Hepatic steatosis and hepatic iron overload modify the association of iron markers with glucose metabolism disorders and metabolic syndrome
Handling Editor: Luca Valenti
Funding information
SHIP is part of the Community Medicine Research Network of the University Medicine Greifswald, which is supported by the German Federal State of Mecklenburg- West Pomerania. MR imaging was jointly funded by Siemens Healthineers (Erlangen, Germany) and the Federal State of Mecklenburg- West Pomerania.
Abstract
Background
Iron status has been linked with impaired glucose metabolism (IGM), type 2 diabetes mellitus (T2DM) and the metabolic syndrome (MetS), but the role of hepatic steatosis or iron overload on these associations remains uncertain.
Methods
We analysed data from 2310 participants without known T2DM of the population-based Study of Health in Pomerania (SHIP-TREND, Germany) through logistic regression models. We tested additive and multiplicative interactions between ferritin and hepatic steatosis or iron overload.
Results
Serum ferritin was positively associated with IGM (OR per 100 µg/L: 1.11 [1.01, 1.23]), T2DM (OR per 100 µg/L: 1.20 [1.06, 1.36]) and MetS (OR per 100 µg/L: 1.11 [1.02, 1.20]) in the total population as well as in participants without hepatic iron overload. However, the synergistic effect of higher ferritin concentrations and hepatic iron overload showed stronger associations with IGM and T2DM. Similarly, while ferritin was positively associated with T2DM and MetS even in the absence of hepatic steatosis, the synergistic effect of higher ferritin concentrations and hepatic steatosis showed stronger associations with IGM, T2DM and MetS. Transferrin was associated with isolated impaired glucose tolerance but not with T2DM and MetS.
Conclusions
Our study suggests that ferritin may be associated with glucose metabolism disorders and MetS even in people without hepatic steatosis or iron overload. However, in individuals with higher ferritin concentrations, the presence of hepatic steatosis may indicate stronger risk for glucose metabolism disorders and MetS, while the presence of hepatic iron overload may indicate stronger risk only for glucose metabolism disorders.
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- ATC
-
- anatomical therapeutical chemical
-
- GGT
-
- γ-glutamyl transferase
-
- HbA1c
-
- glycated haemoglobin
-
- HOMA-IR
-
- homeostasis model assessment-insulin resistance
-
- hs-CRP
-
- high-sensitive C-reactive protein
-
- IFG
-
- impaired fasting glucose
-
- IGM
-
- impaired glucose metabolism
-
- IGT
-
- impaired glucose tolerance
-
- MetS
-
- metabolic syndrome
-
- NGM
-
- normal glucose metabolism
-
- PDFF
-
- proton density fat fraction
-
- T2DM
-
- type 2 diabetes mellitus
Lay summary
The iron marker serum ferritin is associated with prediabetes, diabetes and metabolic syndrome even in people without fatty liver disease or iron overload. But the presence of higher ferritin levels along with either fatty liver disease or iron overload makes the individuals more prone to the risk of these metabolic disorders.
1 INTRODUCTION
Iron is an essential micronutrient required for diverse metabolic processes such as DNA synthesis and oxygen transport. However, excessive iron stores in the liver, pancreas and muscle can be harmful as it has been associated with overproduction of reactive oxygen species, which in turn may be implicated in oxidative stress and cellular damage.1 Hereditary haemochromatosis – a genetic disorder characterized by massive iron overload – has been reported to be involved in the development of type 2 diabetes mellitus (T2DM),2 which suggests that iron overload can also cause diabetes. Ferritin is an indicator of body iron. In accordance, several observational studies have shown associations between ferritin concentrations and increased risk of impaired glucose metabolism (IGM), impaired pancreatic beta cell function, decreased insulin sensitivity, metabolic syndrome (MetS) and T2DM in both Eastern and Western countries.3-7
Despite increasing evidence, the association between ferritin and T2DM and MetS remains inconclusive since ferritin is also an acute phase protein and its synthesis can be stimulated by inflammation, hepatic dysfunction and insulin resistance regardless of iron status. Certain studies have reported that the association of ferritin with T2DM and MetS could be partially explained by hepatic dysfunction on the basis of hepatic enzymes as a marker of hepatic dysfunction.8, 9 However, hepatic steatosis and iron content may contribute further to understand the role of iron and liver dysfunction in the pathogenesis of T2DM and MetS.
The liver serves as a major site for iron storage and in parallel plays a crucial role in iron and glucose homeostasis. Some studies demonstrated that excess iron in the liver may be involved in the pathogenesis of fatty liver,10, 11 while another study observed that fatty liver may disturb iron homeostasis and may lead to iron overload.12 In addition, fatty liver is suggested to be a forerunner in the development of IGM, T2DM and MetS via hepatic insulin resistance.13, 14 While iron, fatty liver and the metabolic disorders like T2DM and MetS all seem to be interlinked to each other, their interplay between them remains unclear. Based on these premises, the association between ferritin, hepatic steatosis and iron overload with IGM, T2DM and Mets was investigated.
Transferrin, an iron transport protein, is another marker of iron metabolism. Its levels increase with the rise in iron requirements. Additional investigations of transferrin may help to further understand the role of iron in the pathogenesis of metabolic disorders. However, studies investigating the relation between transferrin and IGM, T2DM or MetS, in addition to ferritin are sparse.5, 8, 15, 16 Hence, there is only weak and inconsistent evidence to support this hypothesis.
Thus, the aims of the study were to 1) evaluate the association of ferritin and transferrin concentrations with IGM, T2DM and MetS and 2) analyse interactions between ferritin and hepatic steatosis and iron overload on the association with IGM, T2DM and MetS.
2 MATERIALS AND METHODS
2.1 Study population
The present cross-sectional study is based on the second independent cohort of the population-based Study of Health in Pomerania (SHIP-TREND) conducted between 2008 and 2012 in Western Pomerania in the northeast of Germany. A random cluster sample of 8826 eligible adult individuals aged 20-79 years was invited to participate in a comprehensive health examination, of which, 4420 participated (response 50.1%). Details on the study design, protocols and sampling methods have been reported elsewhere.17, 18 All participants provided written informed consent and the study was approved by the medical ethics committee of the University medicine of Greifswald and followed the declaration of Helsinki.
2.2 Interview and physical examination
All participants underwent a standardized computer-assisted personal interview, during which they provided information on sociodemographic and lifestyle factors as well as medical histories and medication use. School education was categorized into 3 groups: <10 years, 10 years and > 10 years, smoking status into current, former and never smokers and alcohol consumption into no (0 g/day), moderate (men 0.1-39.9 g/day and women 0.1-19.9 g/day), and high alcohol (men ≥ 40 g/day and women ≥ 20 g/day) consumption. Participants who exercised for less than an hour/week in their leisure time during summer or winter were classified as physically inactive. Participants were asked to bring all medications taken 7 days before the time of examination. Medication data were obtained online using the IDOM program (online drug database led medication assessment) and categorized according to the Anatomical Therapeutical Chemical (ATC) classification index. Glucose lowering medication was defined by the ATC code A10, and lipid lowering medication by the ATC code C10AB and C10AD.
During the physical examination, standardized measurements of height, weight, waist circumference, hip circumference and blood pressure were performed while the subjects were in light clothing and not wearing shoes. BMI and waist/hip ratio were calculated. Blood pressure was measured 3 times on the right arm in a sitting position after at least 5-min at rest, using an oscillometric device (OMRON HEM 705-CP). Systolic and diastolic blood pressures were calculated as the average reading of the second and third measurements. Participants were classified as hypertensive based on blood pressure readings ≥ 140/90 mmHg or use of self-reported antihypertensive medication.
2.3 Main outcome measurements: impaired glucose metabolism, unknown type 2 diabetes mellitus and metabolic syndrome
Participants without diagnosed T2DM or taking glucose-lowering agents underwent a standard 75 g oral glucose tolerance test. Venous blood was sampled after an overnight fast for at least 8h and 2h post glucose solution intake. Measurements of plasma fasting glucose and 2-h glucose levels were measured using a hexokinase method (Dimension Vista 1500, Siemens Healthcare Diagnostics, Eschborn, Germany).
Based on the criteria of the American Diabetes Association,19 participants were classified as having 1) normal glucose metabolism (NGM) when they had fasting glucose values < 5.6 mmol/L and 2-h glucose < 7.8 mmol/L); 2) impaired glucose metabolism (IGM) when they had fasting glucose values ≥ 5.6 mmol/L but ≤ 6.9 mmol/L (impaired fasting glucose, IFG) and/or 2-h glucose ≥ 7.8 mmol/L but ≤ 11.0 mmol/L (impaired glucose tolerance, IGT) and 3) previously undiagnosed T2DM when they had fasting glucose values ≥ 7.0 mmol/L or 2-h glucose ≥ 11.1 mmol/L.
Based on the criteria of the International Diabetes Federation,20 participants were classified as having MetS when they had central obesity ( defined as waist circumference ≥ 94 cm in men and ≥ 80 cm in women) along with any 2 of the following 4 factors: 1) triglycerides ≥ 1.7 mmol/L or lipid lowering medication (ATC code C10AB or C10AD); 2) HDL cholesterol < 1.03 mmol/L in men and < 1.29 mmol/L in women; 3) blood pressure ≥ 130/85 mmHg or antihypertensive treatment (ATC code C02) and 4) fasting plasma glucose ≥ 5.6 mmol/L or antidiabetic medication (ATC code A10).
Serum fasting insulin and 2-h post-load glucose insulin values were measured by an electrochemiluminescence immunoassay (ADVIA Centaur, Siemens Healthcare Diagnostics, Eschborn, Germany). The homeostasis model assessment-insulin resistance index (HOMA-IR) was calculated as (fasting glucose [mmol/L] X fasting insulin [μU/ml]) / 22.5. Glycated haemoglobin (HbA1c) was determined by high-performance liquid chromatography (Diamat, Bio-Rad Laboratories, Munich, Germany).
2.4 Iron markers and other laboratory measurements
Fasting blood samples were collected without stasis from the cubital vein following a standardized protocol, refrigerated to 4-8°C and shipped on refrigerant packing within 4 to a maximum of 6h to the laboratory. Serum concentrations of ferritin and transferrin were measured by chemiluminescent assays (Siemens Vista, TRF Flex® reagent cartridge, Siemens Healthcare Diagnostics Inc, Newark, DE, USA). Serum levels of total cholesterol, HDL cholesterol, triglycerides, alanine aminotransferase (ALT), γ-glutamyl transferase (GGT), and high-sensitive C-reactive protein (hs-CRP) were measured using the Dimension Vista 500 analytical system (Siemens Healthcare Diagnostics, Eschborn, Germany). Serum creatinine was measured using an enzymatic assay (Dimension VISTA, Siemens Healthcare Diagnostics, Eschborn, Germany). White blood cell count measured in whole blood was analysed within 60 min using a Sysmex XT 2000 Haematology Autoanalyser (Sysmex, Kobe, Japan).
2.5 Hepatic magnetic resonance imaging technique and analysis
Hepatic MR imaging was performed on a 1.5-T MRI system (Magnetom Avanto; Siemens Healthcare AG) with a repetition time of 11.0 msec, 3 echo times of 2.4, 4.8 and 9.6 msec, 10° flip angle, one signal average, bandwidth of 1065Hz/pixel, matrix of 224 × 126 × 32, section thickness of 6.0 mm and a monopolar readout.21 Following image acquisition, MR datasets were processed by using an offline reconstruction algorithm written in Matlab (Mathworks, Natick, Mass) to estimate proton density fat fraction (PDFF) and create R2* maps.22 Post-processing was performed on a MacBook Pro Mid 2012 (2.6GHz Core i7; 16GB RAM, 1600 Mhz DDR3; Apple, Cupertino, Calif). Images were then analysed after an operator defined selection of the liver using the region-of-interest tool in Osirix (version 4.6; Pixmeo, Bernex, Switzerland).
2.6 Definition of hepatic steatosis and hepatic iron overload
After determination of PDFF (as percent) and R2* (as sec−1), patients were classified by using defined cutoffs of liver fat and liver iron content. Cutoffs of PDFF and R2* were based on histopathological calibrations. These calibrations were defined in an external study described elsewhere.22 Hepatic steatosis is defined by a PDFF cutoff of 5.1% or higher and hepatic iron overload is defined by a R2* cutoff of 41.0 sec-1 or greater. Calibration was exclusively based on histopathological grading of liver fat and iron content and did not include biochemical findings such as measurement of triglycerides or iron content.
2.7 Statistical analyses
Baseline characteristics of study participants were expressed as median and interquartile range for continuous data and as absolute numbers and percentages for categorical data. Differences between the subjects with NGM, IGM and T2DM were tested by Mann-Whitney U test for continuous data and χ2 test for categorical data. Partial correlations were calculated between iron markers (ferritin and transferrin) and other continuous covariates after adjusting for age and sex. Multinomial logistic regression analyses were performed to analyse associations of iron markers (ferritin and transferrin) with IGM and T2DM compared to NGM. Further logistic and linear regression models were performed to test associations between iron markers and MetS, fasting glucose, 2-h glucose, fasting insulin and 2-h insulin, HOMA-IR and HbA1c. Associations were analysed based on stepwise adjustment for covariates for all outcomes. The first model was adjusted for age and sex, the second for known diabetes risk factors such as education, smoking, alcohol intake, physical inactivity, BMI, waist/hip ratio, hypertension, triglycerides and total/ HDL cholesterol ratio, renal function markers such as serum creatinine and urinary albumin/creatinine along with the inflammatory markers hs-CRP and leucocytes, and the third additionally for the hepatic enzymes ALT and GGT. For the outcome MetS, the covariates BMI, waist/hip ratio, triglycerides and total/ HDL cholesterol ratio were not used for adjustment because of being single components of MetS.
Effect modifications of sex, hs-CRP concentrations (median cut-off:≥1.18 mg/L vs < 1.18 mg/L), ALT concentrations (median cut off: ≥0.38 µkat/L vs < 0.38 µkat/L), presence of hepatic steatosis and hepatic iron overload on the associations between iron markers and IGM, T2DM and MetS were analysed by including an interaction term between the iron marker and the potential effect modifier in the regression models. Further, serum ferritin concentrations were dichotomized into 2 groups based on sex-specific 4th quartile (Q4) cut point (male: 245 µg/L, female: 102.5 µg/L). Independent associations of ferritin above the 4th quartile, hepatic steatosis and iron overload on IGM, T2DM and MetS were investigated after adjusting for each other. We then examined the joint association of ferritin and presence of hepatic steatosis with the outcomes IGM, T2DM and MetS by defining them into 4 categories 1) ferritin < Q4 and no presence of hepatic steatosis, 2) ferritin ≥ Q4 and no presence of hepatic steatosis, 3) ferritin < Q4 and presence of hepatic steatosis and 4) ferritin ≥ Q4 with presence of hepatic steatosis. Similarly, to examine the joint association of ferritin and presence of iron overload, 4 categories were defined 1) ferritin < Q4 and no presence of hepatic iron overload, 2) ferritin ≥ Q4 and no presence of hepatic iron overload, 3) ferritin < Q4 and presence of hepatic iron overload and 4) ferritin ≥ Q4 with presence of hepatic iron overload.
For sensitivity analyses, we examined i) the associations between iron markers and the 3 different categories of impaired glucose metabolism: isolated IFG, isolated IGT and combined IFG and IGT (IFG + IGT) compared to NGM ii) the association of ferritin with the outcomes (IGM, T2DM and MetS) separately in men and women iii) the effect of interactions between ferritin and hepatic steatosis or iron overload on metabolic outcomes after excluding subjects with excessive alcohol consumption (n = 111).
Effect estimates were reported as odds ratios (OR) with 95% confidence interval (CI) for logistic regression and as ß coefficients with 95% CI for linear regression per 100 µg/L increase in ferritin and per 1 g/L increase in transferrin. All analyses were carried out using STATA 14.2 (Stata Corporation, College Station, TX, USA).
3 RESULTS
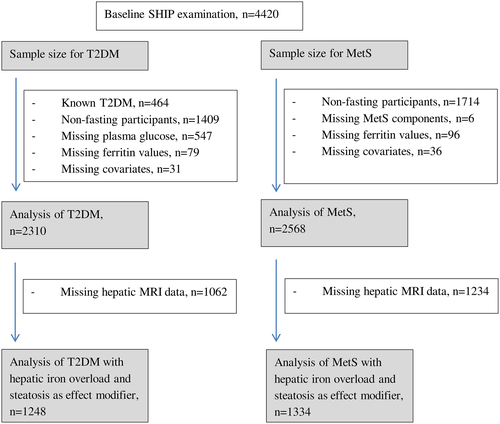
Our study sample size varied according to specific outcome and analysis which included 2310 participants for the analysis of IGM and T2DM and 2568 participants for the analysis of MetS. A detailed breakdown of participants used in the analyses after mainly excluding subjects who were nonfasting or with known diabetes is shown in Figure 1. Of note, subjects who had reported known history of diabetes mellitus were excluded from all analyses.
Clinical characteristics of the study participants stratified by groups of glucose metabolism are shown in Table 1 and Table S1. In our sample, 877 individuals (37.9%) had IGM, 141 individuals (6.1%) had T2DM and 812 individuals (31.6%) had MetS. Overall, subjects with IGM and T2DM tended to be older, were less physically active, had a higher prevalence of hypertension, MetS, hepatic steatosis and hepatic iron overload, and exhibited significantly higher concentrations of ferritin, hepatic enzymes, inflammatory markers and metabolic parameters than subjects with NGM (Table 1).
| Characteristics |
Total n = 2310 |
NGM (n = 1292) |
IGM (n = 877) |
T2DM (n = 141) |
|---|---|---|---|---|
| Age (years)a | 50 (39-61) | 44 (34-55) | 56 (45-64) | 61 (56-68) |
| Sex, malesa | 1,062 (45.97%) | 492 (38.08%) | 492 (56.10%) | 78 (55.32%) |
| Educationa | ||||
| <10 yrs | 349 (15.11%) | 134 (10.37%) | 170 (19.38%) | 45 (31.91%) |
| 10 yrs | 1,262 (54.63%) | 735 (56.89%) | 469 (53.48%) | 58 (41.13%) |
| >10 yrs | 699 (30.26%) | 423 (32.74%) | 238 (27.14%) | 38 (26.95%) |
| Smokinga | ||||
| Never smoker | 899 (38.92%) | 484 (37.46%) | 351 (40.02%) | 64 (45.39%) |
| Ex-smoker | 844 (36.54%) | 432 (33.44%) | 353 (40.25%) | 59 (41.84%) |
| Current smoker | 567 (24.55%) | 376 (29.10%) | 173 (19.73%) | 18 (12.77%) |
| Alcohol intake | ||||
| 0 g/day | 316 (13.68%) | 162 (12.54%) | 127 (14.48%) | 27 (19.15%) |
| 0.01-39.99 g/day in men and 0.01-19.99 g/day in women | 1,883 (81.52%) | 1072 (82.97%) | 708 (80.73%) | 103 (73.05%) |
| >40 g/day in men and > 20 g/day in women | 111 (4.81%) | 58 (4.49%) | 42 (4.79%) | 11 (7.80%) |
| Physically inactivea | 665 (28.79%) | 338 (26.16%) | 280 (31.93%) | 47 (33.33%) |
| Hypertensivea | 962 (41.65%) | 346 (26.78%) | 506 (57.70%) | 110 (78.01%) |
| Metabolic syndrome, yesa, c | 668 (28.92%) | 106 (8.20%) | 454 (51.77%) | 108 (76.60%) |
| Hepatic steatosis, yesb, a | 464 (37.18%) | 156 (21.85%) | 252 (54.19%) | 56 (81.16%) |
| Hepatic iron overload, yesa, b | 219 (17.55%) | 81 (11.34%) | 116 (24.95%) | 22 (31.88%) |
| Hepatic MRI PDFF (%)a, b |
3.68 (2.27-7.32) |
2.78 (2.05-4.73) |
5.56 (3.03-9.87) |
12.81 (6.30-19.14) |
| Hepatic MRI R2 (sec−1)a, b |
34.10 (31.36-38.34) |
33.13 (30.70-36.56) |
35.47 (32.58-40.96) |
36.46 (33.20-41.88) |
| BMI (kg/m2)a |
26.97 (24.16-30.3) |
25.41 (22.89-28.50) |
28.53 (26.03-31.50) |
30.53 (27.80-34.19) |
| Waist to Hip ratioa | 0.87 (0.81-0.94) | 0.84 (0.79-0.91) | 0.91 (0.84-0.97) | 0.94 (0.87-1.0) |
| Triglycerides (mmol/L)a | 1.24 (0.88-1.76 | 1.08 (0.78-0.91) | 1.44 (1.05-1.98) | 1.89 (1.32-2.75) |
| Total cholesterol/ HDL ratioa | 3.76 (3.08-4.64) | 3.52 (2.91-4.25) | 4.14 (3.36-5.04) | 4.33 (3.41-5.30) |
| Alanine aminotransferase (µkat/l)a | 0.37 (0.27-0.52) | 0.33 (0.25-0.45) | 0.42 (0.31-0.58) | 0.51 (0.38-0.71) |
| Serum creatinine (µmol/l)a | 75 (65-86) | 73 (64-83) | 78 (67-89) | 79 (69-91) |
| Urinary albumin/creatinine (mg/g)a | 0.87 (0.58-1.56) | 0.84 (0.56-1.42) | 0.89 (0.58-1.67) | 1.19 (0.73-2.31) |
| HbA1c (%)a | 5.2 (4.8-5.5) | 5.1 (4.7-5.4) | 5.3 (5.0-5.7) | 5.8 (5.4-6.2) |
| Fasting glucose (mmol/L)a | 5.4 (5.0-5.8) | 5.1 (4.9-5.3) | 5.8 (5.6-6.1) | 7.1 (6.3-7.8) |
| 2-hr glucose (mmol/L)a | 6.1 (5.1-7.3) | 5.5 (4.7-6.4) | 6.9 (5.8-8.2) | 12.2 (11.1-14) |
| Fasting insulin (pmol/l)a | 9.4 (6.4-13.9) | 7.6 (5.5-10.6) | 11.8 (8.5-17.1) | 20.1 (14.3-26.7) |
| 2-hr insulin (pmol/l)a | 52.6 (32.4-95.1) | 42.6 (26.2-62.8) | 71 (42.4-141.38) |
162.34 (85.2-224.59) |
| HOMA-IRa | 2.24 (1.46-3.49) | 1.72 (1.20-2.42) | 3.07 (2.15-4.58) | 6.17 (3.8-9.1) |
| Ferritin (µg/l)a | 89 (40-165) | 64 (30-121.5) | 120 (64-208) | 167 (87-314) |
| Transferrin (g/l) | 2.5 (2.3-2.8) | 2.6 (2.3-2.8) | 2.5 (2.3-2.8) | 2.5 (2.3-2.8) |
| γ-glutamyl transferase (µkat/l)a | 0.48 (0.38-0.69) | 0.43 (0.35-0.58) | 0.55 (0.43-0.81) | 0.7 (0.51-1) |
| hs-CRP (mg/l)a | 1.18 (0.62-2.53) | 1.04 (0.53-2.18) | 1.31 (0.72-2.67) | 2.33 (0.98-4.35) |
| Leucocytes (109/l)a | 5.5 (4.71-6.61) | 5.43 (4.64-6.57) | 5.53 (4.76-6.61) | 5.95 (4.94-7.07) |
Note
- Data are median (interquartile range) or number (%).
- Abbreviations: HbA1c, glycated haemoglobin; HOMA-IR, homeostatic model assessment of insulin resistance; hs-CRP, high-sensitive C-reactive protein; IGM, impaired glucose metabolism; NGM, normal glucose metabolism; PDFF, proton density fat fraction; T2DM, type 2 diabetes mellitus.
- a indicates significant difference (P <.05) between the groups of glucose metabolism status based on chi-square tests for categorical variables and Mann-Whitney U test for continuous variables
- b Hepatic MRI imaging data were available for 1248 participants
- c number (%) of participants with metabolic syndrome among the participants with non-missing glucose metabolism status.
In the correlation analyses, ferritin concentrations were strongly correlated with hepatic iron content (r = 0.71), ALT (r = 0.35) and GGT (r = 0.26) concentrations than with inflammatory markers, while transferrin showed only weak correlations with all of them (Table S2). In the regression analyses, a higher serum ferritin concentration was significantly associated with a greater prevalence of IGM (OR per 100 µg/L: 1.11 [95% CI: 1.01, 1.23]), T2DM (OR per 100 µg/L: 1.20 [95% CI: 1.06, 1.36]) and MetS (OR per 100 µg/L: 1.11 [95% CI: 1.02, 1.20]) as well as higher fasting glucose, 2-h glucose, 2-h insulin, HOMA-IR and HbA1c concentrations in the total population independently of known diabetes risk factors, renal function, inflammatory markers and hepatic enzymes (Table 2). However, the association of ferritin with fasting insulin became borderline significant after adjustment for hepatic enzymes.
| Outcome | Iron marker | N | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | |||
| Odds ratios | ||||||||
| IGM and T2DM | ||||||||
| IGM vs NGM | Ferritin | 2310 | 1.31 (1.20, 1.44) | <.0001 | 1.15 (1.05, 1.26) | .003 | 1.11 (1.01, 1.23) | .024 |
| T2DM vs NGM | 1.57 (1.39, 1.76) | <.0001 | 1.32 (1.17, 1.49) | <.0001 | 1.20 (1.06, 1.36) | .005 | ||
| IGM vs NGM | Transferrin | 2310 | 1.45 (1.16, 1.82) | .001 | 1.27 (0.98, 1.63) | .068 | 1.26 (0.98, 1.62) | .078 |
| T2DM vs NGM | 1.88 (1.16, 3.03) | .010 | 1.37 (0.80, 2.33) | .25 | 1.22 (0.70, 2.11) | .486 | ||
| Metabolic syndrome | Ferritin | 2568 | 1.32 (1.22, 1.42) | <.0001 | 1.24 (1.15, 1.34) | <.0001 | 1.11 (1.02, 1.20) | .03 |
| Transferrin | 2568 | 1.57 (1.26, 1.96) | .0001 | 1.38 (1.08, 1.76) | .01 | 1.26 (0.98, 1.62) | .07 | |
| β coefficients | ||||||||
| Fasting glucose | Ferritin | 2310 | 0.15 (0.13, 0.17) | <.0001 | 0.11 (0.09, 0.13) | <.0001 | 0.09 (0.07, 0.12) | <.0001 |
| Transferrin | 2310 | 0.06 (−0.02, 0.13) | .12 | -0.03 (−0.10, 0.04) | .45 | -0.04 (−0.11, 0.03) | .27 | |
| 2-hr glucose | Ferritin | 2304 | 0.40 (0.34, 0.47) | <.0001 | 0.30 (0.24, 0.36) | <.0001 | 0.23 (0.17, 0.30) | <.0001 |
| Transferrin | 2304 | 0.66 (0.45, 0.88) | <.0001 | 0.34 (0.14, 0.54) | .001 | 0.30 (0.11, 0.50) | .003 | |
| Fasting insulin | Ferritin | 2295 | 1.00 (0.76, 1.24) | <.0001 | 0.36 (0.16, 0.56) | .0001 | 0.20 (−0.01, 0.41) | .066 |
| Transferrin | 2295 | 2.07 (1.28, 2.85) | <.0001 | 0.80 (0.15, 1.44) | .016 | 0.71 (0.07, 1.35) | .030 | |
| 2-hr insulin | Ferritin | 2247 | 6.45 (4.77, 8.13) | <.0001 | 3.33 (1.77, 4.90) | <.0001 | 2.10 (0.46, 3.74) | .012 |
| Transferrin | 2247 | 19.36 (14.02, 24.71) | <.0001 | 13.01 (8.05, 17.97) | <.0001 | 12.41 (7.48, 17.33) | <.0001 | |
| HOMA-IR | Ferritin | 2295 | 0.37 (0.30, 0.45) | <.0001 | 0.18 (0.12, 0.24) | <.0001 | 0.12 (0.05, 0.18) | .0001 |
| Transferrin | 2295 | 0.61 (0.37, 0.85) | <.0001 | 0.18 (−0.02, 0.38) | .079 | 0.14 (−0.05, 0.34) | .151 | |
| HbA1c % | Ferritin | 2310 | 0.04 (0.02, 0.06) | <.0001 | 0.03 (0.01, 0.05) | .002 | 0.02 (0.00, 0.04) | .041 |
| Transferrin | 2310 | 0.14 (0.08, 0.19) | <.0001 | 0.12 (0.07, 0.18) | <.0001 | 0.12 (0.06, 0.18) | <.0001 | |
Note
- Model 1: Adjusted for age and sex; Model 2: Model 1 + education, smoking, alcohol consumption, physical activity, BMI, waist/hip ratio, hypertension, total/HDL cholesterol ratio, serum creatinine, urinary albumin/creatinine, hs-CRP, leucocytes; Model 3: Model 2 + alanine aminotransferase, gamma-glutamyl transferase. For metabolic syndrome, variables such as triglyceride, total/HDL cholesterol, BMI and waist/hip ratio which form a component of metabolic syndrome, were not adjusted. Significant associations are shown in bold font.
- Abbreviations: HbA1c, glycated haemoglobin; HOMA-IR, homeostatic model assessment of insulin resistance; NGM: normal glucose metabolism, IGM: impaired glucose metabolism, T2DM: type 2 diabetes mellitus.
In the sex-stratified analyses, associations of serum ferritin concentrations with higher prevalence of IGM and T2DM were stronger in women (IGM OR per 100 µg/L: 1.29 [95% CI: 1.06, 1.56], T2DM OR per 100 µg/L: 1.32 [1.05, 1.67]) than in men (IGM OR per 100 µg/L: 1.05 [95% CI: 0.94, 1.18], T2DM OR per 100 µg/L: 1.19 [1.00, 1.41]) whereas the associations with MetS, fasting glucose, 2-h glucose, HOMA-IR and HbA1c were stronger in men than in women (Table S3). We observed similar effect modifications by sex for the associations of serum ferritin levels with IGM and MetS (Figure 2; P <.1).

In the effect modification analysis of ferritin by hs-CRP, we observed positive associations of serum ferritin concentrations with prevalence of IGM (OR per 100 µg/L: 1.29 [95% CI: 1.13, 1.49]) and T2DM (OR per 100 µg/L: 1.41 [95% CI: 1.17, 1.69]) in participants with hs-CRP concentrations below the median, but no associations in participants with hs-CRP concentrations above the median (Figure 2). No significant effect modification of ferritin by ALT was seen for IGM, T2DM or MetS.
In the effect modification analysis of ferritin by hepatic steatosis, ferritin was significantly associated with MetS in participants without hepatic steatosis (OR per 100 µg/L: 1.29 [95% CI: 1.07, 1.56]) but not in participants with steatosis (OR per 100 µg/L: 0.96 [95% CI: 0.84, 1.10]), while it was significantly associated with T2DM regardless of the presence or absence of hepatic steatosis (Figure 2). The results remained similar even after excluding subjects with excessive alcohol consumption (data not shown). When adjusted for hepatic steatosis, ferritin above Q4 was independently associated with IGM (OR per 100 µg/L: 1.54 [95% CI: 1.11, 2.12]) and T2DM (OR per 100 µg/L: 2.79 [95% CI: 1.51, 5.17]), but the association with MetS (OR per 100 µg/L: 1.37 [95% CI: 0.99, 1.90]) became nonsignificant (Table 3). On the other hand, hepatic steatosis was independently associated with IGM, T2DM and MetS, regardless of the adjustment for ferritin. However, the combined effect of higher ferritin concentrations (≥Q4) and hepatic steatosis showed stronger association with prevalent MetS (OR: 5.43 [95% CI: 3.49, 8.45]), IGM (OR: 3.38 [95% CI: 2.08, 5.48]) and T2DM (OR: 7.66 [95% CI: 2.77, 21.18]) than the single effects of ferritin or hepatic steatosis alone (Table 4).
| Exposure | Adjustment | IGM vs NGM | T2DM vs NGM | MetS vs no MetS | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
|
Ferritin (≥Q4 vs < Q4) |
Main model | 1.59 (1.16, 2.20) | .004 | 2.94 (1.59, 5.43) | .001 | 1.55 (1.25, 1.94) | .001 |
| Main + hepatic steatosis | 1.54 (1.11, 2.12) | .01 | 2.79 (1.51, 5.17) | .001 | 1.37 (0.99, 1.90) | .06 | |
| Main + hepatic iron overload | 1.37 (0.95, 1.96) | .09 | 2.30 (1.15, 4.59) | .02 | 1.87 (1.30, 2.68) | .005 | |
|
Hepatic steatosis (Yes vs no) |
Main model | 1.96 (1.42, 2.72) | <.001 | 2.62 (1.23, 5.59) | .01 | 4.03 (2.94, 5.54) | <.0001 |
| Main + ferritin | 1.92 (1.38, 2.66) | .0001 | 2.42 (1.13, 5.19) | .02 | 4.04 (2.93, 5.57) | <.0001 | |
|
Hepatic iron overload (Yes vs no) |
Main model | 1.71 (1.19, 2.45) | .003 | 2.85 (1.43, 5.65) | .003 | 0.97 (0.67, 1.40) | .87 |
| Main + ferritin | 1.46 (0.97, 2.19) | .07 | 1.84 (0.85, 3.99) | .12 | 0.68 (0.44, 1.04) | .08 | |
Note
- Ferritin was dichotomized into 2 groups as above and below the sex-specific 4th quartile cutpoint (male: 245 µg/L, female: 102.5 µg/L). Main model adjusted for age, sex, education, smoking, alcohol consumption, physical activity, BMI, waist/hip ratio, hypertension, triglycerides, total/HDL cholesterol ratio, serum creatinine, urinary albumin/creatinine, high-sensitive C-reactive protein, leucocytes, alanine aminotransferase, and gamma-glutamyl transferase. For MetS, adjusted for all previously mentioned covariates except BMI, waist/hip ratio, triglycerides and total/HDL cholesterol. Significant associations are shown in bold font.
- Abbreviations: HIO, hepatic iron overload; HS, hepatic steatosis; IGM, impaired glucose metabolism; MetS, metabolic syndrome; NGM, normal glucose metabolism; T2DM, type 2 diabetes mellitus.
| Exposure | Category | N | IGM vs NGM | T2DM vs NGM | MetS vs no MetS | |||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |||
| Ferritin and hepatic steatosis | Ferritin < Q4 and no HS | 656 | 1.00 | 1.00 | 1.00 | |||
| Ferritin ≥ Q4 and no HS | 128 | 1.19 (0.76, 1.86) | .45 | 2.44 (0.74, 7.98) | .14 | 1.93 (1.16, 3.23) | .04 | |
| Ferritin < Q4 and presence of HS | 278 | 1.65 (1.14, 2.40) | .008 | 2.21 (0.81, 6.03) | .12 | 4.83 (3.28, 7.13) | <.0001 | |
| Both ferritin ≥ Q4 and presence of HS | 186 | 3.38 (2.08, 5.48) | .0001 | 7.66 (2.77, 21.18) | .0001 | 5.43 (3.49, 8.45) | <.0001 | |
| Ferritin and hepatic iron overload | Ferritin < Q4 and no HIO | 862 | 1.00 | 1.00 | 1.00 | |||
| Ferritin ≥ Q4 and no HIO | 167 | 1.57 (1.04, 2.38) | .03 | 2.24 (1.03, 4.85) | .04 | 2.06 (1.38, 3.09) | .002 | |
| Ferritin < Q4 and presence of HIO | 72 | 1.87 (1.07, 3.28) | .03 | 1.30 (0.26, 6.48) | .75 | 0.92 (0.47, 1.83) | .82 | |
| Both ferritin ≥ Q4 and presence of HIO | 147 | 1.80 (1.17, 2.77) | .007 | 4.05 (1.88, 8.69) | .0001 | 1.20 (0.79, 1.81) | .39 | |
Note
- Ferritin was dichotomized into 2 groups as above and below the sex-specific 4th quartile cutpoint (male: 245 µg/L, female: 102.5 µg/L). Main model adjusted for age, sex, education, smoking, alcohol consumption, physical activity, BMI, waist/hip ratio, hypertension, triglycerides, total/HDL cholesterol ratio, serum creatinine, urinary albumin/creatinine, high-sensitive C-reactive protein, leucocytes, alanine aminotransferase, and gamma-glutamyl transferase. For MetS, adjusted for all previously mentioned covariates except BMI, waist/hip ratio, triglycerides and total/HDL cholesterol. Significant associations are shown in bold font.
- Abbreviations: IGM: impaired glucose metabolism, NGM: normal glucose metabolism, T2DM: type 2 diabetes mellitus, MetS: metabolic syndrome, HIO: hepatic iron overload, HS: hepatic steatosis.
In the effect modification analysis of ferritin by hepatic iron overload, we found significant associations of ferritin with higher prevalence of IGM (OR per 100 µg/L: 1.31 [95% CI: 1.03, 1.67]), T2DM (OR per 100 µg/L: 1.97 [95% CI: 1.33, 2.93]) and MetS (OR per 100 µg/L: 1.47 [95% CI: 1.17, 1.84]) in participants without hepatic iron overload, whereas we observed no such associations in participants with iron overload (P-value of interaction < .05) (Figure 2). When adjusted for hepatic iron overload, the positive association of ferritin above Q4 was independently associated with T2DM (OR per 100 µg/L: 2.30 [95% CI: 1.15, 4.59]) and MetS (OR per 100 µg/L: 1.87 [95% CI: 1.30, 2.68]), but the association with IGM (OR per 100 µg/L: 1.37 [95% CI: 0.95, 1.96]) became nonsignificant (Table 3). On the other hand, hepatic iron overload was positively associated with IGM and T2DM, but attenuated and became nonsignificant after adjustment for ferritin (Table 3). Nevertheless, the combined effect of higher ferritin concentrations (≥Q4) and hepatic iron overload showed a stronger association with prevalent IGM (OR: 1. 80 [95% CI: 1.17, 2.77]) and T2DM (OR: 4.05 [95% CI: 1.88, 8.69]) than the single effects of ferritin alone (Table 4). In contrast, higher ferritin concentrations in the absence of hepatic iron overload were associated with MetS, but not the isolated effect of iron overload or the combined effect of iron overload and ferritin (Table 4).
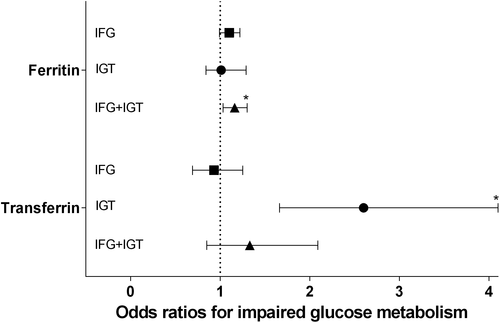
In the analyses of transferrin, a higher value of transferrin concentrations was significantly associated with higher 2-h glucose, fasting insulin, 2-h insulin and HbA1c concentrations independently of known diabetes risk factors, renal function, inflammatory markers and hepatic enzymes (Table 2). When analysing the different categories of impaired glucose metabolism, ferritin was positively associated with combined IFG + IGT (OR per 100 µg/L: 1.16 [95% CI: 1.03, 1.30]), whereas transferrin was positively associated with IGT (OR per 1 g/l: 2.60 [95% CI: 1.66, 4.10]) (Figure 3). We found no significant effect modifications of transferrin by sex, hs-CRP, ALT, hepatic steatosis or iron overload.
4 DISCUSSION
In this population-based sample, serum ferritin concentrations were associated with higher prevalence of IGM, T2DM and MetS as well as higher levels of fasting glucose, 2-h glucose, 2-h insulin, HOMA-IR and HbA1c in the total population independent of inflammatory markers and hepatic enzymes. The association of ferritin with IGM and T2DM was more pronounced in women, while the association with MetS was more pronounced in men. Further, serum ferritin was associated with T2DM and MetS even in the absence of hepatic steatosis. However, the synergistic effect of higher ferritin concentrations and hepatic steatosis showed stronger associations with IGM, T2DM and MetS than the effects of ferritin alone. Similarly, while serum ferritin was associated with IGM, T2DM and MetS even in the absence of iron overload, the synergistic effect of higher ferritin concentrations and hepatic iron overload showed stronger associations with IGM and T2DM than the single effects of ferritin alone. Serum transferrin was associated with IGT, fasting insulin, 2-h post load glucose and insulin, and HbA1c concentrations independently of inflammatory markers and hepatic enzymes.
Serum ferritin has been widely used as a biomarker of iron stores as it is the major iron storage protein in the blood. Previous cross-sectional and longitudinal epidemiological studies3-5, 8, 23-25 demonstrated that serum ferritin levels were associated with an increased risk for T2DM, MetS and also with earlier phases like IGM, which is consistent with our findings. In the stratified analyses, we observed that the associations of serum ferritin concentrations with IGM, T2DM, fasting glucose and 2-h glucose were stronger in women than in men, consistent with few other studies.6, 23 On the contrary, the association of ferritin with MetS and insulin resistance was seen in the total population and in men, but not in women. It is possible that to some extent the association of ferritin with diabetes might be masked by the presence of other risk factors in men, since a combination of risk factors included in the outcome as part of MetS diagnosis showed a stronger association between ferritin and MetS in men. Further, a study which assessed the single components of MetS separately in men and women, reported that serum ferritin was strongly related to higher triglycerides in men and glucose concentrations in women.26 Likewise, our study showed stronger associations of ferritin with glucose-related outcomes in women. In addition, differences in ferritin distributions, genetics and lifestyle characteristics between men and women might partially explain the results as women were less likely to be frequent smokers, hypertensive and diabetic, had lower lipid, ferritin and ALT levels but higher hs-CRP levels in our study (Table S4).
Several possible mechanisms were suggested to explain a link between ferritin and metabolic disorders. One potential cause could be that excess iron may induce oxidative stress and subsequently damage beta cells leading to impaired insulin secretion.27, 28 Likewise, elevated ferritin concentrations might reflect systemic inflammation in addition to increased iron stores since ferritin is also an acute phase protein,29 thus increasing the risk of abnormal glucose metabolism or MetS through systemic inflammation. Our findings, which were adjusted for a range of potential confounders remained unchanged after adjustment for hs-CRP and leucocytes in our study, similar to other previous studies.7, 9 In fact, we even observed that ferritin was associated with IGM and T2DM in participants with hs-CRP concentrations below median. These results further support the role of ferritin in the development of metabolic disorders independent of systemic inflammation.
Another explanation may be that the high iron stores in the liver impair the liver's capability to extract insulin, thereby leading to hepatic insulin resistance and increased hepatic glucose production.27 In our study, we found a stronger correlation between ferritin and hepatic enzymes (ALT and GGT) and the association between ferritin and metabolic outcomes (IGM, T2DM and MetS) was moderately attenuated after adjustment for ALT and GGT in our study. These findings were in line with previously published results.4, 8, 9, 23, 24 These results indicated a role of hepatic dysfunction in the relation between ferritin and metabolic outcomes. On the one hand, several studies suggested a link between hyperferritinaemia and nonalcoholic fatty liver disease,11, 12 thus speculating that excess iron may be involved in the pathogenesis of fatty liver, which in turn contributes to the development of T2DM and MetS via hepatic insulin resistance.13, 14 On the other hand, hepatic iron overload was reported to be strongly associated with insulin resistance irrespective of steatosis or liver damage,30 thus causing insulin resistance directly, which in turn leads to metabolic disorders.31 Hence, we further explored the role of hepatic steatosis and iron overload in the association between ferritin and metabolic outcomes.
Hepatic steatosis is considered to be a common manifestation of T2DM and MetS.10, 13 In our study, we observed an association between ferritin and T2DM regardless of the presence or absence of hepatic steatosis. However, the association between ferritin and MetS was observed in participants without hepatic steatosis but not in participants with steatosis. It is possible that the association of ferritin with MetS might be masked by the presence of hepatic steatosis itself. Further, the association of higher ferritin concentrations with MetS attenuated and became nonsignificant after adjustment for hepatic steatosis, consistent with a study by Zelber-Sagi et al.32 However, the combined effect of higher ferritin concentrations and presence of hepatic steatosis showed stronger associations with prevalence of MetS, IGM and T2DM than the single effects of ferritin or steatosis alone. Our results suggest that hepatic steatosis may play a substantial role in the association between ferritin and MetS and also a minor role in the association between ferritin and IGM and T2DM probably through a pathway greatly overlapping with insulin resistance.33
Hepatic iron quantification is known to be more reliable to assess total body iron stores than laboratory biomarkers. Hepatic iron content was stronger correlated with serum ferritin than hepatic fat content. While a relationship between iron overload and T2DM or MetS has previously been reported,31, 34 our study adds that ferritin was associated with IGM, T2DM and MetS even in participants without hepatic iron overload, consistent with few other studies.4, 31, 35, 36 This association between ferritin and metabolic diseases was not observed in subjects with iron overload. This may be due to less variability in the distribution of ferritin between different glucose metabolic states as the levels were generally higher among iron overload subjects (Table S4). On the other hand, iron overload itself may mask the association between ferritin and metabolic outcomes. However, the combined effect of higher ferritin concentrations and hepatic iron overload showed stronger association with prevalence of IGM and T2DM than the single effects of ferritin alone. On the contrary, neither the combined effect nor the isolated effect of iron overload other than ferritin was associated with MetS. Thus, our results suggest that 1) ferritin may be associated with IGM, T2DM and MetS well below the range of iron overload and 2) iron overload may be more relevant for IGM and T2DM and not for MetS.
Another iron marker investigated was transferrin, a marker of low iron stores. Its production increases when the body iron stores are low; therefore, transferrin and ferritin were inversely correlated. However, we observed positive associations of serum transferrin with isolated IGT, 2-h glucose, fasting insulin, 2-h insulin and HbA1c, which were independent of all risk factors including hepatic enzymes. In accordance, 2 studies have shown transferrin to be associated with higher fasting insulin and 2-h glucose concentrations but not with fasting glucose concentrations, similar to our study. Further, in our study, transferrin showed borderline positive associations with IGM and MetS, but not with T2DM, although positive associations have been reported elsewhere.5, 16, 23, 37 Despite its inverse correlation with ferritin, positive associations of transferrin with metabolic outcomes probably suggest iron-independent pathways.5 However, the possible mechanisms behind these associations remain unclear. Some studies suggest that insulin can lead to upregulation of transferrin expression in human hepatocytes,38, 39 while another study reported that serum transferrin may have an antagonistic effect on insulin action, which can lead to insulin resistance.40 Besides, one could also speculate that the continued state of high glucose concentrations even 2h post meal may have led to upregulation of serum transferrin levels as serum transferrin is said to be highly susceptible to glycation.41
The main limitation of the study is the cross-sectional study design; thus, no causal inferences can be made. However, we have excluded the participants with known diabetes from the analysis to maintain the temporal sequence of association. Despite our large sample size, it should be mentioned, that among previously undiagnosed T2DM subjects (n = 141), the number of subjects with hepatic iron overload (n = 22) was limited, and therefore, our results will need to be validated by further studies. One cannot exclude the possibility of residual confounding, although we adjusted for some important covariates in our analysis. As multiple hypotheses were tested in the same study sample, one cannot rule out the possibility that this approach yielded some false-positive results. However, we would not expect this to be a major limitation because the main findings were relatively consistent between different models. Major strengths of our study include the large population-based study sample, the availability of ferritin as well as transferrin as measures of iron markers, availability of hepatic MRI data and a lot of covariates for potential confounding.
5 CONCLUSION
Overall, we found that serum ferritin was associated with higher prevalence of IGM, T2DM and MetS in the total population independent of inflammatory markers and hepatic enzymes. Serum transferrin was specifically associated with isolated IGT while serum ferritin was associated with combined IFG + IGT. Moreover, our study suggests that ferritin may be associated with these metabolic outcomes even in people without hepatic steatosis or iron overload. However, in individuals with higher ferritin concentrations, the presence of hepatic steatosis may synergistically increase the risk for IGM, T2DM and MetS, while the presence of hepatic iron overload may synergistically increase the risk for IGM and T2DM but not MetS. Thus, our study shows that hepatic dysfunction may play a significant role in the association between ferritin and metabolic disorders.
ACKNOWLEDGEMENTS
We thank all field workers, study physicians, interviewers and laboratory workers for their contribution to data collection. We also thank all participants of the SHIP study for their contribution to the study.
CONFLICT OF INTEREST
The authors do not have any disclosures to report.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.