Incidence and risk factors of anti-tuberculosis drug induced liver injury (DILI): Large cohort study involving 4652 Chinese adult tuberculosis patients
Fanrong Jiang, Huadong Yan and Lili Liang Joint first authors
Funding information
This work was supported by the grants from the Social Development Major Projects of Ningbo City (2016C51005), Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2018ZD039), Zhejiang Provincial Natural Science Foundation (LGF20H030006). The cooperation is to be established and a plaque will be installed – ‘Ningbo No. 2 Hospital Science and Innovation Centre in association with the Faculty of Medicine and Health Sciences, The University of Nottingham. GPA is supported by the NIHR Nottingham BRC (Reference no: BRC-1215-20003).
Abstract
Background and Aims
Anti-tuberculosis drugs remain as an important cause of drug-induced liver injury (DILI) worldwide. Adverse drug reactions reduce the effectiveness of treatment. We aimed to determine the incidence and risk factors associated with anti-tuberculosis DILI (ATDILI).
Methods
Using established criteria and causality assessment methods, risk factors for ATDILI were identified in a contemporary cohort and validated in another cohort prospectively. Independent determinants of ATDILI were identified using Cox regression analysis.
Results
In the derivation cohort (n = 3155), 170 (5.4%) developed ATDILI of which 27 (15.9%) developed jaundice; 9(5.3%) developed acute liver failure (ALF) and 3 died. Among HBsAg positive patients, 11/27 (40.7%) of ATDILI developed after 3 months of starting treatment. In addition, of 218 (6.9%) who developed raised alanine transferase (ALT) levels ≥3 times upper limit normal, 193 (88.5%) resolved and 25 (11.4%) progressed to DILI. Age (HR = 1.014, 95% CI: 1.005-1.023), baseline ALT (HR = 1.014, 95% CI: 1.003-1.024), haemoglobin (HR = 1.011, 95% CI: 1.002-1.020) and HBsAg positivity (HR = 1.516, 95% CI: 1.004-2.290) were independent risk factors for DILI. In the second cohort (n = 1497) of which 85 (5.7%) developed ATDILI. Age (HR = 1.029, 95% CI: 1.003-1.056), baseline AST (HR = 1.036, 95% CI: 1.010-1.062), previous TB treatment (HR = 3.894, 95% CI: 1.304-11.625) and active drinking (HR = 3.624, 95% CI: 1.147-11.454) were risk factors for developing jaundice.
Conclusion
Elevation of ALT of ≥3 × ULN during anti-TB treatment resolves in the vast majority without developing serious consequences. In two cohorts involving 4652 patients, incidence of ALF and death because of ATDILI are low. Age, baseline ALT, haemoglobin and HBsAg positivity are risk factors for the development of DILI and these inform monitoring and management of these patients.
1 INTRODUCTION
Tuberculosis (TB) remains a major health problem worldwide. In 2018, there were an estimated 10 million new cases of TB reported and 1.45 million deaths including 2% of those with human immunodeficiency virus (HIV) co-infection. In China, prevalence of TB is 61 per 100,000 population and 2.6 per 100,000 population dying of TB.1 In order to control the disease, Chinese goverment established the China National Tuberculosis Prevention and Control Scheme in 1990 and implemented directly observed treatment strategy (DOTS) since 1991.2, 3 The main DOTS treatment was the first-line anti-TB treatment including isoniazid (INH), rifampicin (RFP), ethambutol (EMB) and pyrazinamide (PZA) for 6-9 months. However, adverse drug reactions during the course of anti-TB treatment pose an additional challenge. Alternative agents can have greater problems with toxicity and are often less effective, and treatment could be prolonged with attendant challenges to ensure compliance.4, 5
Anti-tuberculosis drug-induced liver injury (ATDILI) is still one of the most important adverse effects with a potential to lead to liver failure and death.6, 7 DILI is the most common adverse reactions resulting in change of treatment or treatment interruption 7 days or more4 and therefore contributes to reduced effectiveness of treatment as well as non-compliance.8 Anti-TB drugs are also one of the common drugs in combination to cause DILI and liver failure in low, middle, and high income countries.9-12 The incidence of ATDILI varies widely between 2% and 28% dependent upon the characteristics of the particular cohort, drug regimens involved and in particular combination of liver enzymes and bilirubin thresholds used to define DILI.9-12 Accurate and timely recognition of DILI is important to prevent serious consequences, however, drug-interruption or withdrawal would reduce its effectiveness.4 In addition, although several risk factors have been reported to be associated with the development of ATDILI,13-16 these studies have important limitations. Tweed et al13 set out ALT threshold of >5 × ULN to identify DILI in 58 out of 1928 patients on three different drug combinations, but, the risk factors for DILI were assessed in 150 individuals with ALT or AST >3 × ULN (not the definition of DILI that was chosen a priori). In addition, authors did not perform multivariate analysis to identify independent risk factors. Singanayagam et al14 described 21 patients with >3 × ULN ALT (including 10 with ALT >5 × ULN) among 288 on anti-TB treatment. Multivariate modelling was again not performed. Abbara et al15 included 105 cases of confirmed or presumed DILI among 1529 patients on anti-TB drugs (including 12 with raised bilirubin without liver enzyme elevation). Of these 77 patients had ALT 3-5 × ULN with symptoms or an ALT >5 × ULN, thereby meeting American and British Thoracic Society criteria for stopping TB therapy were included in the linear regression modelling. In addition to these shortcomings, none of the three studies validated their findings in an independent cohort.
In this context, the present study aims to investigate incidence and risk factors associated with ATDILI in a large, contemporary cohort of consecutive TB patients receiving first line intensive therapy based on international consensus case definitions of DILI.
2 PATIENTS AND METHODS
2.1 Study design
A large contemporary cohort of consecutive TB patients from July 2011 to December 2015 (derivation cohort) and another prospective cohort from January 2016 to December 2017 (validation cohort) in department of infectious diseases and liver diseases, Hwamei Hospital, University of Chinese Academy of Sciences were used. The study fulfilled the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Ningbo No.2 Hospital. Written informed consent was obtained from each participant or their legal representatives before enrolment in prospective cohort. All authors had access to the study data and had reviewed and approved the final manuscript.
2.2 Patients
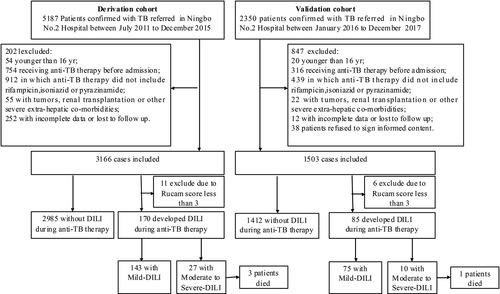
All consecutive patients diagnosed with active pulmonary or extra-pulmonary TB and treated with first line anti-TB drug including RFP, INH, PZA and EMB were eligible for inclusion in this study. Electronic medical record system has in-patients and out-patients’ clinical information recorded including prescriptions, laboratory tests during each visit. There are no particular guidelines that are accepted in practice. But, in general, liver biochemistry is performed 2-4 weeks during the first 2 months and monthly afterwards. Exclusion criteria are listed in Figure 1. (a) Patients younger than 16 years old; (b) patients who received non-standard treatment regimen initially (anti-TB therapy did not include RFP, INH or PZA; (c) Patients receiving anti-TB therapy before admission without baseline data; (d) Patients with tumors, renal transplantation or other severe extra-hepatic co-morbidities; (e) patients who declined consent.
All patients diagnosed with active pulmonary or extra-pulmonary TB receive a standard antituberculosis treatment regimen with INH (5 mg/kg/d), RFP (10 mg/kg/d), PZA (25 mg/kg/d) and EMB (15 mg/kg/d) in the first two months. After two months, all patients were treated INH, RFP and EMB for 4 or more months based on the treatment guideline.17
2.3 Data collection
The following parameters were collected for all the enrolled patients: (a) demographics; (b) tuberculosis history and which organ/s involved and how long; (c) information about the implicated anti-tuberculosis drug including RFP, INH, PZA and EMB. The time of onset after starting treatment and the time of DILI diagnosis and recovery after stopping the drug; (d) Co-prescriptions of other drugs (e) symptoms and signs, including time of occurrence, time of resolution, and symptoms at discharge, were recorded in detail; (f) serum biochemical parameters before anti-TB treatment and during the DILI, including values of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), glutamyl transpeptidase (GGT), serum total bilirubin (TBil), albumin, international normalized ratio (INR), creatinine, white blood cell count (WBC), haemoglobin, platelet; (g) examinations for excluding other causes of liver injury (including hepatitis A virus, hepatitis B virus, hepatitis C virus, hepatitis E virus, Epstein-Barr virus, cytomegalovirus, herpes virus, Wilson disease, and autoimmune hepatitis); and (h) severity and mortality of all enrolled patients during and after hospitalization.
2.4 Causality assessment
Before the diagnosis of DILI is confirmed, causality assessment was completed according to the Roussel Uclaf Causality Assessment Method (RUCAM).18, 19 In two groups, cases with scores equal or greater than 9 (categorised as “highly probable”) and cases with scores equal or >6 (categorised as “probable”) diagnosis of DILI were accepted. Cases with RUCAM scores between 3 and 6 were further reviewed by three hepatologists (Dr Yaoren Hu, Ting Hu and Huadong Yan) with expertise in DILI. Cases judged by at least two of the three hepatologists as “probable” were also enrolled in the study.
2.5 Diagnostic criteria, clinical presentation, severity and management of ATDILI
Case definition for ATDILI was established according to international consensus criteria as20: (a) More than or equal to fivefold elevation above the upper limit of normal (ULN) for alanine aminotransferase (ALT); (b) More than or equal to twofold elevation above the ULN for alkaline phosphatase (ALP; particularly with accompanying elevations in concentrations of 5′-nucleotidase or γ-glutamyl transpeptidase in the absence of known bone pathology driving the rise in ALP level); (c) More than or equal to threefold elevation in ALT concentration and simultaneous elevation of bilirubin concentration exceeding twofold ULN. Severity of DILI was defined and grouped into mild, moderate, severe and fatal according to the International Consensus Criteria.20 The clinical phenotype of DILI was classified based on the R value (ratio) calculated from the liver tests obtained at presentation (R value = [serum ALT/ALT ULN]/[serum ALP/ALP ULN]). Cases were classified as ‘hepatocellular’ if R value ≥ 5.0, ‘cholestatic’ if R value ≤ 2.0 and ‘mixed’ pattern if R value was 2.1-4.9.
In general, during the first two months of chemotherapy, patients underwent routine blood examination, and the liver function test was evaluated every 2 weeks. Subsequently, patients received routine monthly examination. Decision regarding treatment interruptions, withdrawals and reintroductions were individualized by the clinicians in charge TB care and although no protocol was established or guidelines were adopted to determine these, in general, anti-TB therapies were adjusted based on concomitant clinical symptoms.
2.6 Statistical analysis
Continuous variables were expressed as mean standard deviation (SD) or median with interquartile range. Continuous data between the two groups were compared via Student's t test or Mann-Whitney U-test. Nominal variables were expressed as number/percentage and compared using Chi-square test. The risk factors of ATDILI were determined by univariate and multivariate cox regression analysis. Candidate variables (P < .10) after a bivariate analysis were entered into a multivariate Cox regression analysis by a backward-forward approach. For multivariate analysis, the entry and removal probability for stepwise was set as .05 and .10, respectively, and variables with P < .05 were kept as risk factors of ATDILI. The cumulative incidence of ATDILI was calculated using Kaplan-Meier method to deal with the censored data. Statistical analysis were performed using SPSS16.0 (SPSS Inc.), GraphPad Prism 5 (GraphPad Software). A two-sided p value <.05 was set as the significant level.
3 RESULTS
3.1 Patients
As shown in Figure 1, a total of 3155 patients with pulmonary or extra-pulmonary TB were enrolled into the derivation cohort from 5187 initially screened patients. The baseline characteristics of the study cohort are shown in Table 1. There were 2019 (64.0%) males and mean age of the patients was 37 ± 13 years. The median body mass index value of the patients was 19.7 kg/m2 (interquartile range, 4.0 kg/m2). Excess alcohol intake and HBsAg positivity were noted in 304 (9.6%) and 325 (10.3%), respectively; three had coinfection with HIV. In the derivation cohort, 2355 patients (74.6%) had pulmonary TB, 256 patients (8.2%) had extra-pulmonary TB, 544 patients (17.2%) had both. 1469 patients (46.6%) were culture confirmed (Table S1), 222 patients (7.0%) had previous history for TB treatment.
| Characteristic | Derivation cohort (N = 3155) | No DILI (N = 2985) | DILI (N = 170) | P value no DILI/DILI | Validation cohort (N = 1497) | P value derivation/validation |
|---|---|---|---|---|---|---|
| Age (y) | 36 (27) | 36 (27) | 44 (25.3) | .006 | 42 (32) | <.001 |
| Male no. (%) | 2019 (64.0) | 1906 (63.9) | 113 (66.5) | .53 | 969 (64.7) | .63 |
| BMI (kg/m2) | 19.8 (4.1) | 19.7 (4.1) | 20.1 (3.9) | .11 | 19.5 (2.8) | .15 |
| DM no. (%) | 267 (8.5) | 248 (8.3) | 19 (11.2) | .21 | 153 (10.2) | .05 |
| Hypertension no. (%) | 221 (7.0) | 207 (6.9) | 14 (8.2) | .49 | 141 (9.4) | .004 |
| Alcohol use no. (%) | 304 (9.6) | 289 (9.7) | 15 (8.8) | .66 | 154 (10.3) | .49 |
| HBsAg positive no. (%) | 325 (10.3) | 298 (10.0) | 27 (15.9) | .008 | 124 (8.3) | .03 |
| HIV positive no. (%) | 3 (0.1) | 2 (0.1) | 1 (0.6) | .07 | 2 (0.1) | .71 |
| HCV positive no. (%) | 2 (0.1) | 3 (0.1) | 0 | 1.00 | 4 (0.3) | .16 |
| Previous TB treatment no. (%) | 222 (7.0) | 213 (7.1) | 9 (5.3) | .33 | 78 (5.2) | .02 |
| Lab test | ||||||
| ALT (U/L) | 13 (13) | 13 (12) | 16 (15) | .001 | 15 (13) | <.001 |
| AST (U/L) | 19 (9) | 19 (9) | 20.5 (10) | .003 | 18 (9) | .103 |
| Albumin (g/dL) | 37.7 (6.9) | 37.7 (6.9) | 38.8 (7.5) | .10 | 37.9 (7.6) | .09 |
| Total Bilirubin (μmol/l) | 10.5 (8.4) | 10.5 (8.3) | 10.7 (10.1) | .04 | 9.6 (8.8) | <.001 |
| ALP (μmol/l) | 76 (30.3) | 76 (30.1) | 75.5 (29.5) | .90 | 78 (31) | .19 |
| GGT (μmol/l) | 21 (20) | 21 (20) | 25 (22.3) | .26 | 23 (22) | <.001 |
| WBC (109/L) | 6.3 (2.5) | 6.3 (2.6) | 6.3 (2.4) | .45 | 6.4 (2.8) | .07 |
| RBC (1012/L) | 4.4 (0.74) | 4.4 (0.73) | 4.5 (0.78) | .16 | 4.3 (0.76) | <.001 |
| Haemoglobin (g/L) | 128 (23) | 128 (23) | 132 (27) | .003 | 125 (23) | <.001 |
| Platelet (109/L) | 235 (105.3) | 237 (107) | 225 (93) | .13 | 241 (115) | .05 |
| Creatinine (umol/l) | 62.2 (19.9) | 62.2 (19.9) | 62.4 (20.1) | .45 | 59.3 (18.6) | <.001 |
| Triglyceride (mmol/L) | 0.92 (0.54) | 0.92 (0.54) | 0.93 (0.63) | .47 | 0.90 (0.53) | .17 |
| Cholesterol (mmol/L) | 3.76 (1.14) | 3.76 (1.12) | 3.89 (1.24) | .11 | 3.83 (1.2) | .23 |
Note
- Data are expressed as mean ± SD, median (interquartile range) or number (percent). Statistical analysis among groups was performed using Student's t test, the Mann-Whitney U-test, or a Chi-squared test.
- Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate Aminotransferase; BMI, body mass index; DILI, drug induced liver injury; DM, diabetes mellitus; GGT, glutamyl transpeptidase; RBC, red blood cell count; WBC, white blood cell.
3.2 Incidence and clinical characteristics of ATDILI patients at DILI onset
Of 3155 patients identified and followed for one year, 170 patients (5.4%) developed DILI after a median 42.9 days from start of treatment. Overall 64 (37.6%) of patients in the derivation cohort presented with one or more of the symptoms (Table S2), while rest of the DILI were identified based on the liver biochemistry tests. Among them, 141 patients (82.9%) developed DILI within 90 days (Figure S1).
In addition, 218 (6.9%) developed ≥3 times ULN of ALT during drug therapy of which 193 (88.5%) resolved and only 25 (11.4%) progressed to ≥5 times ULN of ALT to be classified as DILI. Comparison of baseline characteristics of developing ≥3 times ULN of ALT patients without further progression and DILI patients are summarized in Table S3. Management of these patients was individualized by supervising physicians, but no treatment-interruption of ≥7 days in the anti-TB regimen4 was found.
Clinical characteristics, biochemical values and severity of DILI are summarized in Table 2. Causality assessment showed that majority of ATDILI were highly probable (87/170, 51.2%) and probable (75/170, 44.1%). Hepatocellular, mixed and cholestatic injury was present in 147 (86.5%), 15 (8.8%) and 8 (4.7%) respectively. Of those with ATDILI, 143 patients (84.1%) were grouped as having mild DILI; 27 patients (15.9%) developed jaundice (moderate DILI); 9 patients (5.3%) had severe DILI and only 3 died.
| Variables | All DILI (N = 170) |
|---|---|
| Days between initial TB treatment and DILI recognition | 42.9 (42.2) |
| Days between initial TB treatment and Jaudice recognition (n = 27) | 33.4 (42.7) |
| Causality assessment | |
| Definite no. (%) | 87 (51.2) |
| Highly probable no. (%) | 75 (44.1) |
| Probable no. (%) | 8 (4.7) |
| Pattern of liver injury | |
| Hepatocellular no. (%) | 147 (86.5) |
| Mixed no. (%) | 15 (8.8) |
| Cholestatic no. (%) | 8 (4.7) |
| R value | 9.6 (7.7) |
| Lab test at DILI onset | |
| ALT (U/L) | 300 (181) |
| AST (U/L) | 226 (187) |
| Albumin (g/dL) | 41.8 ± 6.6 |
| Total Bilirubin (μmol/L) | 13.0 (13.2) |
| ALP (U/L) | 105 (65) |
| GGT (U/L) | 89 (97) |
| Creatinine (μmol/L) | 60.6 (20.0) |
| WBC (109/L) | 5.3 (2.3) |
| Haemoglobin (g/L) | 138 ± 18.9 |
| Platelet (109/L) | 184 (83) |
| Eosinophil count (109/L) | 0.17 (0.30) |
| INR | 1.1 (0.3) |
| Severity of liver injury | |
| Mild DILI no. (%) | 143 (84.1) |
| DILI with jaundice no. (%) | 27 (15.9) |
| Develop jaundice at DILI recognition no. (%) | 22 (12.9) |
| Develop jaundice after DILI recognition no. (%) | 5 (2.9) |
| Severe DILI no. (%) | 9 (5.3) |
| Mortality no. (%) | 3 (1.8) |
| Liver related mortality no. (%) | 2 (1.2) |
| No liver related mortality no. (%) | 1 (0.6) |
Note
- Data are expressed as mean ± SD, median (interquartile range) or number (percent).
- Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate Aminotransferase; DILI, drug induced liver injury; GGT, glutamyl transpeptidase; INR, International normalized ratio; WBC, white blood cell.
3.3 Risk factors for ATDILI
Results of univariate cox regression analysis of risk factors (at baseline) associated with DILI in study cohort are shown in Table 3. Significant predictors of DILI were subsequently introduced into the multivariate cox regression analysis. Independent risk factors predicting DILI were: age (HR = 1.014, 95% CI: 1.005-1.023), baseline ALT (HR = 1.014, 95% CI: 1.003-1.024), haemoglobin (HR = 1.011, 95% CI: 1.002-1.020) and HBsAg positivity (HR = 1.516, 95% CI: 1.004-2.290). Independent risk factors predicting DILI are shown in Table 4.
| Characteristic | Derivation cohort | Validation cohort | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (y) | 1.013 | 1.005-1.022 | .003 | 1.017 | 1.005-1.029 | .004 |
| Male no. (%) | 0.893 | 0.650-1.228 | .49 | 0.718 | 0.448-1.151 | .17 |
| BMI (kg/m2) | 1.042 | 0.991-1.095 | .11 | 1.195 | 0.804-1.776 | .38 |
| DM no. (%) | 1.357 | 0.842-2.187 | .21 | 1.036 | 0.519-2.067 | .92 |
| Hypertension no. (%) | 1.194 | 0.691-2.064 | .52 | 0.999 | 0.483-2.070 | 1.0 |
| Alcohol use no. (%) | 0.907 | 0.534-1.540 | .72 | 1.429 | 0.776-2.631 | .25 |
| HBsAg positive no. (%) | 1.657 | 1.098-2.499 | .02 | 1.807 | 0.981-3.327 | .06 |
| HIV positive no. (%) | 6.479 | 0.907-46.267 | .06 | 0.050 | <0.001-7.240E9 | .82 |
| HCV positive no. (%) | 0.050 | <0.001-1.145E8 | .79 | 0.050 | <0.001-3.912E6 | .75 |
| Previous TB treatment no. (%) | 0.736 | 0.376-1.439 | .37 | 0.655 | 0.207-2.073 | .47 |
| Lab test | ||||||
| ALT (U/L) | 1.017 | 1.007-1.026 | .001 | 1.017 | 1.003-1.030 | .02 |
| AST (U/L) | 1.017 | 1.006-1.027 | .002 | 1.004 | 0.990-1.017 | .62 |
| Albumin (g/dL) | 1.023 | 0.993-1.054 | .14 | 1.020 | 0.981-1.060 | .32 |
| Total Bilirubin (μmol/L) | 1.017 | 1.001-1.033 | .04 | 1.020 | 0.995-1.045 | .11 |
| AKP (μmol/L) | 1.001 | 0.995-1.006 | .77 | 0.991 | 0.983-1.000 | .04 |
| GGT (μmol/L) | 1.002 | 0.999-1.005 | .18 | 0.994 | 0.987-1.002 | .16 |
| WBC (109/L) | 0.979 | 0.912-1.051 | .55 | 0.939 | 0.851-1.036 | .21 |
| RBC (1012/L) | 1.199 | 0.917-1.569 | .19 | 1.100 | 0.761-1.588 | .61 |
| Haemoglobin (g/L) | 1.012 | 1.003-1.021 | .007 | 1.015 | 1.003-1.028 | .02 |
| Platelet (109/L) | 0.999 | 0.997-1.000 | .14 | 0.999 | 0.996-1.001 | .29 |
| Creatinine (μmol/L) | 1.004 | 0.995-1.013 | .40 | 1.004 | 0.990-1.018 | .55 |
| Triglyceride (mmol/L) | 1.081 | 0.892-1.311 | .43 | 0.885 | 0.590-1.327 | .56 |
| Cholesterol (mmol/L) | 1.137 | 0.969-1.334 | .12 | 1.121 | 0.909-1.381 | .29 |
Note
- Cox regression analysis was performed to identify factors associated with DILI.
- Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate Aminotransferase; BMI, body mass index; CI, confidence interval; DILI, drug induced liver injury; DM, diabetes mellitus; GGT, glutamyl transpeptidase; HR, Hazard ratio; WBC, white blood cell.
| Variables | Regression coefficient | HR | 95% CI | P value |
|---|---|---|---|---|
| Risk factors of DILI (TB patients, N = 3155, DILI, N = 170) | ||||
| Age | 0.013 | 1.014 | 1.005-1.023 | .003 |
| Baseline ALT | 0.014 | 1.014 | 1.003-1.024 | .009 |
| Hemoglobin | 0.012 | 1.011 | 1.002-1.020 | .02 |
| HBsAg positive | 0.461 | 1.516 | 1.004-2.290 | .05 |
| Risk factors of moderate to severe ATDILI (DILI, N = 170, moderate to severe ATDILI, N = 27) | ||||
| Age | 0.028 | 1.029 | 1.003-1.056 | .03 |
| Active drinking | 1.288 | 3.624 | 1.147-11.454 | .03 |
| Previous TB treatment | 1.359 | 3.894 | 1.304-11.625 | .02 |
| Baseline AST | 0.035 | 1.036 | 1.010-1.062 | .006 |
Note
- Cox regression analysis was performed to identify factors associated with DILI.
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate Aminotransferase; CI, confidence interval; DILI, drug induced liver injury; HR, Hazard ratio.
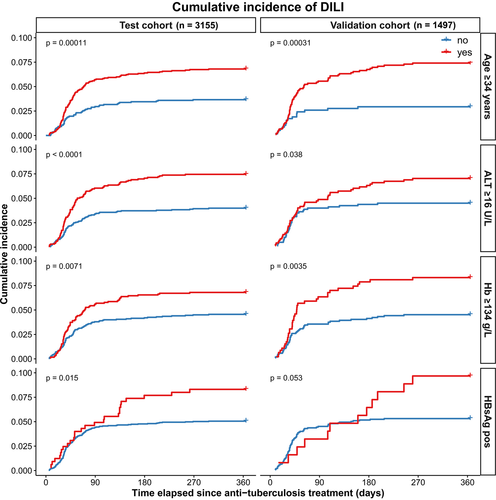
Cumulative incidence of ATDILI in patients with age ≥34 years, baseline ALT ≥16 IU/L, haemoglobin ≥134 g/L and HBsAg positivity were significantly higher than age <34 years, baseline ALT <16 IU/L, haemoglobin <134 g/L and HBsAg negative (P < .001, P < .001, P = .007, P = .015 respectively).
In those who were HBsAg positive 16/27 (59.3%) of ATDILI developed before 3 months compared to those who were HBsAg negative where 125/143 (87.4%) of ATDILI developed before 3 months.
3.4 Risk factors for moderate to severe ATDILI
Of 170 DILI patients, 27(15.9%) developed moderate to severe ATDILI after a median 33.4 days from start of treatment. Results of univariate regression analysis of risk factors associated with moderate to severe DILI are shown in Table S4. Independent risk factors predicting moderate to severe DILI were shown in Table 4: Age (HR = 1.029, 95% CI: 1.003-1.056), baseline AST (HR = 1.036, 95% CI: 1.010-1.062), previous TB treatment (HR = 3.894, 95% CI: 1.304-11.625), and Active drinking (alcohol consumption >14 units in women and >21 units in men; HR = 3.624, 95% CI: 1.147-11.454). In the validation cohort, age and AST levels were higher in patients developing ATDILI compared those who didn't, although because of relatively low number of ATDILI in this cohort we were not able to do multivariate modelling in this cohort.
3.5 Characteristics and results from patients in the validation cohort
A prospective cohort of 1497 patients confirmed with tuberculosis constituted the validation cohort. Their clinical characteristics and biochemical values at baseline are summarized in Table 1. For the whole validation cohort, 85 (5.7%) developed DILI. 10 (11.8%) developed moderate to severe DILI. Results of univariate analysis of risk factors associated with DILI are also shown in Table 3. Age, baseline ALT, haemoglobin and HBsAg positivity were still strongly predictive of ATDILI in the validation cohort. Cumulative incidence of ATDILI in validation cohort patients with age ≥34 years, baseline ALT ≥16 IU/L, haemoglobin ≥134 g/L and HBsAg positivity were still significantly high than those with age <34 years, baseline ALT <16 IU/L, haemoglobin <134 g/L and HBsAg negative (P < .001, P = .04, P = .004, P = .05 respectively).
3.6 Investigations of alternative causes
Two patients each (four in total) were excluded from the derivation and validation cohorts as the events were classified as HBV flare/reactivation by causality assessment with RUCAM score <3 (Figure 1).
Of 325 HBsAg positive patients in the derivation cohort 55 (16.9%) had HBeAg positive, 27 patients developed DILI. In those 27 DILI patients, ALT in all of the 27 were normal before anti-TB treatment. Multiple HBV-DNA levels were monitored as clinically indicated in 22, 10 patients were on antiviral treatment (ETV). In validation cohort, of 124 patients were HBsAg positive, 23 (18.5%) were HBeAg positive, 10 patients developed DILI. Of these, nine patients had regular HBV-DNA monitoring as clinically indicated. Seven patients were on antiviral treatment (ETV and TDF).
Within two cohorts, 464 of 4652 (10%) were taking traditional and complementary medicine (TCM). TCM were excluded as an aetiology for DILI in these patients using RUCAM as described in methods section. In derivation cohort, 24/170 (14.1%) in DILI group and 287/2985 (9.6%) among those who did not develop DILI (P > .05) were on TCM treatment. In validation cohort, 13/85 (15.3%) in DILI group and 140/1412 (9.9%) among those who did not develop DILI (P > .05) were on TCM treatment.
3.7 Long-term outcome
Management of patients with raised liver enzymes were individualized by supervising physicians. In general, investigation to exclude explanations including HBV flare (as appropriate) were performed and liver biochemistry tests were monitored closely. Prompt withdrawal of anti-TB medication was observed in all with DILI, but, drugs and reintroduction regimen of re-introduction was determined by treating physicians. Key outcomes during a 2-year follow-up in patients with 3 × ULN of ALT as well as those who developed DILI have been presented in Table S5.
4 DISCUSSION
Reported incidence of ATDILI varies widely between 2% and 28% because of combination of factors related to patient characteristics, drug regimens as well as DILI case definition.16 In two large cohorts involving 4652 consecutive TB patients receiving a combination of first line anti-TB drug therapy, 5.5% developed ATDILI associated with jaundice and ALF in 0.8% (37/4652) and four patients died. Interestingly, 218/3155 (6.9%) patients in the derivation cohort developed ALT elevation 3-5 times ULN and of these 193/218 (88.5%) resolved without ≥7 days treatment interruption. Only 25 of those with ALT elevation 3-5 times ULN progressed to DILI (as defined by ALT ≥5 times ULN) of which none in the latter group developed ALF or died.
We have identified age, baseline ALT, haemoglobin and HBsAg positivity as risk factors for the development of ATDILI. These risk factors demonstrated in a large contemporary cohort, have been validated in an independent cohort of 1497 patients receiving same drug-regimen. Among 5.7% (85/1497) who developed DILI in the validation cohort, age ≥34 years, baseline ALT (≥16 IU/L), haemoglobin (≥134 g/L) and HBsAg positivity were still predictive of DILI. Among those who have developed DILI, age, baseline AST, active drinking and previous anti-TB treatment were independent risk factors for developing jaundice and ALF.
Our study has a number of strengths. We have established two homogenous and large cohorts of anti-TB patients treated with the standard combination therapy to estimate the incidence of DILI in this group. Case definition of DILI, severity and processes of causality assessment adhered to those established by the international DILI Expert Working Group.20 Case definitions used in this study are those established for all DILI,18 where threshold set for defining DILI include ALT elevation of ≥5 × ULN. It is important to note that 218/3155 (6.9%) patients developed ALT elevation of ≥3 × ULN, 88.5% of these patients were able to complete ATB treatment. None among the rest of 11.5% who progressed to develop DILI resulted in acute liver failure. On long-term follow-up, this group had 90.7% (175/193) TB cure rate (Table S5). This provides reassurance for patients and clinicians regarding the threshold of liver enzyme elevation set for the diagnosis of DILI when there is a need for drug withdrawal. Observation that patients develop self-resolving liver test abnormalities during anti-TB therapy has long been recognized, however, it should be noted that the original study which included patients treated with INH in which such resolution or non-progression of DILI was described reported fatality among 13/114 (12.3%) patients.21 Using clear case definitions, we have been able to report the incidence and natural history of this phenomenon often referred to as ‘adaptation’, mechanisms underlying which are yet to be elucidated.22 In addition, although we have adhered to ALT ≥5 × ULN as the threshold to identify DILI, ALF (0.2%, 10/4652) and mortality 0.09%, 4/4652) is still low compared to the large cohort of DILI because of all drugs from a USA-based multicentre study.23 On the other hand, in a subset of patients with anti-TB DILI related ALF, spontaneous survival of 7/25 (28% [95% CI 12%-49%]) has been reported.24 In our group, this was 6/9 (67% [95% CI 30%-93%] with a wide and overlapping confidence intervals mean that the difference in outcomes of two studies is not statistically significant and both groups are too small to compare. Moreover, only 18.8% in the former study24 discontinued the medication before the onset of symptoms and another 14.3% stopped because of symptoms before presenting with jaundice. In our cohort, 84% stopped medication before developing jaundice which may explain the better outcome. Considering the wide use of this particular affordable regimen globally, anti-TB therapy remains an important contributor to the burden of DILI across the world.11, 24, 25
Although there are number of studies that have investigated a variety of potential host factors that increase the susceptibility to anti-TB DILI,22 the information is derived from heterogeneously defined and inconsistently characterised cohort; none has used large, longitudinal and independent cohorts to validate these. We have identified and validated age, ALT at the start of Anti-TB therapy, HBsAg, and high HB as independent risk factors for DILI occurrence. Age and baseline AST were also risk factors for severe DILI with jaundice and ALF, further strengthening the validity of our findings. Age cut-off of 34 years that defines the risk category in our study is in agreement with some studies,26, 27 although age cut-offs of 55 years or over have been proposed as risk factors in other studies previously.18, 19 Overall, even when age cut-offs derived from different cohort studies vary, relationship of age with increased risk of DILI appears to be consistent. In addition, although age specific prevalence rate ratio of TB globally (calculated using prevalence in 15-24 year age group as a baseline) increases with the age, demography of both testing and validating cohorts in this study with regards to age distribution (Figure S2) is not dissimilar than expected from that of the population of china 2010-2020.
Baseline ALT and AST were independent risk factors for ATDILI and its severe manifestations respectively. In addition, the cut-off values at which ALT level (≥16 IU/L) is associated with DILI is within the normal range, First of all, normal range for most laboratory values have come from evaluating samples from a modest sized cohort of apparently healthy individuals and in general normal ranges have not been established in large longitudinal population based cohorts against clinical outcomes nor in fact assessed in different ethnic groups. A seminal study involving >180,000 people with 8 years of follow-up demonstrated that ALT of 20-29 IU/L at baseline was associated had excess risk of all-cause mortality, cancers, cardiovascular and digestive system diseases.28 Another longitudinal population based cohort study demonstrated that rise in ALT within the normal range (attributed to accumulation of liver fat) can predict incident diabetes in the long-term.29 Although fatty liver has been associated with increased risk of DILI, in the context of tuberculosis patients, we do not think that is the likely explanation for our findings.30 As baseline ALT and AST were identified as risk factors independent of HBV, alcohol intake and unrelated to BMI (which is not associated with DILI), we don't think these reflect undiagnosed underlying liver disorder. Active drinking was a risk factor for the development of jaundice, but, was not associated with the susceptibility to ATDILI itself. As Kupffer cells, resident macrophages of the liver are involved in the uptake and clearance of enzymes such as ALT and AST, levels of latter enzymes may be a reflection of Kupfer cell numbers.31 We speculate that our findings of association of baseline ALT within the normal range being associated with the risk of developing DILI may be because of Kupffer cell numbers or function influencing the degree of oxidative stress in relation to drugs. Higher proportion (11/27; 40.7%) of patients who were positive for HBsAg developed DILI after 3 months when compared to others where DILI appeared earlier in the course of Anti-TB therapy, indicating potentially different mechanisms, such as immune reconstitution where recovery of innate and adaptive immune system may influence DILI risk in this particular group.32 We suggest that in a high-prevalence area of hepatitis B, patients receiving anti-TB therapy should be routinely screened for HBV status and HBsAg positivity should trigger monitoring during the whole period of anti-TB treatment.
Interestingly, high baseline haemoglobin level was one of the risk factors for DILI, replicated in an independent validation cohort. In a population based prospective study based in Ningbo involving >7400 NAFLD free subjects, those with higher baseline haemoglobin had higher incidence of NAFLD over 3 years follow-up.33 Association of Hb was in the normal range and independent of body mass index, Type 2 diabetes and other metabolic liver diseases. It has been hypothesized that free haemoglobin induced oxidative damage contributed to the development of NAFLD.34 Haptoglobin, one of the acute phase protein binds to free plasma haemoglobin to reduce oxidative stress induced by free haemoglobin. Therefore, we speculate that the positive association of DILI with baseline Hb may reflect lower degree of physiological defence against oxidative stress related to the drugs. We did not estimate haptoglobin levels in either of the cohorts, hence, are not able to assess this any further.
The current study has a few limitations. Firstly, the study comes from a specialist centre for infectious diseases which while ensuring homogeneity and consistency in clinical practice, might reflect specific case-mix and severity of TB that may not be reflective of patients in the general population. Secondly, we chose a large retrospective cohort to develop the multivariate model; this inevitably introduces some inconsistencies in data acquisition. Even though we identified age, baseline AST, previous TB treatment and active drinking as risk factors for developing jaundice, because of a small number of life threatening complications, we are not able to develop multivariate models to predict ALF or death related to DILI. In addition, 15/170 (9.2%) of patients with DILI were lost to follow-up (Table S5). Observational nature of the study precludes investigations of mechanisms underlying the how risk factors influence the incidence of DILI, and its serious outcomes. We have not evaluated the genetic factors that may have particular influence on the risk of DILI in this ethnic group; therefore, these findings may not be applicable to another ethnic group. Finally, although people co-infected with human immunodeficiency virus (HIV) were not excluded, overall there were only five patients with HIV in two cohorts limiting the evaluation of the role of co-infection as a risk factor for DILI. Our evaluation of concomitant drugs was limited to information provided by the patient as only prescription drugs were automatically recorded in the database. In addition, therapeutic regimen involving combination of anti-TB drugs does not permit assessment of individual agent's causality in ATDILI.
In summary, first-line anti-tuberculosis DILI and related death are lower than that has been reported in these large cohorts of patients. We have demonstrated that 88.5% of those who develop raised ALT while on ATB therapy resolve without ≥7 day drug interruption and therefore are able to complete the crucial treatment. Age, baseline ALT, haemoglobin and HBsAg positivity are risk factors for the development of DILI. Patients with positive HBsAg develop DILI later, which means that monitoring should be adjusted to suit this particular population. Considering the fact that baseline ALT and haemoglobin level cut-offs in the normal range determines the risk, it is unlikely that any change in monitoring is necessary for this group. Focus on genetic factors has the potential to improve the efficacy and safety of anti-TB therapy further.4
CONFLICT OF INTEREST
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest related to this manuscript.
AUTHOR CONTRIBUTIONS
HY and GPA conceived the study; TC, HY and FJ designed the study; HY, GPA wrote the manuscript; FJ, LL, JD and SJ carried out the statistical analyses; FJ, LL, JD, SJ, SY, HW collected clinical data and biochemical data. All authors have read, critically reviewed and approved the final version.