angiogenesis and fibrosis progression (Figure 1). In animal models, TLR4 knockout mice are protected from fibrosis and PHT following bile duct ligation.39, 40 Similar results were observed in mice treated with rifaximin after bile duct ligation.39, 40 In humans, a trend towards HVPG reduction was observed in controlled study using rifaximin, but these finding remain controversial.41, 42 A similar reduction in portal pressure was observed in patients with acute on chronic liver failure treated with anti-TNF; however, this treatment was not adopted due to high rates of infection after anti-TNF treatment.43 Human data are scarce; furthers studies are needed to confirm the dialogue between VEGF, DAMPS and PAMPS in PHT progression.
3 ANGIOGENESIS AND CHRONIC INFLAMMATION ALSO CONTRIBUTE TO HEPATOCARCINOGENESIS
Levels of VEGF are increased in HCC and correlate with tumour angiogenesis and progression. In fact, high levels of VEGF are associated with rapid disease progression and reduced survival, and VEGF gene amplifications have been described in 4%-8% of HCCs.44 Interaction between VEGF and its receptor results in endothelial proliferation and migration and formation of new tumour blood vessels generating rapid growth and dissemination. In addition, the neovessels have abnormally leaky vasculature resulting in areas of high interstitial pressure and severe hypoxia and acidosis, who further drive malignant potential, induced epithelial-mesenchymal transition and the recruitment of immune cells.45 VEGF induces tumour proliferation via the induction of hepatocyte growth factor by macrophages.46 VEGF also leads to disruption of hepatocellular tight junctions that may promote HCC spreading into normal liver parenchyma.47 Besides inducing tumour angiogenesis, VEGF also mediates immunosuppression within the tumour microenvironment (TME) by promoting immunosuppressive cells such as regulatory T cells, myeloid-derived suppressive cells and tumour associated macrophages but also by suppressing antigen suppressive cells and cytotoxic T lymphocyte through TOX activation after binding VEGFR248-51 (Figure 1). Thus, the use of anti-VEGF therapy results in vascular normalization and thereby reduces tumour hypoxia that improves the anticancer activity of infiltrating immune cells.52, 53 However, results of phase 2 trial using anti-VEGF either alone or in combination with chemotherapy were disappointed in HCC patients.54 Recently, Ramucirumab, an VEGFR-2 antagonist, was associated with improved overall survival in a phase III trial when compared with placebo in a subgroup of patients with baseline alpha-fetoprotein ≥ 400 ng/ml (8.5 vs 7.3 months, P = .0199); nevertheless, Ramucirumab is poorly used in practice due to a limited increase in survival.55
Chronic inflammation is a major trigger of HCC carcinogenesis and tumour progression. Infiltration of inflammatory cells within the premalignant environment produces various cytokines such as IL-6 that enhance the transformation of the liver progenitor cells into a more cancerous phenotype,56 but also synthesis of growth factors, chemokines and proangiogenic factor that create a TME that supports the transformation of hepatocytes and also induces their survival through activation of anti-apoptotic pathway and inhibition of immune surveillance (Figure 1). Chronic inflammation also leads to tissue remodelling through the crosstalk between immune cells, HSCs and LSECs that promotes ECM degradation, the release of growth factors and consequently tumour growth. In addition, platelets also interact with immune cells and play a role in the hepatocarcinogenesis process. Once activated, platelets recruit inflammatory cells and induce growth factors production such as PDGF that mediates cellular proliferation and angiogenesis.57 High levels of platelets correlate with large HCC. In addition, platelets interact with LSECs that secrete specific chemokines regulating the recruitment and adhesion of leukocytes. Recently, the use of antiplatelet therapy allowed to prevent non-alcoholic steatohepatitis and subsequent HCC development in several dietary and genetic mouse models.58
Immune cells also prevent tumour occurrence and growth: there is a complex interaction between pro-tumoral and anti-tumoral signals mediated by the immune system, the tumours cells and the TME, which can evolve over time resulting either in tumour elimination or tumour progression. TME of HCC is strongly immunosuppressive, as a result of VEGF interaction with immune cells48-51 and also because HCC tumours cells may directly recruit TReg and tumour associated macrophages.59 TME, through HSCs, also promotes the differentiation of blood monocytes into myeloid derived suppressor cells through IL-6 signalling that enhances immune check point signalling, decreases anti-tumoral immune response mediated by natural killer and T cells cytotoxicity and contributes to an immunosuppressive TME.60 Immune check point factors such as programmed death-1 (PD-1), PDL-1, and cytotoxic t-lymphocyte antigen 4 (CTLA-4) down regulate the amplitude of immune response such as inhibition of T cell activity (CD8+ effectors and CD4+ helpers) that participates in suppressing tumour immunity and appears as a major mechanism of immune resistance.61 Immune checkpoint inhibitors (ICIs) allow to maintain the physiological cell-mediated immune response against tumour cells and are the new promising approaches in oncology. However, even if phase III trials using nivolumab vs sorafenib in first line for advanced HCC or using pembrolizumab vs placebo in second line showed interesting results regarding overall survival and disease free survival, statistical analysis did not reach significance.62, 63
4 A LINK BETWEEN HEPATOCELLULAR CARCINOMA AND PORTAL HYPERTENSION
Clinically significant portal hypertension is predictive of HCC occurrence independently of the severity of the underlying cirrhosis.7, 8 HCC also increases HVPG through the presence of arteriovenous shunting within the tumour and modifications of liver architecture. Moreover, HCC can be associated with vascular invasion of the portal and/or its branches and contributes to PHT, thus exposing the patients to AVB risk. However, PHT evaluation remains difficult, especially in patients with advanced HCC. Baveno criteria64 do not seem accurate to evaluate PHT as platelets level may be impacted by HCC presence and the presence of a large tumour, especially in the right lobe, may interfere with the elastometry results. Moreover, the presence of portal invasion makes HVPG measurement not always accurate. Thus, appropriate evaluation of PHT remains a major issue in patients with advanced HCC, and regular endoscopy should be preferred for the screening of oesophageal varices, especially in patients with vascular invasion.
Clinically significant portal hypertension impacts the choice of HCC treatment. CSPH is a well-identified predictive factor for liver decompensation and death after liver resection. Currently, optimal surgical candidacy for resection is based on a multiparametric evaluation including compensated Child-Pugh class A liver function with MELD score <10, to be matched with grade of PHT, acceptable remaining parenchyma and the possibility of a laparoscopic/minimally invasive approach.6, 65 CSPH is also associated with decrease overall survival after ablation and chemoembolization.66, 67 Moreover, the presence of clinically significant ascites precludes any locoregional of systemic therapy. Interestingly, the occurrence of AVB and clinically significant ascites is associated with poor prognosis in HCC patients.10, 11, 68 Interestingly, secondary prophylaxis after AVB was associated with increased overall survival justifying the need to treat both PHT and HCC.68
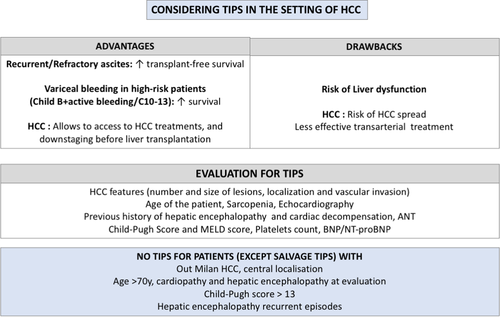
A treatment of CSPH by TIPS placement may be beneficial in some situations as it increases overall survival in case of AVB in high-risk patients (concept of pre-emptive TIPS) and transplant free survival in case of recurrent/refractory ascites.69-71 Moreover, in HCC patients, TIPS can also allow the access to HCC treatments (either alone or in a downstaging perspective before liver transplantation).69, 72, 73 However, HCC is generally considered as a contra-indication to TIPS placement, due to the fear of HCC spread, and the poor outcome of these patients.74-77 There is also a concern regarding the potential increased risk of liver failure after locoregional therapies and a decrease in efficacy of HCC treatments.74-77 In fact, few data are available regarding these points, and whether or not TIPS is a real risk factor for higher toxicity after locoregional therapy remains uncertain. At present, TIPS should be considered when needed in cirrhotic patients with Milan-In HCC to improve survival and as a bridge to liver transplant, in its classical indications (salvage, pre-emptive, failure of secondary prophylaxis and recurrent/refractory ascites). As for all cirrhotic patients, salvage TIPS should be performed when needed. However, there must be a careful selection of candidates in all other settings, and TIPS should be contraindicated in patients with heart failure, significant hepatic encephalopathy and advanced liver failure (Child-Pugh score > 13 in case of pre-emptive for AVB, high bilirubin/low platelets count or a MELD score > 19 in case of ascites) (Figure 2).70 Moreover, as far as HCC treatment is concerned, one can envision pre-operative TIPS placement before liver resection in patients with CSPH, and in order to treat ascites and allow locoregional treatments, either as curative options or in a perspective of downstaging.
5 IMPACT OF SYSTEMIC THERAPY FOR HEPATOCELLULAR CARCINOMA ON PORTAL HYPERTENSION
During the last decades, tyrosine kinase inhibitors (TKIs) were the standard of treatment for advanced HCC. Sorafenib and Lenvatinib were used in first line treatment while Regorafenib and Cabozantinib in second line.78-81 All of these TKIs target the VEGFR and may impact PHT pathophysiology (Table 1). Inhibition of VEGF-mediated angiogenesis by drugs such as sorafenib allowed to reduce portal pressure, portosystemic collateralization and fibrosis in animal models.15, 26-29, 82 Similar results were observed in rodents models treated with regorafenib and exposed to fibrotic models such as bile duct ligation, chronic carbon tetrachloride injections and partial portal vein ligation83 and with Lenvatinib.84 In humans, portal venous area but not portal venous flow velocity was also significantly decreased, as well as the congestion index (portal venous area divided by portal venous flow velocity) in 25 Child-Pugh A patients after 2 weeks administration of Sorafenib, suggesting a potential beneficial effect on PHT.85 On the contrary, the congestion index was significantly worsened in 28 Child-Pugh A patients who received two weeks of Lenvatinib for advanced HCC.86 Contrary to Sorafenib, Lenvatinib also targets fibroblast growth factor (FGF) receptors, and FGF19 and FGF 21 are known to promote liver regeneration and the maintenance of hepatic metabolism which could impact the underlying liver disease and favour PHT; however, further studies are needed to address this point. As discussed before, AVB is a major issue in patients with HCC, and similar PHT bleeding complications rates were observed in the phase 3 studies for advanced HCC (less than 3%) (Table 1).13, 55, 62, 78-81 However, in these series, patients were well selected as recent history of bleeding was an exclusion criterion, and real-life data are needed (Table 2). Interestingly, neoplastic portal vein thrombosis was independently associated with AVB in patients treated with Sorafenib.87 Such patients should benefit from regular screening and adapted bleeding prevention therapy.88
| Systemic treatment | Target of systemic treatment | Effect on portal hypertension | |
|---|---|---|---|
| Mice models | Humans’ data | ||
| Tyrosine kinase inhibitors | |||
| Sorafenib |
VEGFR-2, VEGFR-3 PDGFR-ß CRAF, BRAF, V600E BRAF c-KIT, FLT-3 |
↓ Angiogenesis26, 27 | ↓ PHT28, 85 |
| Lenvatinib |
VEGFR-1, VEGFR-2, VEGFR-3 PDGFR, c-KIT, RET FGFR-1, FGFR-2, FGFR-3, FGFR-4, |
↓ Angiogenesis84 | ↑ PHT?86 |
| Regorafenib |
VEGFR-1, VEGFR-2, VEGFR-3 PDGFR, TIE2, FGFR c-KIT, RET, RAF-1, BRAF, V600E BRAF |
↓ Angiogenesis83 | No data |
| Cabozantinib |
VEGFR-2, c-MET TIE2, AXL,RET, ROS1, TYR03, MER, KIT, TRKB, FLT3 |
No data | No data |
| Monoclonal antibody | |||
| Bevacizumab | VEGF | No data | No data |
| Ramucirumab | VEGFR-2 | No data | No data |
| Immunotherapy | |||
| Atezolizumab | PD-L1 | No data | No data |
| Nivolumab | PD1 | No data | No data |
| Pembrolizumab | PD1 | No data | No data |
- Abbreviations: HCC, hepatocellular carcinoma; PDGF, platelet-derived growth factor; PDGFR, Platelet-derived growth factor receptor; PHT, portal hypertension; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
| Study | Design of the study | Exclusion criteria regarding bleeding events | Bleeding events related to PHT |
|---|---|---|---|
| First-line treatments | |||
|
Llovet et al. NEJM 200878 |
SHARP Study Phase 3 RCT Sorafenib (n = 299) vs Placebo (n = 303) |
Gastrointestinal bleeding within 30 days prior to randomization |
Sorafenib (2.69%) Placebo (4.3%) |
|
Kudo et al. Lancet 201880 |
REFLECT study Phase 3 RCT Sorafenib (n = 476) vs Lenvatinib (n = 478) |
Gastrointestinal bleeding and interventional treatment for varices within 28 days prior to randomization Antiplatelet agents were prohibited Thrombotic disorders or use of anticoagulants requiring therapeutic INR monitoring |
Sorafenib (1.05%) Lenvatinib (1.47%) |
|
Finn et al. NEJM 202013 |
IMBRAVE 150 study Phase 3 RCT Atezolizumab/Bevacizumab (n = 336) vs Sorafenib (n = 165) |
A prior bleeding event due to PHT within 6 months prior to randomization Untreated or incompletely treated oesophageal and/or gastric varices with bleeding or high-risk for bleeding |
Atezolizumab/Bevacizumab (2.6%) Sorafenib (0.4%) |
|
Yau et al. Ann of Oncol 202092 |
CHECKMATE 459 Phase 3 RCT Nivolumab (n = 371) vs Sorafenib (n = 372) |
No specific exclusion criteria regarding bleeding history |
Nivolumab (1.09%) Sorafenib (1.93%) |
| Second-line treatments | |||
|
Bruix et al. Lancet 201779 |
RESORCE study Phase 3 RCT Regorafenib (n = 379) vs Placebo (n = 194) |
Gastrointestinal bleeding within 30 days prior to randomization |
Regorafenib (1.60%) Placebo (0.52%) |
|
Abou-alfa et al. NEJM 201881 |
CELESTIAL Study Phase 3 RCT Cabozantinib (n = 470) vs Placebo (n = 237) |
Subjects with untreated or incompletely treated varices with bleeding or high risk for bleeding Concomitant anticoagulation, at therapeutic doses, with anticoagulants |
Cabozantinib (1.71%) Placebo (2.52%) |
|
Zhu et al. Lancet 201955 |
REACH-2 Study Phase 3 RCT Ramucirumab (n = 470) vs Placebo (n = 237) |
Any bleeding episode considered life-threatening, or any Grade 3 or 4 gastrointestinal bleeding episode in the 3 months prior to randomization Oesophageal or gastric varices that require intervention or represent high bleeding risk. |
Ramucirumab (1.02%) Placebo (1.05%) |
|
Finn et al. J Clin Oncol 202062 |
KEYNOTE-240 study Phase 3 RCT Pembrolizumab (n = 279) vs Placebo (n = 134) |
Oesophageal or gastric variceal bleeding within the last 6 months |
Pembrolizumab (1.08%) Placebo (0.75%) |
Note
- Data obtained on clinical trial.gov.
- Abbreviations: INR, international normalized ratio; PHT, portal hypertension; RCT, randomized-controlled trial.
6 PORTAL HYPERTENSION AND THE NEW COMBINATION BEVACIZUMAB AND ATEZOLIZUMAB
Bevacizumab is an antibody directed against VEGF who plays a major role in PHT pathophysiology as described before. Atezolizumab is an immune checkpoint inhibitor (anti-PDL1) which aims to restore anti-tumour immunity and may contribute to PAMPs and DAMPs elimination. Therefore, these two molecules could theoretically limit the onset of PHT. However, in the Imbrave 150 study, bleeding events were more frequently observed with the combination Bevacizumab and Atezolizumab than with Sorafenib (25.2% vs 17.3%), including 7% and 4.5% of gastrointestinal bleeding, respectively, and 2.4% and 0.6% of AVB linked to PHT.13 In this study, the patients were well selected, with a majority of patients with controlled viral hepatitis who benefitted from optimal PHT prophylaxis. In series where prophylactic treatment to prevent AVB was less standardized, 10% of patients included in Phase II trials of HCC using bevacizumab presented bleeding complications related to PHT.54 Therefore, the impact of the combination of Bevacizumab and Atezolizumab on PHT remains to be determined. Indeed, even if VEGF contributes to PHT through angiogenesis process, this growth factor is also required for sinusoidal homeostasis. Inhibition of VEGF may impact preserved liver sinusoids in the non-tumoral liver leading to sinusoidal alterations, impairment of oxygen and nutrient supply to hepatocytes causing cell death and the recruitment of inflammatory cells, consequently worsening the underlying liver disease and PHT. In addition, the use of Atezolizumab, which promotes cytotoxic lymphocytes, may also contribute to increase production of proinflammatory cytokines that might activate innate immune cells, as well as HSCs and LSECs, and consequently drive liver fibrosis, chronic inflammation and PHT,89 especially in NASH and alcoholic liver disease where chronic inflammation plays a major role in the progression of the liver disease. Immunotherapy agents have also been linked to the occurrence of colitis.90 This immuno-mediated side effect might promote bacterial translocation in the setting of cirrhosis and favours chronic inflammation. Thus, promoting inflammation in a setting of diseases where chronic inflammation plays a major role could be also detrimental. Last, but not least, hepatic sinusoidal obstruction syndrome leading to PHT was also noticed after nivolumab treatment, and one can hypothesize that this is also the case with other immunotherapies.91 Hence, further studies are needed to evaluate the impact of the new combination Atezolizumab and Bevacizumab in real life, especially in patients with more severe PHT status. Until then, one can only recommend strict screening and monitoring of PHT, by regular endoscopies, and optimal prophylaxis of AVB.
In conclusion, PHT and HCC are linked in a pathophysiological point of view through angiogenesis process and chronic inflammation but also in the clinical management of the patients. Combination of PHT and HCC is associated with poorer prognosis in patients with cirrhosis. A better understanding of the crosstalk between these two complications of cirrhosis is mandatory to improve the outcome of these patients, especially in the era of Atezolizumab and Bevacizumab as a first line for advanced HCC.
ACKNOWLEDGEMENT
None.
CONFLICT OF INTEREST
The authors do not have any disclosures to report.