Hepatitis E: An update on One Health and clinical medicine
Thirumalaisamy P. Velavan and Srinivas Reddy Pallerla contributed equally.
Abstract
The hepatitis E virus (HEV) is one of the main causes of acute hepatitis and the de facto global burden is underestimated. HEV-related clinical complications are often undetected and are not considered in the differential diagnosis. Convincing findings from studies suggest that HEV is clinically relevant not only in developing countries but also in industrialized countries. Eight HEV genotypes (HEV-1 to HEV-8) with different human and animal hosts and other HEV-related viruses are in circulation. Transmission routes vary by genotype and location, with large waterborne outbreaks in developing countries and zoonotic food-borne infections in developed countries. An acute infection can be aggravated in pregnant women, organ transplant recipients, patients with pre-existing liver disease and immunosuppressed patients. HEV during pregnancy affects the fetus and newborn with an increased risk of vertical transmission, preterm and stillbirth, neonatal jaundice and miscarriage. Hepatitis E is associated with extrahepatic manifestations that include neurological disorders such as neuralgic amyotrophy, Guillain-Barré syndrome and encephalitis, renal injury and haematological disorders. The risk of transfusion-transmitted HEV is increasingly recognized in Western countries where the risk may be because of a zoonosis. RNA testing of blood components is essential to determine the risk of transfusion-transmitted HEV. There are currently no approved drugs or vaccines for HEV infections. This review focuses on updating the latest developments in zoonoses, screening and diagnostics, drugs in use and under development, and vaccines.
Key points
- Hepatitis E virus (HEV) is also transmitted zoonotically and should be considered under a One Health concept to prevent the spread from animal hosts to humans.
- An acute HEV infection can be aggravated in pregnant women, organ transplant recipients, patients with pre-existing liver disease, HIV-infected and cancer patients receiving chemotherapy.
- HEV infections are associated with extrahepatic manifestations including neurological disorders.
- HEV RNA in blood and stool indicates an acute or chronic infection, and the risk of transfusion-transmitted HEV is increasingly recognized.
- There are no approved drugs and the only vaccine Hecolin® is solely licensed in China.
1 INTRODUCTION
Hepatitis E virus (HEV) is one of the leading causes of acute hepatitis in developing and developed countries, infecting about 20 million people in developing countries, resulting in 3.3 million symptomatic cases and 44 000 deaths per year.1 HEV not only infects hepatocytes, but also extrahepatic tissues.2 Studies on HEV seroprevalence have shown that it causes a significant burden, and one-third of the world population is infected at least once in their lives.1 HEV is a single-stranded RNA virus and belongs to the genus Orthohepevirus with the species Orthohepevirus A, B, C and D within the Hepeviridae family. It is known that the Orthohepeviruses A, C and D infect mammals.3 Orthohepevirus A comprises eight genotypes, HEV-1 to HEV-8,4 and all eight belong to a single serotype.5 The HEV-1 to HEV-4 and HEV-7 genotypes are known to infect humans.
HEV-1 and HEV-2 are transmitted via the faecal-oral route. On the other hand, HEV-3 and HEV-4 infect humans and animals mainly in developed countries and are transmitted zoonotically through the consumption of undercooked pork, contact with wild animals or via blood transfusion. A recent review indicates varying seroprevalences (0.03%-52%) in western countries, with the highest prevalence reported from the Netherlands, Germany and France.6 Most acute HEV infections are self-limiting and resolve within a few weeks without treatment. However, deaths are reported because of fulminant hepatitis in 0.5%-4% of patients in different geographical regions.7 Currently, there are no approved drugs or vaccines against HEV infections, but patients are treated with broad-spectrum antiviral drugs, PegIFN2alpha and ribavirin (RBV) as mono- or combination therapy. The only HEV vaccine Hecolin®, is solely approved and available in China.
This review of hepatitis E aims to provide updates on the current literature relevant to One Health and clinical medicine.
2 ZOONOSES AND ONE HEALTH
The spillover of pathogens from animal hosts to people termed ‘Zoonotic transmission is increasingly recognized and is a major contributor to many emerging infectious diseases.8 The One Health notion acknowledges the interdependence of human, animal and environmental health.9, 10 It is a collaborative, multi-disciplinary approach that aims in the promotion, improvement, and defense for the health and well-being of all species.11 Since HEV infections can be transmitted zoonotically, they should be considered under the One Health concept to prevent the spread of HEV from animal hosts to humans.
HEV infections are identified in a wide range of animal species12, 13 (Table 1). This includes domestic animals, companion, zoo and wild animals. Some animal species harbour specific HEV genera or genotypes, adapted to them and are predominantly found within these species as a virus reservoir.12 In other animal species, only a few isolated infections have been reported with low prevalence.13
| Orthohepevirus A | Orthohepevirus B | Orthohepevirus C | Orthohepevirus D | |
|---|---|---|---|---|
| Host/source | Human and mammals | Avian HEV (AHEV), primarily chicken, wild birds | Rodents, rat, ferret, mink, bandicoot, Asian musk shrew, Chevriers field mouse, Pere Davis mole, humans32 | Bats |
| Genotypes (Gt) | Gt1-8 (HEV-1 to HEV-8) | Gt1-Gt4 (putative Gt5) and several unassigned strains from wild birds149 | Gt C1 (rat) and Gt C2 (ferret), putative Gt C3 and GT C4 | Gt D1 and Gt D2150 |
| Distribution |
Gt1, Gt2: mainly in resource-limited countries; Gt3, Gt4: mainly in industrialized countries; Gt5, Gt6: wild boar Gt7: Middle East, Africa, Arabia; Gt8: China |
Gt1: Australia, Korea Gt2: Europe (Poland), USA, Korea Gt3: Europe, China, USA Gt4: Hungary, Taiwan Gt5: China (Hebei) |
Gt C1: Germany, Australia, France, Spain, Italy, USA, Vietnam, China and several other countries Gt C2: Japan, USA, Netherlands |
Germany, China |
| Impact on one health and public health |
Gt1 and Gt2: larger outbreaks in humans, high morbidity and mortality in pregnant Gt3, Gt4, Gt7: zoonosis Gt3, Gt4: chronic hepatitis E |
Main causative agent of hepatitis-splenomegaly syndrome (HSS) and big liver and spleen disease (BLSV); Hepatic rupture haemorrhage syndrome (HRHS) | Zoonosis | No zoonotic impact |
| Transmission |
Gt1and Gt2: Faecal-oral Gt3, Gt4, and Gt7: zoonotic |
Faecal-oral | Zoonotic potential and cross-species transmission | n.d. |
- Abbreviation: n.d., not defined.
There is increasing incidence of hepatitis E throughout Europe,14 and much is still unknown about its distribution, sources and transmission routes in humans and animals.15, 16 A collaborative network "HEVnet" (https://www.rivm.nl/en/hevnet) uses the "One Health" approach to understand the HEV sources and transmission routes by molecular epidemiology, thus supporting the prevention and control.15 Similarly, several other initiatives follow the "One Health" concept and create One Health surveillance databases to understand the epidemiology of hepatitis E distribution in humans, animals and environmental samples in European countries.17, 18
Within Orthohepevirus A (Table 2), the human-pathogenic HEV-3 and -4 have been shown to be widely distributed in domestic pigs and wild boars, indicating a potential virus reservoir.12 In addition, these genotypes have been detected in a wide range of other mammalians including cattle, horse, goat, dog, cat and sheep; however, in most cases they occur at low prevalences.13 In addition, HEV-3 has been detected in several deer species and human hepatitis E cases have been described after consumption of deer meat.19 However, recent analyses indicate that deer is only infected by close contact to infected wild boars, thus arguing against a true virus reservoir function for these animals.20 Furthermore, HEV-5 and HEV-6 have only been sporadically detected in wild boars in Japan so far. However, a recent study showed that HEV-5 can infect cynomolgus monkeys, thus, indicating a zoonotic potential.21 HEV-7 is specifically observed in camel populations and is prevalent in dromedary camels in North and East Africa, United Arab Emirates and Pakistan.22 A case of chronic human infection with HEV-7 has been described in 2016 in an immunosuppressed patient who regularly consumed camel meat and milk.23 HEV-8 has only recently been identified in Bactrian camels from China.24 Although it has not been detected in humans so far, it was recently demonstrated to be capable of infecting cynomolgus macaques indicating a HEV-8 related emerging zoonosis.25
| Host/source | HEV subtypes | Distribution | Transmission | Impact on one health and public health | |
|---|---|---|---|---|---|
| HEV-1 | Humans, higher primates such as Rhesus monkeys (Experimental); Chimpanzees (Natural and Experimental); questionable in horses152 | HEV subtypes 1a-1g |
Predominantly in resource-limited countries 1a: India, Asia; 1b: Asia, Pakistan, Cuba 1c: Asia, Uzbekistan; 1d: Morocco, Algeria 1e: Africa |
Faecal-oral | Responsible for larger outbreaks in humans |
| HEV-2 | Humans, higher primates such as Rhesus monkeys (Experimental); Chimpanzees (Natural and Experimental) | HEV subtypes 2a and 2b |
Predominantly in resource-limited countries 2a: Mexico 2b: Africa |
Faecal-oral | Responsible for outbreaks in humans in Africa153 |
| HEV-3 | Humans, domestic pigs, wild boars, deer and other mammalian animals: rodents, rabbits, cattle, horses, goats, sheep, dogs, cats, mongoose, bottlenose dolphin |
HEV subtypes 3a-3m, 3ra (rabbit) divided in three clades: 3.1: 3a, 3b, 3c, 3h, 3i, 3j 3k, 3I, 3m 3.2: 3e, 3f, 3g 3.3: rabbit strains 3ra 3c, 3e, and 3f are predominant in Europe151 |
Predominant in industrialized but also in source-limited countries 3a: Asia, Europe; 3b: Asia, Canada 3c: Netherlands, France, Germany 3d: Taiwan; 3e: Greece, France, Spain, Germany, UK; 3f: Germany, UK, France, Spain 3g: Kyrgyzstan; 3h: Italy, Uruguay, New Zealand; 3i: South America; 3j: Canada, Australia, Mexico 3l and 3s: Switzerland154, 155 |
Zoonotic | Zoonosis, acute and chronic infection, extra hepatic manifestation |
| HEV-4 | Humans, and mammals: domestic pigs, wild boars, cattle, goats, sheep, deer, leopard, black bear, yak. Questionable in birds (crowned crane, silver pheasant) | HEV subtypes 4a-4i |
Predominant in Asia, circulating in Europe 4a: China; 4b: Asia; 4c: Asia; 4d: China, Italy 4e: India; 4f: Asia, Germany; 4g: China |
Zoonotic | Zoonosis, acute and chronic infection, more frequent fulminant hepatitis156 |
| HEV-5 | Wild boar157 | HEV subtypes 5 and 5a | Japan | Not defined | Zoonosis unclear |
| HEV-6 | Wild boar157 | HEV subtypes 6 and 6a | Japan | Not defined | Not defined |
| HEV-7 | Dromedary camel, humans158 | HEV subtypes 7a (DcHEV-178C) and 7 (DcHEV-180C) | Middle East, Africa, Saudi Arabia | Zoonotic | Zoonosis |
| HEV-8 | Bactrian camel158 | HEV subtypes 8a (12XJ) and 8 (BcHEV-GP) | China | Not defined | Not defined |
Rabbits are infected with viruses from a specific clade of genotype 3 (rabbit HEV or genotype 3ra), pointing out for a host specificity for this virus.26 This virus type is broadly distributed in domestic and wild rabbits in many countries of the world.27 The first human infections with rabbit HEV were described in France in 2017, where one immunocompetent patient with underlying cirrhosis and four immunocompromised patients were affected.28 Recently, additional rabbit HEV infections have been reported in three solid organ transplant recipients from Switzerland.29
Orthohepevirus C contains viruses infecting rats, ferrets and minks. The rat HEV was first identified in 2010 in wild Norway rats from Germany.30 Meanwhile, it has been shown to be widely distributed in different species worldwide indicating its broad circulation in rats.31, 32 As the rat HEV genome shares only <56% nucleotide sequence identity with HEV-1 to -4, the zoonotic potential of this virus was initially assessed to be low.33 However, a first indication, that the virus can also infect mammals, came from its detection in a brown bear in 2017.34 Thereafter, a first human case of persistent hepatitis caused by rat HEV was published in 2018 affecting an immunosuppressed liver transplant patient in Hong Kong.19 One year later, rat HEV was described as the etiological agent of severe acute hepatitis in an immunocompetent patient from Canada.35, 36 Meanwhile additional seven patients with rat HEV infection have been identified in Hong Kong,35 indicating an emergence of this virus.37 Although similar rat HEV strains have been identified in rats from Hong Kong, the distinct transmission pathways to the human cases could not be clarified so far.35
Representatives of the other virus species within the family Hepeviridae have not been found in humans so far and are therefore not considered as zoonotic agents. Orthohepevirus B is known to occur in birds, especially in chicken,38 whereas Orthohepevirus D in bats.38 The viruses of the genus Piscihepevirus have so far only been detected in cutthroat trouts and are suspected to solely infect fish species.38
Well-investigated transmission pathways of zoonotic HEV genotypes from animals to humans include occupational exposure,39 consumption of undercooked meat and meat products prepared from infected animals.12 However, contamination of other food types, eg shellfish or berries, by contaminated irrigation water or fertilizer originating from pigs, have been supposed as another possible foodborne transmission route. In addition, transmission through contact with environmental water sources contaminated by animals, has also been suggested.
The foodborne transmission of HEV is of particular interest, because it is assumed to be one of the most important routes and measures of food hygiene may be applied to prevent virus transmission. Therefore, studies have investigated the presence of HEV RNA in meat and meat products, indicating prevalences of 0%-21% in domestic pig livers and 2%-38% in wild boar livers in different countries of the world.12, 40, 41 In processed meat products containing porcine liver such as liver sausages, the detection rates are even higher (between 16% and 47%), which may be explained by mixing the livers from several animals into one product. However, detection of HEV RNA does not necessarily mean the presence of infectious virus as HEV may be inactivated during the food production process. Because of the lack of a robust and easy-to-perform cell culture system for HEV infectivity measurement, only a few studies could demonstrate infectivity directly in food.42 Inactivation of HEV has therefore only been analysed using cell culture-adapted strains or experimental inoculations into pigs.43 A cell culture study indicated, that heating for 70°C for 2 min is sufficient for a >3.9 log decrease of infectivity.44 In contrast, heating at 71°C for 20 min was necessary in order to completely inactivate HEV present in a liver paté as assessed by experimental inoculation into pigs.45 Other factors usually applied for conservation of food are lowering pH and increasing salt concentrations. However, recent investigations using a cell culture isolate indicate a very high stability of HEV against pH 2-9 as well as against the highest salt concentrations usually applied during meat production.46, 47 Consumption of under-cooked raw meat products, should therefore be avoided by individuals who are at high risk for development of severe hepatitis E.
3 HEPATITIS E IN DISTINCT POPULATIONS
3.1 Pregnant women
Hepatitis E virus-1 during pregnancy is associated with increased rates of premature delivery, miscarriage and stillbirths, in developing and low-middle income countries.48 The clinical complications often include acute liver failure, bleeding and eclampsia gravidarum. It is believed that hormonal (progesterone and oestrogen) and existing immune conditions, high viral loads, nutritional status and host factors may promote fatal HEV-1 courses during pregnancy.49, 50 In endemic regions, pregnant women infected with HEV-1 in the third trimester are at high risk with relative mortality and morbidity of ~15%-60%.51 HEV-1 related deaths were high among pregnant women in Bangladesh.52 The 1986-1988 outbreak in China reported 119 000 cases causing 707 deaths, including 414 pregnant women.53 Also, HEV seroprevalence among pregnant women in Africa is between 29% and 84% among women in Egypt, with a low seroprevalence of 6.6% in Gabon.54 Despite high seroprevalence in Egypt, no related deaths have been documented. Several host and viral factors could be responsible for the variations in the outcomes. Host genetic factors such as mutations in progesterone receptors (PROGINs: 320bp Alu insertion in intron G, V660L and H770H) were shown to modulate immune responses.55 The PROGIN polymorphisms are associated with the clinical course in pregnancy.56 Acute and chronic HEV-3 infections in pregnancy were also reported in pregnant women, but the infections resolved eventually without any complications.57, 58
3.2 Solid organ transplant recipients and other immunocompromised individuals
A subgroup of patients undergoing solid organ transplantation (SOT) (eg heart, kidney, liver and renal pancreas) appear to develop chronic HEV infection. Natural course of infection is not fully understood, but rapid progression to cirrhosis has been reported. These patients are high-risk groups for HEV infection because of their immunosuppressive status.59 Patients on immunosuppressive drugs, such as mTOR inhibitors, have a higher risk of chronic infection.60, 61 In addition, lymphocyte counts and viral diversity have been associated with chronic progression of HEV infection in 40%-70% of immunosuppressed patients.62, 63 The overall prevalence of HEV infection in SOT recipients in Western Europe varies between 0.7% and 1.5%, but is higher (3.2%) in lung transplant recipients.64-66 Persistent HEV infections are reported in patients with inherent immunodeficiencies67 or individuals receiving immune-modulating agents for autoimmunity and malignancies.60, 68, 69
3.3 New implications
Hepatitis E virus primarily infects liver tissue, but also replicates in non-hepatic tissue such as brain, placenta, kidney, small intestine and spleen, possibly associated with extrahepatic manifestations.2 In addition, cross-reactive immune responses may lead to tissue damage in other organs.70 The most commonly observed extrahepatic manifestations include neurological disorders such as neuralgic amyotrophy (NA), Guillain-Barré syndrome (GBS), encephalitis, renal injuries and haematological disorders.71, 72 Studies show that about 5%-10% of GBS patients were diagnosed with HEV infections.73-76 HEV RNA was detected in cerebrospinal fluid samples from patients with GBS and NA.77-80 The nucleic acid sequences from GBS patients revealed HEV quasispecies compartmentalization,80 and this explains the emergence of novel neurotropic HEV variants. Neurological symptoms are seen among patients infected with HEV-1 in Asia,74 and in acute and chronic patients infected with HEV-3.73 In vitro studies show that HEV-3 proliferates efficiently in neuroblastoma cells.81 Moreover, In addition to central nervous system infections, HEV can cause impairment of kidney function.82
4 SCREENING AND DIAGNOSIS
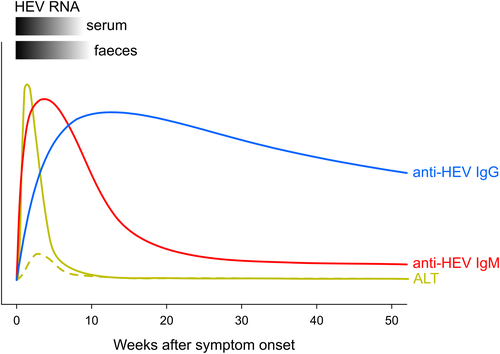
The clinical symptoms of acute hepatitis E are non-specific and resemble the symptoms of hepatitis A.83 The incubation period of HEV lasts 2-9 weeks (15-64 days) in case of an acute infection (Figure 1). RNA can usually be detected in blood 2-6 weeks after infection and in stool for about 2 weeks longer. HEV-specific immunoglobulin M (IgM) antibodies appear in the circulation 3-4 weeks post-infection and persist between four and six months,84, 85 whereas immunoglobulin G (IgG) antibodies remain detectable for several years after recovery.84, 86, 87
4.1 Serology
Enzyme-linked-immune-assays (ELISAs) or immunoblot assays are widely used in serology. Serological markers, either antigen (HEV antigen) or antibodies (IgM and IgG) are used in the diagnosis of acute and chronic infections. Many of these tests use antigens or peptides of the immunodominant epitopes (ORF2 and/or ORF3), and recombinant antigens show higher sensitivity than shorter peptides.,88 HEV antigens are detected between one to three weeks after infection and may persist up to seven weeks in the serum.89 HEV antigen is expressed higher in immune-compromised patients than immune-competent individuals.90 ORF2 Ag assays are used for direct viral detection. This assay detects capsid protein in serum samples with a specificity of 100%.90 Because of its limited sensitivity the ORF2 Ag ELISA is not commonly used in routine diagnostics.91 However, there are data showing that detection of ORF2 Ag may indicate an acute infection more reliably than a positive anti-HEV IgM result.92 As an alternative, anti-HEV IgM based point-of-care immune-chromatographic assays are in use in resource-limited countries.
4.2 Nucleic acid testing
The presence of HEV RNA in blood and stool is an indication of acute or chronic HEV infection. HEV nucleic acid testing (NAT) assays are sensitive, reliable and can detect RNA concentrations as low as 7 IU/mL and can be used together with serological assays to confirm the diagnosis of HEV infection.93 There are several commercial and in-house NAT assays for the detection of HEV in blood and stool. Detection of HEV RNA in urine for diagnostic purposes is currently not recommended since there is only limited data available from chronically infected patients with diagnostic sensitivities of <50%.94 Although reliable, there is little variation in test results, largely because of the primer targets, between genotypes and sub-genotypes.95 Furthermore, the sensitivity of NAT differs among commercial kits and laboratories, because of RNA input volume.96 To address these discrepancies, WHO has developed international standards and reference panels for HEV-1 to HEV-4.96, 97 Assays used for screening of HEV in blood, stool, solid organs and stem cell transplants include conventional reverse transcriptase-PCR (RT-PCR), nested PCR, real-time RT-PCR, digital droplet real-time RT-PCR, transcription-mediated amplification (TMA), loop-mediated isothermal amplification assay (RT-LAMP).82, 98, 99 HEV RT-PCR, nested PCR and LAMP assays can be used as qualitative, whereas, real-time RT-PCR, digital droplet real-time RT-PCR and TMA methods can quantify HEV copies with a limit of detection as low as 7-20 IU/mL.84, 93 TMA methodology is used in high throughput screening in blood samples.100 One-step RT-LAMP single-tube test is able to detect 11 copies/reaction and has been suggested for use as a point-of-care diagnostics.98
4.3 Blood donors
RNA-testing of blood components are essential in order to determine the risk of transfusion-transmitted HEV. The screening of blood components is performed either selectively (in individuals or in pools) or universally, in accordance with national guidelines.101 A first systematic prospective study conducted in the United Kingdom in 2012-2013 revealed that immune suppressed patients were at risk of acquiring HEV through blood transfusions.102 HEV RNA testing was introduced in UK since 2016 to prevent transfusion transmitted HEV infection, and later implemented as universal screening. Since November 2012, over 3.2 million blood donor samples have been tested for HEV RNA in the EU and the study reports that one in 3,109 donations is HEV RNA positive.101 Transfusion associated HEV infections were well-documented in UK, Japan and among other European nations.103 As there is a high risk of transfusion-transmitted HEV infections, seven countries in the European Union including Ireland, Netherlands, France, Germany, Spain, Austria and Luxembourg implemented universal and/or selective HEV RNA screening.101 It is important to note that blood donor screening has to be performed with HEV-RNA testing as HEV antibodies are frequently negative in viraemic asymptomatic blood donors.104
5 TREATMENT OPTIONS
Most of acute HEV infections are self-limiting, as the disease appears mild in immunocompetent patients and therefore supportive management can be recommended. However, acute infection can be aggravated in immunocompromised patients, in particular organ transplant recipients, HIV-infected patients and cancer patients receiving chemotherapy. Acute-on-chronic liver failure because of HEV infection is an increasing concern in western countries. Currently there are no approved drugs for treatment, but patients are treated with broad-spectrum antiviral drugs, PegIFN2alpha and RBV. However, both drugs are contraindicated for use in pregnant women. Treatment indications and algorithms have been suggested in guidelines developed by the European Association for the Study of the Liver in 2018.105 Several RBV-associated mutations have been described,106 with G1634R being the most prominent and best characterized so far.107-109 However, a recent multicenter study showed that these mutations had no effect on the RBV treatment outcome.110 Furthermore, the lack of a robust HEV cell culture models has hampered the development of new antiviral HEV drug candidates. Only little is known about potential virus-host interaction partners.111 A newly developed system raises hope, that this fact will be banned from future articles and new leverage points for intervention strategies can be identified.112 Two strategies are currently used to identify lead compounds: drug repurposing and de novo screening from natural products or raw extracts or chemical screens. The drug candidates can target viral proteins or host proteins; direct-acting antiviral drugs are the drugs that act against the viral proteins. In contrast, indirect-acting antiviral drugs act on the host target proteins.113
Sofosbuvir (SOF) is a nucleotide prodrug, used to treat chronic HCV infections in combination with RBV, PegIFN2alpha and others.114 The drug binds to NS5B-RNA-dependent RNA polymerase of HCV and inhibits viral replication.115 Several in vitro studies in Huh7 and HepG2 cells against HEV-3 genotype and in induced pluripotent stem cell-like cells against HEV-1 to HEV-4 showed promising antiviral activity.116 However, no antiviral activity was observed in HEK293, U-87MG and Huh7 cell lines.117 Furthermore, clinical case studies with SOF in combination with RBV showed mixed results; some patients cleared HEV and some not.118-120 Recently, a multicenter HepNet pilot study was conducted in Europe to treat chronic hepatitis E with SOF only in those patients who did not respond to RBV treatment (Clinical trial. gov ID: NCT03282474).121 This study documented that SOF was able to reduce HEV viral load but was unable to eliminate HEV in immunocompromised patients.
Netzler et al investigated few approved or potential antiviral drugs in various preclinical phases of development against other viruses such as HCV, influenza and dengue virus.122 Two candidates, NITD008 and GPC-N114, showed promising strong anti-HEV activity, and the combination of these drugs showed strong synergistic activity. However, the candidate NITD008 in preclinical studies in rats and dogs reported cytotoxic activity.122
Qu et al screened a library of over 1000 FDA approved drugs and found deptropine, a histamine H1 receptor antagonist, to be a potent inhibitor of HEV replication.123 As HEV causes hepatic and extrahepatic manifestations, the drugs were screened in multiple cell lines. In the Hep3B cell model, treatment with deptropin potentially inhibits HEV without affecting cell viability, excluding the antiviral effect of deptropin through non-specific cytotoxicity. Nisiyama et al performed an extensive screening of 27 antiviral compounds using Gaussia luciferase reporter assays. Several classes of repurposed drugs have been tested, including RBV and SOF.124 This study found that combinations of interferons (IFNs), RBV, SOF and 2'-C-methylguanosine (2CMG) show promising synergistic effects.124
Silvestrol, a natural compound, is known to inhibit the host-directed eukaryotic RNA helicase, required for the unwinding of RNA in the 5′-UTRs of mRNAs.125 Originally, this drug was first shown to be a potent anti-cancer agent in human breast, prostate and hepatocellular carcinoma graft models and is well-tolerated.126 This compound has been screened for antiviral activity for other viruses.127 It was recently evaluated for potential antiviral activity against HEV-3 infected A549 cells, and was shown to inhibit HEV replication without cytotoxicity.128 In another study, the antiviral activity of silvestrol was tested on HepG2/C3A, Huh7.5 and hepatocyte-like cells from human embryonic and induced pluripotent cell lines infected with HEV-1 to HEV-4 genotypes.129 It is shown that silvestrol decreases the HEV replication in a dose-dependent manner. In vivo inhibitory effect in human chimeric liver mice infected with HEV is also reported.129 Both in vivo and in vitro studies show promising antiviral activity and may be a potential candidate.
Recently, FDA-approved T-cell specific immunotherapies for the treatment of cancers.130 It is known that pathogen-specific effector T-cells play an essential role in controlling acute viral infections, making T-cell therapy an attractive alternative to currently used therapies.131 Several T-cell-based therapies are now in development for the treatment of viral and bacterial infections.132 Soon et al showed that HLA-A2-restricted HEV-specific T-cell epitopes targeting HEV RNA helicase and RNA-dependent RNA polymerase were identified in patients with acute-resolving HEV infection. In vitro studies have shown that engineered HEV-specific CD8+ T-cells from chronic hepatitis E patients could recognize the HEV-specific epitopes and kill the target cells.133
Interferons are cytokines secreted by host cells as cellular immune response to viral infections. IFNs trigger interferon-stimulated genes when binding to IFNR, the key antiviral factor in fighting infections.68 Type I IFNs have been used for the standard treatment of HCV and HBV infections.134 Todt et al evaluated the antiviral activity of IFN-α (type I), IFN-γ (type II) and IFN-λ3 (type III) against HEV alone or in combination with RBV in cell culture models and found that the interferons IFN-γ and IFN-λ3 have a moderate activity and the IFNα2a subtype has the strongest antiviral activity.135 However, there were no synergistic effects observed when interferon combined with RBV. Another study showed that the interferons IFN-α2b, IFN-λ1, IFN-λ2 and IFN-λ3 in combination with SOF or 2CMG were synergistically able to inhibit growth and clear HEV in cultures.124
6 VACCINES
Currently, several HEV vaccine candidates are under development. However, Hecolin® is the only licensed vaccine available in China since 2012.136 The vaccine is a recombinant truncated ORF2 protein HEV239 (aa368-606) containing 23nM VLPs. This vaccine has undergone several clinical trials, with the administration of three doses (0, 28 and 180 days). The phase III clinical trial conducted in 112 604 healthy adults (16-65 years) showed that this vaccine was safe and efficacious.136 This vaccine had >99% efficacy against HEV among adults who were followed up for four and half years.137 However, the primary endpoint of the trial was prevention of symptomatic acute hepatitis but not prevention of infection. Thus, the vaccine does yield sterilizing immunity. Another trial evaluated the immunogenicity and safety for an accelerated vaccination regimen (0, 7 and 21 days) and was safe (Clinical trial.gov; ID: NCT03168412).138 This dosage regimen can be recommended for travellers visiting an HEV-endemic regions or used during an HEV outbreak. In addition, another clinical study (Clinical trial.gov; ID: NCT02417597) conducted in healthy adults >65 years of age concluded that Hecolin®, with protective efficacy of 96%, is safe and well-tolerated.139 These clinical trials were conducted in China, where HEV-4 is the most predominant circulating genotype. Thus, the vaccine provided cross-genotype efficacy as Hecolin® is based on a HEV-1 sequence. In parallel, a small phase Ia/Ib clinical trial in USA is underway, to address the safety and tolerability in western population (Clinical trial.gov identifier: NCT03827395). Although a handful of clinical studies with a protective efficacy of more than 90% have been documented, further studies are warranted in high-risk populations such as pregnant women, solid transplant recipients and immune-compromised populations. An efficacy study is currently underway in Bangladesh among non-pregnant females (age 18-45) with Hecolin® vaccine (Clinical trial.gov identifier: NCT02759991).
Along with Hecolin®, several HEV candidate vaccines are currently being developed and target ORF2 structural capsid protein that coats viral particles.140-142 Initial primate studies have shown antibodies raised against ORF2 complete or truncated proteins protected against infections.143 HEV vaccines in various stages of development have been reviewed Cao et al, 2018.140 Most of these vaccines are in preclinical stages and use ORF2 truncated protein as an immunogen. Different expression systems are used for the development of these vaccines.144, 145 It is reported that large-scale vaccination campaigns are not as cost-effective as the infections are mostly benign in the developing world and were under reported.146 Because of the economic viability, few vaccines were dropped from further development. For example, the HEV GSK candidate vaccine was dropped, inspite of a good efficacy in phase II clinical trial.147 Few vaccines are under development in providing combined efficacy against two distinct pathogens; hepatitis A and E, HEV-HBsAg and trivalent HEV-RV-AstV vaccines.148
7 CONCLUSION AND PERSPECTIVES
Hepatitis E virus infection is much more common than previously thought. We are just in the beginning to understand all aspects of HEV virology, immunology, the animal reservoir, modes of transmission and clinical relevance. Moreover, therapeutic strategies and vaccine development need further research.
ACKNOWLEDGEMENTS
The author CTB, RJ, ES acknowledges grants from the German Federal Ministry of Health with regard to a decision of the German Bundestag by the Federal Government (CHED-project grant no: ZMVI1-2516-AUK-701/BMG: 321-4471-02/157) and (NiCaDe-project grant no: ZMVI1-2519GHP711/D81667). The author TPV acknowledges grants from the German Federal Ministry of Education and Research (nos. BMBF01DP19006A, BMBF01DP17047).
AUTHOR CONTRIBUTIONS
TPV, CTB and HWM contributed to the design and wrote the review. TPV and SRP wrote the first draft. RJ contributed to the section on zoonoses and one health. ES and DT contributed to the section on treatment options. JJW and MS contributed to the sections screening and diagnosis and serology. JH contributed to the section on screening and diagnosis. JWKS contributed to the section on vaccines.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.